Back to Journals » International Journal of Nanomedicine » Volume 15
Subcellular Performance of Nanoparticles in Cancer Therapy
Authors Liu CG, Han YH, Kankala RK , Wang SB, Chen AZ
Received 6 August 2019
Accepted for publication 16 December 2019
Published 5 February 2020 Volume 2020:15 Pages 675—704
DOI https://doi.org/10.2147/IJN.S226186
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 2
Editor who approved publication: Dr Linlin Sun
Chen-Guang Liu, 1, 2,* Ya-Hui Han, 1, 2,* Ranjith Kumar Kankala, 1–3 Shi-Bin Wang, 1–3 Ai-Zheng Chen 1–3
1Institute of Biomaterials and Tissue Engineering, Huaqiao University, Xiamen, Fujian 361021, People’s Republic of China; 2College of Chemical Engineering, Huaqiao University, Xiamen, Fujian 361021, People’s Republic of China; 3Fujian Provincial Key Laboratory of Biochemical Technology (Huaqiao University), Xiamen, Fujian 361021, People’s Republic of China
*These authors contributed equally to this work
Correspondence: Ranjith Kumar Kankala Email [email protected]
Ai-Zheng Chen Tel/Fax +86 592 616 2326
Email [email protected]
Abstract: With the advent of nanotechnology, various modes of traditional treatment strategies have been transformed extensively owing to the advantageous morphological, physiochemical, and functional attributes of nano-sized materials, which are of particular interest in diverse biomedical applications, such as diagnostics, sensing, imaging, and drug delivery. Despite their success in delivering therapeutic agents, several traditional nanocarriers often end up with deprived selectivity and undesired therapeutic outcome, which significantly limit their clinical applicability. Further advancements in terms of improved selectivity to exhibit desired therapeutic outcome toward ablating cancer cells have been predominantly made focusing on the precise entry of nanoparticles into tumor cells via targeting ligands, and subsequent delivery of therapeutic cargo in response to specific biological or external stimuli. However, there is enough room intracellularly, where diverse small-sized nanomaterials can accumulate and significantly exert potentially specific mechanisms of antitumor effects toward activation of precise cancer cell death pathways that can be explored. In this review, we aim to summarize the intracellular pathways of nanoparticles, highlighting the principles and state of their destructive effects in the subcellular structures as well as the current limitations of conventional therapeutic approaches. Next, we give an overview of subcellular performances and the fate of internalized nanoparticles under various organelle circumstances, particularly endosome or lysosome, mitochondria, nucleus, endoplasmic reticulum, and Golgi apparatus, by comprehensively emphasizing the unique mechanisms with a series of interesting reports. Moreover, intracellular transformation of the internalized nanoparticles, prominent outcome and potential affluence of these interdependent subcellular components in cancer therapy are emphasized. Finally, we conclude with perspectives with a focus on the contemporary challenges in their clinical applicability.
Keywords: organelle, proton sponge effect, intracellular pathways, cancer therapy, nanocomposites
Introduction
Despite the significant advancements in understanding the origination, development, and maturation of cancer on one end, and the development of numerous therapeutic strategies in its eradication on the other end, the research is still under progress for the development of highly advanced therapeutic strategies for efficient ablation of cancer.1 In this framework, enormous efforts over the past few decades have been dedicated to the tremendous development of a series of several therapeutic strategies, including chemotherapy,2,3 radiotherapy,4 surgical therapy,4 or even palliative therapy,3,5 which have been under practice to fight against this fatal disease. Nevertheless, these traditional strategies suffer from several shortcomings of each method. Along this line, although there has been a significant decline in the overall mortality rate and an increase in the life span of patients, however, the traditional chemotherapy utilizing several chemotherapeutic molecules either alone or in combination has been facing several hurdles. Predominantly, severe adverse effects are instigated in patients on systemic administration of chemotherapeutic drugs due to the undesired accumulation of drugs and metabolites in the vital organs. Secondly, the efficiency of administered drug dose might not be effective as anticipated due to the acquired multidrug resistance (MDR) by the cancer cells through cell surface efflux pumps for cell defense. These predominant consequences often result in poor therapeutic outcomes, leading to the high recurrence rate and utilization of altered therapeutic regimens at high doses.6,7 To a considerable extent, there has been significant progress in the modification of drugs to augment their intracellular bioavailability through various approaches such as chemical functionalization. However, regardless of its success in overcoming the non-specific distribution, this chemical modification approach substantially reduces the efficacy of drugs and can be pragmatic to certain drugs with limited chemical functionalities.
Broadly speaking, various therapeutic approaches known since antiquity are predominantly based on the palliative care, i.e., which has been merely focused on the delivered responses of the sensual systems of a person, by attempting to achieve the temporary respite from the alleviated pain or just to ease their mind as humanistic care. However, eradicating the growth of tumors has remained the predominant goal of therapy at those times.8 In comparison, it is increasingly recognized that the progressively emerged experimental therapeutics possessed the near-standard trials to save patients enduring with miserable diseases,9 which significantly fascinated by dealing with the primary symptoms in the late 19th century. Subsequently, researchers have been more concentrated on targeting the tumor site specifically, by conducting the stereotactic therapy for patients, who were in the early stage with the infeasible surgery practices.10 With the progression of modern society, the technological advancements over several decades have garnered great potential in overturning the aforementioned conventional strategies of discovery, diagnosis, and therapy, to precisely achieve clinical goals relevant to early diagnosis and effective treatment of diseases including cancer.
In this modern era, nanotechnology has garnered significant interest from researchers in various fields for the generation of materials with diverse compositions and morphological attributes.11–14 In the past two decades, these interesting features have significantly influenced the researchers to explore enormous varieties of innovative nanobiomaterials with engineering characteristics and ideal functions through involving supramolecular, nanocrystal growth, and sol-gel chemistries.15,16 The application of nanotechnology to medicine has sparked enormous interest in cancer treatment and diagnosis due to its given special attributes in generating materials with typically controlled multi-dimensional (1–3 D) structures on the nanoscale range (approximately 1–100 nm in one of the measurable dimensions) for drug/gene delivery, diagnostic probes for radioactive or other advanced therapeutic strategies.17 This technology offers enormous advantages in fabricating materials through fine-tuning of physicochemical properties by altering the sizes, shapes, and composition, among others.11 Compared to various traditional chemotherapeutic agents, these engineered materials as drug delivery platforms hold a great promise in offering controlled release of drugs/genes/therapeutic peptides,18–24 exhibiting cancer microenvironment responsiveness for drug release,6,7,25,26 and other sophisticated external stimuli-response delivery including photothermal therapy (PTT),25,27–30 photodynamic therapy (PDT),30–32 ultrasound-responsive and immunotherapy, among others.33–36 Among such delivery systems, a few of them have been approved for cancer therapy, and numerous others are currently active and/or recruiting additionally under clinical trials.37
Compared to their bulk counterparts or precursors, the fabricated nanoparticles of specific composition exhibit two intrinsic advantages of high surface-to-volume ratio and abundant surface chemistry.38 The high surface-to-volume ratio of nanoparticles enables the encapsulation of diverse therapeutics molecules (drugs/genes/contrast agents/therapeutic peptides) either in the interior or over the surface through physical adsorption or electrostatic interactions.39 In addition, the availability of abundant functionalization surfaces facilitates the ease of immobilizing therapeutics guests, and diverse biomacromolecules for altering their behavior in vivo, and feasible targeting ligands for augmenting their bioavailability.40 Broadly speaking, all the available nanomaterials used for biomedical applications can be categorized based on the chemical composition into three major divisions of organic, inorganic, and inorganic/organic hybrid systems. The carbon-based organic materials, for instance, liposomes, emulsions, among others, are relatively highly compatible and degradable in the physiological environment, which have driven their earlier translation to clinics, for example, Abraxane, Doxil, DaunoXome, and, Myocet, among others.37,41 Despite their success, numerous formulations based on the organic materials have been withdrawn, and many struggling to pass further clinical translation procedures due to their intrinsic features of poor stability in the physiological fluids and deprived drug encapsulation efficiency.38 Comparatively, the inorganic-based nanobiomaterials offer exceptional advantages like intrinsic thermal and colloidal stabilities, resistance, unique structural and chemical optical features, as well as morphological attributes, ease of tailoring the architectures, but relatively low degradation rates.42 Specific examples of well-known inorganic materials include layered materials, magnetic nanoparticles, semiconductor quantum dots, metal nanoparticles (MNPs), mesoporous silica materials, which are of particular interest in diverse biomedical applications.6,7,43,44 However, the translation of these stable constructs have been encountering a great challenge due to the safety and degradability concerns in the biological environment.38,45–47 Finally, the hybrid systems combined with the intrinsic advantages of both organic as well as inorganic offer great benefits of compatibility, degradability, and stimuli-responsive delivery of therapeutic guests.48,49
In general, the functional nanoparticles for cancer therapy are predominantly administered through the intravenous route, which could circulate systemically throughout the blood circulation due to their tiny sizes providing extended circulation in vivo. Appropriate design and specific modifications resulting in augmented physiochemical attributes of nanoparticles facilitate their precise adherence to different proteins in the systemic circulation, which prolongs the circulation time and conveniently facilitates reaching the tumor site.50 Further, the cellular internalization could be achieved either by passive (Enhanced Permeation and Retention, EPR) or active targeting modes, which could lead to better therapeutic outcomes over conventional therapeutic strategies. Nevertheless, they still suffer from significant limitations of nonspecific distribution and deprived therapeutic efficiency, which yet to remain unaddressed. Moreover, it should be noted that rate of cellular internalization and final bioavailability of the carrier is affected by the size, shape or surface charge of the nanoparticle. The final size of the materials in the nanoscale range plays a crucial role in creating these complex structures, which accelerate experts in shifting their attention from targeting macro-/or micro-sized pathological tissues or organs to specific cells concerning their promising, safe and effective delivery as well as subsequently understanding the mechanisms of action and degradation pathways. For instance, Tammam et al51 used chitosan nanoparticles as models, where different-sized nanoparticles showed size-dependent cellular internalization efficiency and subsequent protein delivery. Similarly, the final particle size, as well as surface charge of the nanoparticles, significantly affected their subcellular localization. Moreover, numerous reports indicated that the ultra-small positively-charged nanoparticles had a higher affinity toward specific organelles, such as mitochondria and nucleus, facilitating their permeability. Similar to the cell surface, these membranes also possess surface charge enabling the electrostatic interactions with the guest species.52–54 Furthermore, via triggering phase transformation, free energy release, reorganization, and dissolution, these internalized nanoparticles subsequently respond to the cell membrane proteins, and organelles as well as nucleic acid content to establish various kinds of interfaces that could sustain by the colloidal forces and dynamic biochemical reactions. Substantially, these consequences promote the formation of the protein corona, particle encapsulation, intracellular uptake, and biocatalysis during the course of transportation in the biological microenvironment.55
Notably, there exist specific distinctions between organs and microscopic cellular substructures in possessing unique specific morphology and structural as well as functional attributes. In this context, numerous reports have demonstrated targeting the cancer cell surfaces using different ligands on nanoparticles and their subsequent fate in cells by focusing on the basic transport or transfer mechanism during the internalization process. In addition, a step forward, a few of the reports have explored some specific ligands that could target a few of the organelles and reconnoitering their specific pathways that act through.38,56–58 In this vein, some of the intriguing reports by Steichen38 and Varkouhi56 reported the behavior of nanoparticles concerning their entry into cells. These precise reviews gave a summary of selected information on the formation of vesicles that encapsulate nanocarriers from the early endosome to the late endosome with a pH transformation from 7.4 to 5.0 for degradation and their escape mechanisms for their delivery in the cytosol, contemporary pore formation, pH-buffering effect, and protein fusion.38,56 As mentioned earlier, the specific interactions and precise effects between nanoparticles and the organelles play a crucial role in determining the behavior and the fate of nanoparticles intracellularly, which could explicitly provide the evidence in designing the efficient delivery systems.
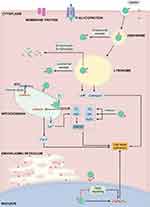
In general, the intracellular route map of nanoparticles is explicitly discussed hereunder. As shown in Figure 1, nanoparticles are predominantly engulfed by cells through different pathways depending on their size and surface charge, which significantly depend on various modifications over the nanocontainers and intrinsic properties of the core material. Broadly speaking, the particles with larger size (>1 μm) can be generally entered into the cells by micropinocytosis. Contrarily, the nanoscale products are most commonly internalized through the endocytosis pathway, which is dynamic, convenient, and well-regulated internalization route for any particle.59 However, the type of endocytosis for the entry of nanoparticles is different, which significantly depends on the eventual size of the particles. Small-sized particles (~60 nm) could conveniently enter the cell by the caveolin-dependent endocytosis, in which the nanoparticles could be exposed to neutral pH in a caveosome. On the other hand, the large-sized nanoparticles (~120 nm) could be internalized through clathrin-dependent/independent endocytosis, which predominantly depends on the modifications over the nanoparticle surface by ligands and their types as well as the shape of the particles.60 The nanoconstructs that can pass through the cell membrane via endocytosis is exposed to the acidic microenvironments in the endosome and subsequent lysosome.61 More often, the internalized nanoparticles are encapsulated in the endosome-based liposome packets, which further pass them to the lysosomes with a highly acidic microenvironment, where they undergo enzymolysis and hydrolysis or discharged the inert nanocontainers unmodified into the cytoplasm through lysosomal escape pathways.59 Further, these liberated nanoparticles into the cytoplasm continue to interact intracellularly with other specific organelles, for example, reacting to the components in the acidic environment of endocytosis/lysosome, activating electron transport chain or the production of reactive oxygen species (ROS) in mitochondria, or Golgi apparatus and regulating DNA-control in nucleus or mitochondria. In some instances, after interacting with the specific organelles and performing their predetermined specific tasks, such as delivering the therapeutic agents, and photoactivation, the nanoparticles, such as polymer-based soft composites that are sensitive to these microenvironments, can be degraded along with the cell deterioration after death, whose remnants can be then dealt with by macrophages like all other components of cells. In the case of non-degradable materials, they may continue to internalize into the adjacent cells.45,62 To this end, the non-biodegradable inorganic-based nanoparticles could be taken up by the lymphatic drainage and eliminated subsequently after prolonged circulation in the physiological environment. Hence, specific structural attributes, biodegradability behavior, and the appropriate acting mechanisms of specific organelles reconnoitering the facts behind the subcellular performance and the fate of nanoparticles should be explored for better insights to exploring new innovative technologies.
In the further sections, we first give emphasis on recent advancements concerning the escape pathways of internalized nanoparticles through endosome/lysosome, highlighting the advantages and limitations. We then focus on the intracellular performance as well as the fate of nanoparticles with the essence showcasing the precise interactions between nanoparticles and different key organelles, such as mitochondria, nucleus, endoplasmic reticulum, and Golgi apparatus. Finally, we conclude the prominent outcome, contemporary challenges, and the potential influence of these interdependent subcellular components in cancer therapeutics.
Subcellular Performance and Intracellular Fate of Nanoparticles
A cell is a typical unit of the body possessing several organelles, which are much like organs in the human body as every organelle has its specific duties to perform in providing all needs to the cell, such as supplying nutrients, removal of waste, cell repair, growth, and reproduction, among others. In fact, the word organelle refers to a mini-organ. Along this line, various subcellular key organelles play a crucial role in handling nanoparticles-based cancer therapeutics after their successful internalization such as endosomes as well as lysosomes for digestion of the internalized foreign bodies, autophagy, and first line of cell defense,63 mitochondria for monitoring the apoptotic cascades, calcium cycle, and ATP synthesis,64 nuclei for DNA alteration, gene expression, and cell proliferation,65 and endoplasmic reticulum, as well as Golgi complex apparatus, facilitate the protein synthesis and their transportation to other organelles.66,67 Meanwhile, they also play different and necessary roles in relevant to cancer ablation and diverse metastasis inhibition pathways, such as apoptosis, autophagy, necrosis, and Ferroptosis, among others.68 Considering these aspects, it could be beneficial in driving these prominent biological effects of various organelles towards the therapeutic opportunities in eradicating cancer. For instance, some of the drugs, such as cisplatin and doxorubicin (DOX), precisely exhibit their effects in the nucleus.69,70 Thus, it is highly required to augment the availability of such drugs in the respective organelles and activate the specific cell death signal. In addition to therapeutic efficiency, these organelle-targeting strategies of conveying therapeutic species could address a significant limitation of high dosage necessity due to premature leakage and drug resistance. These consequences result in achieving high therapeutic index by overcoming numerous biological barriers to reduce the adverse effects of such cytotoxic drugs. Herein, we emphasize the discussions on all these aspects, highlighting the critical behavior of the nanoparticles and the treatment received from the organelles (Table 1).
 |  |  |
Table 1 Examples Presenting Different Organelle-Targeted Nanoformulations for Cancer Therapy |
Endosome/Lysosome
Typically, the endosomes are membrane-bound organelle compartments of endocytic transmembrane that represent the primary sorting endomembrane system in cells, involving the transportation of different substituents through major sorting pathways. These resultant endomembrane pockets predominantly act as primary doorsteps for nanoparticles toward their cellular internalization and selective conveyance to the interior of the cell through the inner compartments called lysosomes. Distinguished by three different phases of early, late, and recycling compartments, the endosomes significantly provide the acidic environment for endocytosed materials to be sorted before reaching the degradative lysosomes, which is the terminal point of the endocytic pathway, that also stemmed from the trans-Golgi membrane system.71,72 While on their way toward the target site intracellularly, the endocytosed nanoparticles face an extreme shift in the pH of their surrounding medium from an environmental pH of 7.4 in the extracellular medium to approximately 6.0 in the early endosomes, and further to 4.5 in the lysosomes due to excess proton supply in the endocytic medium.73 Such a concomitant shift in pH has become an efficient biological trigger in delivering the active therapeutic guest molecules from the bulk of the containers. These consequences involve renowned changes through the spatiotemporally controlled way by utilizing the stimuli-responsive molecular switches that contain ionizable weakly acidic/alkaline groups, e.g., disulfide linkage, hydrazone, and dinitroimidazole, among others.74,75
In addition to the conveyance of the guest species, the endosomes facilitate the recycling of the guest molecules via fusion, where they are transported from the plasma membrane to early endosomes or delivered through the vesicles back to the plasma membrane.76 This unidirectional transport from the late endosomes to lysosomal compartments is the unique characteristic feature of the entire internalization process, as a result of consumed fusion with lysosomes. However, in some of the peculiar cases of extremely tiny constructs (size approximately less than 1 nm) that can pass directly through the phospholipid bimolecular layer, the cellular internalization process of most of such nanoparticles, no matter the passage through which pathway they select such as clathrin-dependent/independent endocytosis, or micropinocytosis and phagocytosis driven by specialized actin, would come in early endosomes and subsequently toward the late endosomes.76,77 In addition, it should be noted that there exist some specific instances, where if no effective or specific modifications were made on the surface of the nanoparticles to increase the surface charge or enable proton sponge effect, the nanoparticles would stay in the late endosome till it adjoins with the lysosomal compartment.73,78 More often, the nanocarriers that are modified with specific modification with ligands can escape through various pathways resulting in further events of ablation, while the particles without any surface modification that are prone to the lysosomal environment get subsequently degraded in this compartment. However, the degradation behaviour of unmodified particles stringently depends on the intrinsic stability of nanocarriers. Herein, the intracellular behavior and fate of nanoparticles that correlating with the several functions of these organelles are discussed in relevance to the ablation of cancer cells, highlighting the degradation and escape mechanisms.
Degradability
Since their discovery, lysosomes have been considered as the “recycling bins” of the cells, due to their capability of degradation by high amounts of destructive enzymes like hydrolases in their lumen.79 Notably, lysosomes act as degradative compartments for most of the resultant products of biosynthesis that often participate in various cellular processes, such as membrane repair, pathogen defense, cell autophagy, and signal transduction, as well as foreign bodies internalized through endocytosis.79 From this perspective, Grant and Donaldson classified the roles of caveolar endocytosis containing the dynamin-dependent caveolin coat for the internalization of glycosphingolipids and some specific viruses, or independent modes like cell division control (CDC) protein-42 homolog, ADP-ribosylation factor (ARF)-1 and actin, among others.76 In addition, the authors demonstrated that the nanoparticles without any specific surface modification are highly prone to the acidic environment in lysosomes similar to all other intracellular constituents as they are exposed to multiple hydrolases of the lysosomal lumen. Further, the surface of nanoparticles was gradually dissociated, and the intact structure of nanoparticles was eventually broken, resulting in the small compartments as well as highly-charged ions. However, it should be noted that the resultant products significantly depend on the molecular structure and chemical composition of the nanomaterials.80 These tiny resultant substituents, such as metal ions or other molecules at a high concentration released into the cytoplasm, can significantly affect the normal biochemical activities of cells and homeostasis, leading to the significant imbalance in the intracellular biochemical components and other cytoplasmic constituents. Moreover, in some instances of highly-charged metal species, these consequences might also lead to changes in the expressions of intracellular proteins and genes, resulting in the ablation of cancer cells through apoptosis.
In general, the nanoparticles predominantly aggregate in the lysosomal compartment, which, however, depends on the physicochemical properties as well as morphological attributes of materials, e.g., particle size, surface charge, shape, and chemical composition.78,81 In addition to the aggregation behavior, the highly acidic environment facilitates the degradation of acid-prone architectures, which are discrepant and predominantly based on the chemical composition. These specific nanoarchitectures are highly attractive as the lysosomal targeted systems eventually reach after their internalization through receptor-mediated endocytosis. In this framework, the organic material-based nanoparticles, usually the so-called biodegradable polymers (Poly-L-lactide, PLA),81 can be designed by a kind or more of monomers linked by various sensitive ionizable weakly acidic and basic linkages (polymers from natural origin, dextran and chitosan and synthetic origin that possessing acid and amine functional groups),82 as well as specific molecular switches, such as disulfide, hydrazone, dinitroimidazole-based linkages.81,83–86 In fact, these molecules are highly responsive to the acidic environment and can significantly facilitate the degradation of the construct.87,88 To this end, some of the inorganic nanoparticles, such as metal-organic frameworks (MOFs), periodic mesoporous silicas (PMOs), and other stable nanoparticles such as gold and platinum nanoparticles, that are immobilized with acid-responsive linkages can be degradable in the endosomal/lysosomal microenvironment, resulting in the small-sized nanocrystals or even free ions.78,89–91 These stimuli-responsive linkers offer enormous advantages in drug delivery over conventional carriers, including the deprived premature release of therapeutic guests in the physiological fluids, improved encapsulation efficiency, and reduced undesirable adverse effects, among others.82,92 To the other end, some of the inorganic nanomaterials are acid-prone such as layered double hydroxides (LDHs), which can not only facilitate the precise release of drugs in the lysosomal environment through controlled degradation but also conveniently enable degradation in the physiological fluids.49,68
Escape Pathways
In addition to the specific internalization pathways through active or passive modes, the surface-modified nanoparticles functionally for controlled drug release, and conformational or photothermal conversion are directed out of the lysosomes through various discharge pathways. Numerous efforts over the past decade have been dedicated to designing different versatile nanoparticles-based formulations with several modifications on their surface that precisely guide them to the site of specific intracellular organelles for exhibiting specific functions, namely organelle targeting, Various examples of organelle-targeting ligands include mitochondriotropic moieties like triphenylphosphonium (TPP), dequalinium (DQA), and mitochondrial penetrating peptides (MPPs).93–95 However, it should be noted that the modified particles are required to escape from the lysosome and subsequently drive themselves toward the targeted organelle as well as facilitate the release of therapeutic cargo in the cytoplasm or targeted organelles.57 The mechanism of the endosomal/lysosomal escape of nanoparticles happens to be favorable in various ways: 1) Formation of membrane apertures in the endosomes or lysosomes, 2) Proton sponge effect, 3) Fusion with the membrane, and 4) Photochemical disruption of endosomal or lysosomal membranes.
Lysosomes, the multivesicular bodies (MVBs), are generated from the matured vacuolar elements that pinched-off from the regions of perimeter endosomal membrane bud toward the luminal compartment.96 Indeed, these are the inseparable targets of late endosomes as well as the destination of the endocytic pathway. However, the degradation membrane relies on the superficial pore dynamics involving the subsequent opening and closure of the gap, which can be predominantly controlled together by the line tension as well as the membrane tension, enabling the expansion or shrinkage of the pore, respectively. However, the initiation of pore formation happens to be conducive at such circumstances of an upsurge of the membrane tension or reduction of the line tension, and vice versa (Figure 2). In this context, Chen et al demonstrated the fact lying behind the transition of alamethicin from S state (embedded in the head group regions on account of the hydrophobic interaction) to I state (forming transmembrane pores subsequently killing cells), in which the concept of membrane elasticity and the transformations tangled between barrel-stave pores and toroidal pores were involved.97,98 Furthermore, utilizing the water-soluble amphipathic antimicrobial peptide, Lee et al confirmed the formation of equilibrium pores that induced by peptides while monitoring of giant unilamellar vesicles (GUVs),99 in which these kinetic pores-implanted single membranes significantly exhibited optimized effects with stable size. Along this line, the superficial modification of nanoparticles are formulated with peptides or the similar components of polypeptides, such as melittin,100–102 bovine prion protein (bPrPp),103 listeriolysin O,104 and streptococcal streptolysin O,105 among others, have shown excellent affinity to the edge of the pores, which significantly reduced the line tension after associating with the edge of the pores. However, the loss of balance between the membrane tension and the line tension could not stabilize the original pore size, facilitating the subsequent expansion of pores to form irreversible apertures in the membrane of lysosome or endosome.106
 |
Figure 2 Proposed interactions between peptides (rectangles) and lipid bilayers. (A) Peptides are soluble in water but have a high affinity for binding to lipid bilayers. (B) Peptides inserting into the head group region stretch the membrane area, cause thinning of the chain region, and thus create an internal membrane tension. (C) Peptides preferentially bind to the edges of the pores, which have the consequence of loosening the internal membrane tension. (The depicted pores are called toroidal, or wormhole models. Some peptides make barrel-stave pores. It should be noted that the mechanism is the same for both types of pores.) Reprinted figure with permission from Huey W Huang, Fang-Yu Chen, and Ming-Tao Lee, Physical Review Letters, 92(19), 2004.] Copyright 2004 by the American Physical Society.106 |
Although the lysosomal escape pathway of internalized nanoparticles is often debated and elusive, the proton sponge effect hypothesis is generally accepted. Oftentimes, in the high pH conditions, the lumen of lysosomes allows the inflow of excess protons by enabling the membrane pumps, which subsequently drive the intramolecular exchange with the chloride ions and water. Further, these consequences enable the infiltrate swelling, resulting in the burst of lysosome and subsequently release the intraluminal constituents into the cytoplasm. Oftentimes, this phenomenon is significantly achieved through specific modifications, involving the unsaturated amino chelation, in which the continuously opened proton pump (V-ATPase) induces the exchange of chloride ion and a water molecule retention for each proton. Then, the excess inflow of various ions and water molecules triggers the swelling and substantial outburst of the lysosome.56 Considering the polyplexes, Nel et al55 supposed that the superficial unsaturated amines could chelate protons and retain the operating pumps, which were significantly responsible for the acidification of lysosomes. For every proton carried by the nanoparticles during the internalization to the lysosomes, an equivalent number of chlorides, as well as water molecules needed to be retained for maintaining the homeostasis (Figure 3). However, the particles could be escaped from the swelled and ruptured lysosomes due to the influx of chloride and water molecules, and eventually deposited in the cytoplasm. Simultaneously, the leakage of lysosomal content and calcium ions (Ca2+) might result in the reduction of ATP and the increment of a cytotoxin, inducing further apoptosis. In one instance, the escape pathways of nanoparticles with similar size and shape, but different charges of positive (MNPs, MIL-101, Zeolitic imidazolate framework (ZIF)-8 and nanoparticles modified with amine functionality), neutral (UiO-67 and nanoparticles coated with polyethylene glycol, PEG), and negative (SiO2, MOF-801 and nanoparticles modified with carboxyl functionality) potentials were examined. It was concluded that the nanoparticles with positive charge could only be feasible to escape from the lysosome, and nanoparticles with negative or neutral charge could almost be retained in the lysosome before enzymatic hydrolysis. These consequences happened to be conducive as the positively-charged nanoparticles (for instance, PEI) could be efficiently internalized into the lysosome due to the differences in charge. Moreover, a large number of chlorine ions were pumped-in to neutralize the charge density in the lumen of the lysosome, leading to the expansion and disruption due to a sharp enhancement of osmotic pressure in the lysosome.107 Although the substantial exchange of ions happened, it should be noted that these consequences have no significant influence on the change in the lysosomal pH.
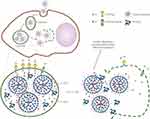 |
Figure 3 Schematic illustrating the proton sponge effect leading to the lysosomal damage and the induction of cytotoxicity by the cationic nanoparticles. Cationic (for example, PEI-coated) nanoparticles bind with high affinity to lipid groups on the surface membrane and are endocytosed in the tight-fitting vesicles. Reprinted by permission from Springer Nature, Nature Materials, Understanding biophysicochemical interactions at the nano-bio interface, Nel AE, Madler L, Velegol D, et al., Copyright 2009.55 |
This critical phenomenon of escape by membrane fusion can be often observed in the cell transport and endocytosis processes, usually involving the participation of fusion molecules with a similar composition like peptides or lipids (liposomes, emulsions, or similar biomimetic molecules) coated over nanoparticles favoring their escape. With respect to the physicochemical properties of subcellular targeted nanoparticles, the intracellular concentration, exposure duration, and the surface reactivity are all indeed the predominant attributes that are responsible for driving the successful interactions between them prior to the escape of the guest species.55 Notably, the nanoparticles immobilized with specific fusion peptides (e.g., Green Fluorescent Protein (GFP)-tagged Rabs, Rab4, Rab5, Rab7, Rab11, GTPases) on the surface can interact with the lysosomal membrane and comprehensively achieve the escape of nanoparticles through facilitating the membrane fusion phenomena.108 For instance, the confocal microscopic analysis indicated that the green fluorescent assemblies of the perinuclear aggregate in Enhanced Green Fluorescent Protein (EGFP)-tagged Rab7 wt-expressing cells were partly co-labeled with the late endosome marker CI-MPR (Figure 4A–C) but at a high degree of the lysosome markers Lamp-1 (Figure 4D–F), Lamp-2, and cathepsin D (Figure 4G–I). Nevertheless, CI-MPR and Lamp-1 markers presented a certain degree of overlapping. Similarly, Song et al109 used wheat germ agglutinin-functionalized polymeric nanoparticles (WGA-NP) to explore the mechanisms of transcellular passage in Caco-2 cells, which exhibited that the various molecular weights of surface PEG resulted in altered cellular association, transcellular conveyance, and endocytosis pathway efficiencies. However, the core material, PLGA significantly contributed to a better lysosome escape and higher WGA-NP transcytosis after incubation for 2 h. In addition to the type and varied length of the polymer, the size and shape of the eventual nanoconstructs had shown a significant influence on the phagocytic fusion mechanism. In an attempt to elucidate these attributes, Chithrani and coworkers110 opted for the colloidal gold nanoparticles (sizes from 14 to 100 nm) as the ideal model system for uptake and evaluated their subsequent internalization. In comparison, the authors demonstrated that the spherical-shaped nanostructures showed higher entanglement to the cells and subcellular compartments over the rod-shaped nanoparticles. Further, Rejman and coworkers111 revealed that the intracellular routes of nanoparticles were intensively dependent on their particle diameters, where the internalization of particles with diameter less than 200 nm was predominantly favored by clathrin-coated cavities. Together, the authors concluded that the caveolae-mediated internalization was highly conducive owing to the increase of particle diameter. However, the destination of particles with a diameter greater than 500 nm was not unconcealed to the lysosomes.
 |
Figure 4 The EGFP-Rab7 wt fusion protein is mainly associated with lysosomes. Green corresponds to the EGFP signal (A, D and G) and red to the immune-detected markers CI-MPR (B), Lamp-1 (E), and cathepsin D (H). (C), (F), and (I) show the merged images, where yellow indicates co-localization. Note that the co-localization of EGFP-Rab7 wt with CI-MPR is only partial (C), whereas there is a more distinct co-localization of EGFP-Rab7 wt with Lamp-1 (F) and cathepsin D (I). Bars, 20 mm. (J, K and L) Immuno-gold labeling of cells expressing the EGFP-Rab7 wt. The panels show examples of large vesicular structures forming tightly packed aggregates. These structures appear as multi-vesicular bodies with numerous small, internal vesicles, or they have a more typical lysosome appearance with a dense content of membranous material. Note that all of these aggregated, late endosome/lysosome–like structures (Ly) are distinctly labeled for EGFP (10-nm gold; arrowheads) on the cytoplasmic surface of their outer membranes, as well as for Lamp-1 internally (15-nm gold, small arrows). Correspondingly, note that minimal cytosolic labeling for EGFP is seen. Bar, 250 nm. Republished with permission of American Society for Cell Biology, Rab7: a key to lysosome biogenesis, Bucci C, Thomsen P, Nicoziani P, Mccarthy J, Bo VD, 11(2), 2000; permission conveyed through Copyright Clearance Center, Inc.108 |
In regard to the utilization of nucleosomes and lysosomes, the photochemical internalization (PCI) approach is reckoned as a localized hydrophilic process. Notably, the photosensitizer-mediated chemiluminescence reaction happens to be favorable upon its stimulation, resulting in the generation of ROS that significantly destroys the membrane structure. Moreover, it leads to the evacuation of nanoparticles from the lysosomes or other membrane-bound organelles such as the nucleus.112 Upon conducting the experiments on the xenotransplantation models of over 80 different cells and 10 different tumor cells, Selbo et al113 demonstrated these implications of PCI for the first time that the nanocarriers could escape and release the therapeutic guests into the cytoplasm.114,115 Further advancements on the PCI approach resulted in driving them to Phase I/II clinical trials in 2010.113 Irrespective of the mechanism, either active or passive mode of disruption, the integrity of the endosomal/lysosomal membrane is either partially or completely destroyed due to the deadly ROS. In one of the recent studies, it was observed that the disruption of lysosomal membrane often led to the release of cathepsins through a process of lysosome membrane permeabilization (LMP) pathway due to the toxic ROS, resulting in the activation of endogenous apoptotic stimuli.116 These consequences could be more evident in cancer cells due to the higher availability of cathepsins compared to the normal cells.
Correlating to these aforementioned facts, we recently demonstrated that the 2D layered materials, LDHs with excess hydroxyl groups on their surfaces, could also induce the enrichment of cathepsins release, which might have initiated the cell death through activating apoptotic cascades.68 In another case, See et al demonstrated that the CALNN-capped gold nanoparticles were digested by the protease cathepsin L, and could be used for biosensing application.78 Similar to the fusion-like escape, the particle diameter also significantly influences the PCI-based approach. However, contrarily, the interference of the nanoparticles with the lysosomal membrane is inversely correlated to the particle diameter, indicating that the impact of diameter (>500 nm) is insignificant.111 Regardless of their mechanism of lysosomal escape, it also results in evacuating the nanoparticles from lysosome and substantial disruption of lysosomal membrane and ablation of the cancer cell. In addition, specific mechanical abrasion modes such as alternating magnetic fields can also be applied for improving the LMP. Owing to their non-invasive and excellent controllability attributes, this strategy can effectively break down the lysosomal membrane, which not only drives the nanoparticles but also augment the release of lysosomal enzymatic cascades and substantial lysis of the organelle.117 Further, the improved accumulation of nanoparticles through various lysosomal targeted systems facilitating the lysosomal disruption mechanisms enhance their delivery efficiency and substantial antitumor efficacy.
Lysosomal Cell Death Mechanisms
In addition to recycling cellular constituents through the degradation of intracellular and internalized foreign bodies for maintaining homeostasis, lysosomes act as crucial sites in initiating and accomplishing various cell death pathways such as apoptosis and autophagy or necrosis.118,119 Amongst various cell death mechanisms, autophagy is the lysosome-dependent self-destructing approach, which often degrades or recycles biomacromolecules and undesired organelles.120 Lysosomal cell death pathways are often rapidly executed while altering the LMP-mediated pathways and substantial discharging of their hydrolytic contents into the cytoplasm.73,121 LMP can be significantly induced in lysosomes through various mechanisms, involving ROS generation, and utilization of lysosomotropic agents, and some cell-specific mortality effectors, such as Bcl-2 family proteins and microtubule toxins.122 In this framework, inhibition of various enzymes can also induce the stimulation of LMP in the lysosomal lumen. Contrary to the normal cells, lysosomes possess significantly larger volumes of proteases in the tumor cells. For instance, acid sphingomyelinases (ASM) that catalyze the hydrolysis of sphingomyelin can be inhibited by utilizing the cationic amphiphilic drugs (CADs) by interacting with the lysosomal membrane, accompanying the integrity of lysosomal membrane to stimulate LMP (Figure 5). Contrarily, the release of cathepsins into the extracellular space that can engage in the degradation of basement membranes and exterior matrix sequentially promotes the development of the tumors.123–126
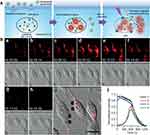 |
Figure 5 (A) Lysosome-aimed strategy for simultaneously targeted delivery, lysosomal imaging and destruction, and real-time self-feedback of therapeutic efficacy. Real-time monitoring of fluorescence and morphology during PDT. (B) a–h) Real-time fluorescence images at λEx/Em of 633/660–720 nm (top) and bright-field images (bottom) of Apt-TNP-loaded MDA-MB-231 cells under 808 nm irradiation at 100 mW cm−2. i) Merged fluorescence and bright field image at the starting time of PDT. Scale bar, 20 mm. j) Time course of fluorescence intensity collected at dots 1–4 shown in (i), corresponding to the lysosomes (1, 3) and cytosol (2, 4). Tian J, Ding L, Ju H, et al. A multifunctional nanomicelle for real-time targeted imaging and precise near-infrared cancer therapy. Angew Chem Int Ed. 2014;53(36):9544–9549. With permission from Copyright 2014 John Wiley and Sons.63 |
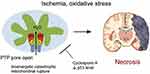 |
Figure 6 Schematic illustrating the p53 accumulation in the mitochondrial matrix and triggering the opening of mtPTP and substantial necrosis by the physical interaction with the PTP regulator Cyp-D. Reprinted from Cell, 149(7), Vaseva AV, Marchenko ND, Ji K, Tsirka SE, Holzmann S, Moll UM, p53 opens the mitochondrial permeability transition pore to trigger necrosis, 1536–1548, Copyright 2012, with permission from Elsevier.145 |
Apart from these tumor-accelerating functions, the cathepsins also show a strong ability toward suppressing the growth of the tumor, which could be potential targets in ablating tumors.63,127,128 Specific consequences of lysosomal escape that can alter LMP or destruct the lysosomal membrane lead to the release of cathepsins significantly. Further, the destruction of the lysosomal membrane leads to the discharge of hydrolytic enzymes, initiating the unselective damage to the cell constituents, and further triggers the process of necrosis through cytosolic acidification.122 Kashkar et al demonstrated that the UV irradiation-triggered ASM activation played a crucial role in inhibiting tumor metastasis and an essential prerequisite for Bax conformational change.129 Contrarily, in the ASM-deficient cells, the discharge of enzymes was highly concentration-dependent, which in turn activated the caspase-independent necrosis. It was also observed that the matured lysosomes were more anticipated to be induced by activated LMP, while the lysosomes in the close proximity to the mitochondria suffered from slight membrane injury.122 In another case, Xue et al demonstrated that the cells with antiapoptotic oncoproteins (26-kDa Bcl-2 and 24- to 33-kDa Bcl-xL protein) loss due to the light-induced damage could be more sensitive to apoptosis.130 Although there have been some pieces of evidence on the potential of nanoparticles in overcoming MDR, it was highly anticipated that this light-induced lysosomal death pathway could be one of the targets of action for exploring the therapeutic strategies in tumor ablation, especially in PDT.63,131,132 However, in-depth analyses on developing specific markers for tracing the cathepsin moieties are required to explore their release into cytoplasm. Despite their promising effects involved in cancer therapy, lysosomes have not received much attention compared to other organelles such as mitochondria and nucleus. Further efforts are required to explore the mechanistic insights involved in the endosomal or lysosomal-targeted systems.
Mitochondria
Mitochondrion, referred to as the powerhouses of the cell, is a bilayered film-coated semi-autonomous organelle, reserving its own genetic material,133–135 including pivotal oxidative phosphorylation (OXPHOS) genes.136,137 This semi-autonomous organelle significantly participates in the differentiation, signaling, and apoptotic events of cells. Notably, the outer membrane, with ultra-small Parson enclosing the entire organelle, contains a wide variety of enzymes engaged in diverse physiological activities. Cytochrome C, one of such electron transport proteins situated at the intermembrane localization, is involved in the bio-oxidation process. The inner space of mitochondrion contains an extensive impermeable membrane under the contribution of numerous cristae. Over the decades, several advancements have been made and realized that numerous essential functions involving the cell regulation systems take place in the mitochondria including the generation of energy (primary source of ATP synthesis), regulation of redox reactions and resultant ROS generation, immunity regulation, integration of reticulum, transduction of signals, differentiation of cells, balance of intracellular calcium ions, and the biological precondition of the synthesis of partial cell plasma, e.g., acetyl coenzyme A and pyridine, to adjust the metabolism and control the cell cycle.138 In addition to these normal physiological and biochemical activities, mitochondria are also involved in some specific cell death mechanisms such as apoptosis and antiviral responses of the cells relevant to diseases, such as chronic lung infections.139–141 These critical biological effects of this organelle offer enormous potential towards effective cancer treatment by precisely targeting the mitochondria over conventionally disposing the drugs in the cytoplasm.
It is increasingly recognized that apart from the ability of endoplasmic reticulum in regulating apoptosis independently, and many acquaintances need to be coupled with the other organelles. However, the most crucial organelle involved among them is mitochondria, from which BH3 (Bcl-2 homology domain protein) is the only activated protein domain that induces the apoptosis by stimulating the pro-apoptotic effector protein, such as BAX or BAK, after which the triggered oligomerization leads to the mitochondrial outer membrane permeabilization (MOMP).142 It should be noted that the mitochondrial permeability transition (MPT) is the crucial factor in initiating the apoptotic pathway under the circumstances of oxidative stress with the loss of potential and impairment of the membrane integrity.143 The mitochondrial membrane permeability transition pore (mtPTP), known as the polyprotein transmembrane channel permitting the transit of <1.5-kDa solutes,144 is often regulated by specific conduits, cyclophilin (Cyp) D, voltage-dependent anion channel (VDAC), as well as adenine nucleotide translocator (ANT). Responding to the oxidative stress, mtPTP is activated by the change of membrane osmotic pressure or opened by p53, a central stress sensor (Figure 6).145 Further, the apoptosis protein signal is excited by the cytochrome C release into the cytosol, in which the apoptosome is formed by the incorporation between cytochrome C and APRF-1, activating downstream cysteine proteases, for example, caspase-3, eventually resulting in the disassembly of cell cluster leading to phagocytosis.64,142,146 Consequently, the release of cytochrome C also induces mitochondrial DNA damage, resulting in cell necrosis.58 Moreover, the mitochondria in cells show an enormous impact on diverse life activities, especially the occurrence and development of tumors. In the late 1950s, Warburg demonstrated that the cancer cells, even in the aerobic environment, could obtain energy and produce excess lactic acid through low productivity and oxygen-free glycolysis pathway called “aerobic glycolysis or Warburg effect”. Meanwhile, Warburg and coworkers also demonstrated that the metabolic changes in the mitochondria are one of the predominant sources for the origin of cancer.147 In this context, the strategy of using nanoparticles toward targeting at mitochondria in stimulating apoptotic events toward its ablation has been recognized as the momentous finding in cancer therapy.
Targeting Strategies
Based on the above-mentioned facts and considerations relevant to the initiation of apoptosis, several efforts have been dedicated to the advancements of nanoparticles-based therapeutic designs by choosing mitochondria as a potential target toward inhibiting tumor metastasis. In this framework, one of the potential approaches includes the targeting of membrane minimal constituents, including ANT, VDAC, and Cyp-D, that are associated with the entry of nanoparticles into mitochondria. Various other promising strategies include the conveyance of antioxidants, activation of MPT, reduction of ROS, up-regulated expression of pro-apoptotic genes.52,93,94,148 However, the targeting approaches utilized for directing various nanoparticles intracellularly, and factors influencing their internalization are predominantly discussed in this section.
In general, the particles with a diameter lesser than 500 nm and molecules larger than 40 KDa could be feasibly accumulated in the tumor microenvironment and limiting their penetration into healthy cells due to the EPR effect. Although numerous innovative supramolecular designs for the delivery of therapeutic guest molecules are available at arbitrary size ranges, it is highly challenging to internalize such composites into the mitochondria.148 Recently, it was concluded that only a few of tiny metal nanoparticles (gold,53 silver,149 and some certain metal oxides as well as quantum dots52 whose size smaller than 10 nm) could permeate through the mitochondrial membrane into the matrix through the VDAC, while the nanoparticles at a larger size were being obstructed.150 In an attempt to demonstrate this fact, Salnikov and coworkers53 utilized calibrated 3-nm-sized gold nanoparticles to stride cardiac mitochondria through VDAC pores ranging from 3 to 6 nm, substantiating the breach caused by osmotic swelling. Meanwhile, these particles could affect the transport of respiratory chain and mitochondrial permeability, and their internalization was accompanied by the synergy between permeability alteration and mitochondria expansion, enhancing the mitochondria-targeted ability significantly. Although the entry of particles is restricted due to the size limitations, it is feasible to deliver the therapeutic cargo at the proximity of mitochondria using the large-sized constructs. Further, these designs could be reprogrammed by attaching several mitochondrial membrane targeting ligands, such that they could be well retained in the proximity of mitochondria, successfully enabling the delivery of drugs.
Mitochondria possesses a very precise membrane along with the internal structure, which provides a basis for ascertaining specific sites of some polypeptide sequences.151 Along this line, Ma and coworkers152 constructed a gold core (20 nm) stabilized with a layer of biotinylated CALNN (Cys-Ala-Leu-Asn-Asn)-based peptide, which was further immobilized with tetrameric streptavidin for effective functionalization of biotinylated molecules, (i.e., a cytotoxic peptide, KLA: (KLAKLAK)2) toward the specific identification of mitochondria. In another case, Yamada and Harashima153,154 synthesized a lipid derivative immobilized with mitochondrial targeting signal peptide, which was then used to fabricate peptide-modified liposomes. They confirmed that these innovative vesicles efficiently delivered therapeutic cargo via targeting endogenous mitochondrial proteins. Although there exist some strategies for targeting the membrane proteins of mitochondria for efficient therapy, it should be noted that the surface charge of the mitochondrial membrane significantly influences the convenient delivery of therapeutic cargo. Lemeshko and coworkers155 demonstrated that the cationic fluorescent probe safranin O prominently reduced the swelling percentage of mitochondria initiated by BTM-RP1 or by tryptophan derivatives, indicating that the superficial electrical charge has shown a critical impact on the membrane permeabilization and selectivity via cationic peptides. These consequences indicate that the cytotoxicity and selectivity of the appropriate peptide moieties can rely on the agents that are specifically responsible for the superficial charge modulation.
Indeed, the superficial charge of the mitochondrial membrane specifically controls the circulation of nanoparticles in the cytosol during drug delivery. Accordingly, it is highly feasible to achieve mitochondrial targeting by lipophilic architectures with delocalized cations.148 Owing to the high-density phospholipids yielding high membrane potential (constant negative charge), mitochondria are highly selective for the entry of some of the common lipophilic structures, including TPP,93,156 rhodamine 123 (Rh123),157,158 flupirtine,159 and MKT-077,160–162 which could be selectively retained in the mitochondria. Amongst them, TPP holds a better balance of lipophilicity and charge with a large area of three benzene moieties in its chemical structure, which can be efficiently used for mitochondrial targeting of nanoparticles by immobilizing it over various inorganic nanoparticles94 such as cerium and ferromagnetic particles or polymer, drug-polymer block system.93,156 In this vein, Biswas and coworkers163 fabricated a mitochondrial targeting design by conjugating the dye Rh123 to the amphiphilic PEG-phosphatidylethanolamine (PEG-PE) for paclitaxel (PTX) delivery. They demonstrated that the targeted design resulted in larger amounts of dye in the mitochondria and exhibited better efficacy compared to those of non-targeted ones.
Intrinsic Generation of ROS
In recent times, there has been a growing interest of researchers towards the utilization of ROS science for cancer ablation as most of the drugs and trace amounts of metal substrates participate catalytically in the molecular pathways, resulting in the deadly free radical species through Fenton- and Fenton-like chemistries. Broadly speaking, ROS, highly oxidizable, and metabolically active species, are categorized into two major divisions of oxidants, ie, non-free radicals, and free radicals. Various non-free radicals include hydrogen peroxide (H2O2) and single state oxygen (1O2), which could be catalytically converted to uncharged highly reactive short-lived, free radical molecules having an unpaired valence electron mediated by superoxide dismutase pathway, such as superoxide anion radical (O2−), and hydroxyl radical (·OH), among others. In addition to these conventional types of ROS, various other types of free radicals include nitric oxide (NO·), lipid peroxide (LOOH), alkoxy free radical (RO·), peroxyl radical (·OOH), and metal-oxygen complexes, among others, which can gather to complete the redox reaction in the body and participate in various metabolic processes of normal cells. In 1961, Jensen164 observed that partial oxygen was involved in the mitochondria redox reaction of NADH or succinic acid, which then resulted in H2O2, indicating the generation of ROS. Subsequently, they extended their studies on mitochondria and the generation of various types of ROS and established various complex relationships between them. However, it should be noted that the formation and subsequent release of ROS are closely related to mitochondrial complex II, mitochondrial complex I, and mitochondrial complex III, which can regulate the mitochondrial oxidative respiration (electron transport) chain. Simultaneously, the redox state of NAD(P)H/NAD(P)+, NADPH oxidase, monoamine oxidase (MAO), p66, and α-glycerophosphate dehydrogenase also play crucial roles in the formation of ROS in mitochondria.165 Xing and coworkers,166 designed an ROS-responsive nanoparticle system for the delivery of a cellular respiration inhibitor, camptothecin (CPT). The CPT species significantly induced the mitochondrial ROS (mtROS) upregulation, therefore accomplishing successive self-circulation of CPT discharge and mtROS burst, endowing the long-term high oxidative stress and substantial apoptosis of cancer cells.
In addition, some of the chemotherapeutic drugs (for example, Dox), and trace metal species (copper and iron), and combination of both catalyzing the non-free radical oxidant species in the normal aerobic environment generate free radicals efficiently. These chemotherapeutic drugs delivered from the nanoparticles ultimately augment the apparent intracellular levels of non-free radical oxidants, H2O2, through a series of events: i) Reductive metabolic activation; ii) Conversion to its semiquinone derivative; iii) Transformation to superoxide through the one-electron reduction; and iv) Dismutation reaction catalyzed by the superoxide dismutase (SOD) enzyme, resulting in the eventual deadly hydroxyl ions.7,90,91 Similarly, the trace amounts of first-row transitional metal ions, such as iron and copper, intrinsically participate in the catalysis of H2O2, resulting in the formation of deadly free radicals through Fenton- and Fenton-like reactions, respectively.43 Due to their apparent availability, these H2O2 species can be used as effective targets for the ablation of tumors through ROS science. However, specific limitations of ROS, such as poor stability and aggregation over the surface of the organelles and specificity, are remained to be addressed. Furthermore, ROS produced by mitochondria, in return, also contributes toward the enhancement of LMP. The consequence of the 15 min contact with H2O2 manifested that the lysosomal rupture can almost instantly even if restricted, results in the significant discharge of lysosomal constituents, leading to the cell damage. Meanwhile, during oxidative stress, phospholipase A2 can be activated to undermine the stability of phospholipid organelle membranes, causing lysosomal fracture laterally.167–169
Effects of Internalized Nanoparticles in Mitochondria
Indeed, the actively-targeted nanoparticles using specific ligands that with high affinity to the membrane can efficiently infiltrate mitochondria and exhibit specifically defined tasks of a delivery system toward cancer ablation effects. However, the structural damage of the mitochondria is often affected by the particle’s physicochemical properties and externally applied triggers (light-induced hyperthermia and ultrasound) that significantly influence the inner microenvironment of mitochondria. Zhang et al170 fabricated a near-infrared (NIR) fluorescence/photothermal double response probe based on the methylene blue, which could enter the mitochondria of cancer cells. Moreover, the weak alkaline microenvironment maximized the photothermal efficacy of the probe. Subsequent changes in the permeability of mitochondria membrane and enhanced light-induced hyperthermia-induced the apoptosis/necrosis of cancer cells. In another study, Lv et al171 demonstrated the effects of low-intensity (0.6–0.8 W/cm2) ultrasound dynamic therapy on the human oral squamous carcinoma cell line using 5-aminolevulinic acid (ALA). The significant rise of ROS levels in the treatment groups resulted in the morphological changes of swollen mitochondria, indicating the inception of mitochondrial signaling pathways toward the ablation of cells. Similarly, indocyanine green (ICG), as a fluorescent agent, is one of the United States Food and Drug Administration (US-FDA)-approved NIR dyes possess both photothermal and photodynamic effects, which are effective after when the nanoparticles carrying the dye being phagocytosed by the cells.92 Although the advanced strategies, such as ultrasound- and light-based therapies have resulted in significant progress, there existed specific problems associated with such therapies of low penetration depth and limited localization of irradiated light, which yet remained to be unresolved.172
Engulfment of certain foreign bodies, for instance, nanoparticles carrying chemotherapeutic agents, and their resultant deadly ROS stimulates the cytochrome C release from mitochondria, which sequentially activates the downregulation of Caspase-3/9 precursor, and initiates the mitochondrial permeability transition pores, resulting in the membrane permeability to exchange the mitochondrial and cytoplasmic constituents. These consequences eventually result in the organelle edema and cause substantial damage. For instance, in response to the internalization of nanoparticles into the cells, some of the organelles such as endoplasmic reticulum (ER), respond to defend such foreign bodies and release large amounts of calcium ions into the cytoplasm in response to the stress. These large amounts of calcium ions are taken up due to the membrane potential gradient, and calcium overload in the mitochondria can be recognized as an apoptosis factor. Moreover, the nanoparticles can also trigger the change of membrane potential, the downtrend of matrix pH, and the formation of cereal cystine peptide, which lead to the initiation of exogenous mitochondrial death pathway, inducing the elevation of apoptotic events in the cell.173 In addition, the translocated cytochrome C from the mitochondria to the endoplasmic reticulum interacts with the 3-phosphoinositide receptor, forming positive feedback to apoptosis. In an attempt to demonstrate these issues, Fang and coworkers174 demonstrated that the natural cyclopeptide RA-V inhibited the coaction of 3-phosphoinositide-dependent protein kinase 1 and AKT, due to the loss of membrane potential, the liberty of cytochrome C and the excitation of the caspase cascade.
Apoptotic stimulators, such as TNF-α and lipopolysaccharide, are capable of inducing the generation of ROS in mitochondria, which can significantly change the arrangement of mitochondrial membranes, instead of lysosomal membranes. Indeed, the lysosomal enzymes promote the productivity of H2O2 in mitochondria, which strengthens the cytoplasmic acidification and then initiates Bax mitochondrial translocation, involving the recruitment of cellular apoptosis.175 Previous reports indicated that the Ferroptosis, related to the ROS gathering and glutathione depletion, resulted in the shrinkage of mitochondria, increase of membrane density, disappearance of the mitochondrial ridge, and eventual breaching of the mitochondrial outer membrane. Numerous such reports based on the utilization of specific metal ions released from the nanoparticles, such as copper, iron, and manganese, demonstrated that these ions generally induced the Fenton and Fenton-like reactions, which showed enormous potential in the cancer therapy based on Ferroptosis.43,44 In addition, the mitochondrial DNA is responsible for the structural protein-encoding of the crucial electron transfer chain and 2 ATP synthase subunits, such that the nanoparticles may also cause disturbances or mutation of mitochondrial DNA, its associated electronic transmission, and oxidative phosphorylation. These consequences further enhance the ROS levels, which contribute to the endogenous apoptosis. Finally, the interactions between the internal and external factors form a vicious circle, causing the apoptotic cell death.176,177
Nucleus
As the characteristic feature of eukaryotic cells, the nucleus is the central organelle, predominantly containing most of the cell’s genetic material, which plays a significant role in specific functions such as cell growth, proliferation, differentiation, and apoptosis. Notably, the nucleus is considered as the control center of cells as it regulates various cell activities through managing the gene expressions. Once the nuclear DNA is damaged, both endogenous and exogenous conditions may cause severe diseases to cells and tissues. For instance, during autophagy, the transcription factor EB that regulating the lysosomal expression network is translocated to the nucleus to trigger this protective mechanism.
Typically, the genetic content of the nucleus is enclosed in a bilayer phospholipid membrane made of different protein compositions, including the speckle domains, the early promyelocytic nucleosome (PMLNB), and Cajal bodies, among others.178,179 The subcellular localization of the genetic material, chromosomes, and nucleosomes in the nucleus is not random, and the specific loci or chromosomes are in a specific region, known as the chromosome territories (CTs).180 These proteins act as objective receptors for various diseases (proteins, nuclear receptors, and DNA) in the nucleus, which can provide great potential toward targeting the nanoparticles to the nucleus. Thus, tremendous progress in the past few decades has been evidenced by the development of various targeting strategies in guiding the nanoparticles toward the nucleus, specifically gene-based therapies. These strategies often favored the conveyance and delivery of therapeutic cargo in the nucleus.51,181,182 In general, the internalization of nanoparticles toward the nucleus is facilitated by two predominant pathways. One of these practical ways includes the interaction of the composite structure of phospholipids or similar particles with the nuclear membrane, facilitating the fusion of the outer layer of phospholipids with the nuclear membrane, which consequently enables the intervention through the membranous barriers, and release of the internalized nanoparticles into the nucleus.183 To substantially meet the requirements of membranous fusion and nuclear uptake, the nanosystem is usually modified with specific ligands that can activate nuclear receptors and enable their internalization.184 In an attempt to address this issue, Qiu and coworkers182 developed a codelivery system based on drug-silver-gold nanorods, in which one of the ends carried a specific aptamer for specifically internalizing these composites into the nucleus, achieving the responsive aggregation-induced cell death. Another way of nuclear internalization includes the passing of nanoconstructs through the nuclear pores or occupy nucleoplasm that for transferring nucleoprotein. However, the extremely small-sized functional diameter of the nanoparticles (9–40 nm)182 limit their nuclear diffusion. In the beginning, it was motivated to utilize nuclear-targeted nanoparticles to carry DNA, mediated by unique endocytic pathway and fossa protein, facilitating the bypass the acid environment in the lysosome and reaching the nucleus directly. Nevertheless, over the decades, there has been increasing interest in the fabrication of various advanced strategies toward targeting the nucleus and are explicitly discussed hereunder.
Active Targeting Using Peptides
The substantial entry of internalized nanoparticles into the nucleus through the nuclear membrane predominantly depends on the average size of nanoparticles and the surface-modified ligands, such as protein and peptides. However, the peptide sequences immobilized over the nanoparticles significantly instruct their entry as well as fate intracellularly. Paunesku et al modified the TiO2-DNA nanoparticles on the surface of isopropyl dehydrated glycerol and grafted with 1–5 dopamine-modified DNA molecules. This nano-sized particulate system specifically identified the mitochondrial DNA sequence of NADH dehydrogenase II (ND2) and the ubiquitous R18S ribose in mammals. The body RNA gene-matched peptide molecule not only possessed nuclear targeting ability but also resulted in the mitochondrial targeting effect.185 Among all the peptides, nuclear localization sequence (NLS) has been widely applied for targeting the nucleus. In fact, the conventional nuclear import pathway is predominantly facilitated by importin α and β. Amongst them, the importin-α binds to the importin β through NLS utilizing its NLS binding domain. The NLS and the intracellular factor interact with each other, resulting in the formation of a stable complex and impede at the nuclear pore complex. Further, the nanoparticles are then diffused into the nucleus through an active transport process once the sequence is recognized by the particular protein receptor. The nuclear input pathway results in the entry of nanoparticles to the nucleus. For instance, the NLS/RGD-modified gold nanolet186 with silver nanoparticles,187 in which the number of nuclear location sequences is not proportional to the difficulty of nanoparticle entering the nucleus. In another case, the Ebola virus VP24 protein has bounded the untypical NLS binding site of karyopherin alpha 5 (KPNA5) with high affinity, which interrupted the transport of tyrosine-phosphorylated STAT1 and maintained the delivery of other cargos.188 Salma and coworkers51 fabricated chitosan nanoparticles in the diameter range of 25 and 150 nm and subsequently modified them with the NLS peptide of multiple density gradient. The experimental results showed that the smaller-sized chitosan nanoparticles could be located in the nucleus in a more significant amount without the nucleation sequence, and the results were contradictory to the intuition for the nuclear location sequence. Moreover, fewer NLS modified nanoparticles have more nuclear localization, and similar results were shown in their further studies.189 In another instance, Sahin and coworkers69 fabricated a nuclear-targeted complex system by immobilizing a 17 amino acid oligopeptide NLS (DRQIKIWFQNRRMKWKK) to DOX-loaded nanoparticles. These nuclear-targeted systems resulted in the substantial localization merely at the nuclear membrane and ablated cancer cells compared to the free DOX molecules, whose internalization was predominantly based in the cytoplasm.
Amongst these peptides contributed by simple modification, TAT (YGRKKRRQRRR) is one of the most widely used NLS peptides. Such NLS peptide, in combination with highly basic functional groups, is predominantly used for nuclear targeting of various organic or inorganic nanoparticles. Several examples include DOX@MSN@TAT/RGD layer structure from Pan et al,190 TAT/PEG-Mal-grafted iron oxide nanoparticles from Peng et al,191 and TAT-Cerium doping system from Yu and coworkers.192 Notably, all these studies were proposed using TAT to change the nuclear properties and promote the nuclear penetration of nano-sized particles. In most of the instances, these nucleus-targeted systems efficiently delivered the therapeutic cargo in the proximity of the nucleus for their enhanced theranostic efficacy.191 In another case, Wang and colleagues193 fabricated the peptide-modified magnetic nanoparticles, which significantly resulted in synergetic targeting of nuclei and mitochondria. However, it was evident that the NLS peptide-modified nanoparticles only sieged the nucleus instead of the nuclear internalization. To this end, various specific chemical groups can also be used, which precisely exhibit affinity toward the nucleus. For instance, Jana et al194 synthesized acridine-benzbutyrate nitrogen mustard nanoparticles, which combined the DNA with the DNA alkylation group to target at nucleus by the acridine molecule.
Effects of Internalized Nanoparticles in the Nucleus
The internalized nanoparticles in the nucleus predominantly interact with the intranuclear constituents, resulting in different types of mutations and severe DNA damage, the block of DNA cycle, and the activation of the apoptosis signaling pathway.195 In the early 1970s, scientists began to explore the predominant reasons for human cancer, and increasingly recognized that there exists a close relationship between the genetic changes and dysfunction of cancer.196–198 Since then, it has been well acknowledged that mutation, whether a gene, a sequence of genes, or even the whole chromosomal transformations, have shown a powerful impact on the origination as well as differentiation of various cancers.199–201 It should be noted that the some of the inorganic nanoparticles cause nuclear DNA damage through delivering various therapeutic molecules such as drugs, protein and nucleic acids (DNA and RNA) among others, which can directly interact with DNA by intercalation, leading to the inhibition of replication, transcription and translation-assisted macromolecular biosynthesis. Although the DNA damage in cells can trigger the self-repair mechanism, the damaged large area of DNA subsequently hinders the cell cycle, resulting in the prolonged G1 and G2 phases, and substantial loss of ability to repair by itself prior to next synthesis by mitosis. Henceforth, the apoptotic signal transduction pathway gets activated, driving to eventual cell death.202,203
In addition to diverse therapeutic molecules that precisely interact with the nuclear constituents resulting in cell death, various nanoparticles participate in cell death through various mechanisms similar to their interaction with mitochondria, inducing the generation of enormous levels of ROS. In addition, the enhanced levels of intracellular ROS affect not only various membrane-covered organelles, such as mitochondria and lysosomes, but also show a significant impact on the nuclei. In this context, the ROS scavenger may also substantially purge the nuclear translocation, leading to the activation of an autophagy mechanism.204 Various inducers in the nucleus can elevate the activation of NADPH oxidases and the generation of NADPH oxidases-derived ROS, triggering the nuclear translocation,205 reducing the ratio between Bcl-2 and Bax afterward, as well as stimulating caspase-3/-9 in mitochondrial approach. Currently, the generation of oxidative lesions caused by stress in the nucleus is widely studied, using specific biomarkers of oxidative stress, for example, 8-hydroxy-2ʹ-deoxyguanosine (8-OHdG). The utilization of this 8-OHdG biomarker is widely considered due to hydroxyl radical and enenyl ring addition, which can be predominantly formed during lipid peroxidation, associated with the formation of mutated electrophilic reagents, such as malondialdehyde, and 4-hydroxyl nonylaldehyde, among others.206 In addition, this biomarker not only acts as a mere biomarker for oxidative stress but also a remedy for oxidative stress-induced gastrointestinal diseases. The oxidative attack on DNA is a predominant reason for DNA damage induced by many nanoparticles and the intracellular toxicity. On nearly all mutations or tumor suppressor genes, the changes, such as deletion or addition of base pairs caused by DNA oxidative damage could be observed.207 In addition, the DNA skeleton (deoxyribonucleic acid chain) under oxidative attack, or DNA in the process of oxidative damage repair, can also detect the single strand damage of DNA in the cell.
ER and Golgi Apparatus
ER is a eukaryotic organelle with cisternae membranous structure that held by the cytoskeleton intracellularly. However, the organelle ER manages its adaptation to the unique tumor microenvironment, depending on the number of folds of transmembrane and secretory proteins, and diverse functionalities such as biosynthesis of lipids, detoxification of drug molecules, and the storage of calcium ions, among others.208 The rough ER that with the embedded ribosomes on its surface participates in the synthesis of various lysosomal enzymes. Further, the transport vesicles carrying lipids and proteins from rough ER deviate such functional substituents to the Golgi apparatus from the smooth ER. For weakly alkaline nature of ER, the acidic feature of lysosomes has been extensively applied for stimulus-triggered cancer therapy. Notably, ER stress has been acknowledged as a crucial pathway toward cancer treatment. Several biocompatible metal complexes can be used to target ER stress for highly efficient cancer therapy.209
To this end, the Golgi apparatus is one of the vital organelles that organizes, processes, and packages various proteins that are produced in the organelle ER. This apparatus is generally consisted of three different compartments such as the cis-Golgi network (CGN), the Golgi stack of cisternae (cis-Golgi, medial-Golgi, and trans-Golgi), and the trans- Golgi network (TGN).210 Interesting feature of Golgi complex is that these compartments possess a pH-gradient across the three compartments from CGN (pH 6.7) to TGN (pH 6.0).210 Golgi apparatus receives the proteins-contained vesicles synthesized in ER and secrets these cargos through exocytosis, for instance, ANQ-IMC-6 probe.210 After combined with specific cyclooxygenase-2 (COX-2) in the Golgi apparatus, the probe performed intense fluorescence by pacifying the photo-induced electron transfer.
More often, the variations in the glycosylation pathway of tumor cells ascend in the significant effects in pH homeostasis as well as function of the organelle, which subsequently leads to the disorganization of the organelle structure and scattering of hypertrophic elements in Golgi apparatus.211 Henceforth, these consequences could be used as an objective for pH-responsive therapeutic delivery of drugs and imaging probes. In a case, Xue and coworkers25 fabricated a Golgi apparatus-activated pH-triggered asymmetric cyanine dye (pH-PTT in Figure 7), which could be sensitive in the specific pH range (6.2–7.5). In such acidic environment, the pH-PTT dye was transformed into a superior conjugated structure with strong absorption efficiency at 808 nm, which could able to harvest heat energy. Furthermore, this pH-PTT dye could assemble with bovine serum albumin (BSA), resulting in the BSA-pH-PTT nanoparticles, which could favorably accumulate in the Golgi complex of tumor cells due to the hypertrophic morphology.212 Therefore, these designed BSA-pH-PTT composites were represented as a smart delivery system as they could be significantly activated in the Golgi apparatus to achieve the PTT effect. It should be noted that these ER and Golgi apparatus-targeted formulations are still in the infant stage as the mechanisms of delivery and targeted pathways are still unclear.
 |
Figure 7 (A, B) Illustration of pH-PTT as a smart drug system for specific Golgi apparatus-activated PTT. (C) BSA-pH-PTT/Mito tracker (c and d) and BSA-pH-PTT/Golgi green. (1) Red channel: 650 ± 25 nm for BSA-pH-PTT (25 mM), λex = 543 nm; (2) green channel: 530 ± 25 nm for Lysotracker (200 nM), Mito tracker (500 nM) or Golgi tracker (200 nM), λex = 488 nm; (3) merged images; (4) bright field and (5) enlarged images of 3, scale bar: 50 mm. Reproduced with permission of Royal Society of Chemistry from A smart drug: a pH-responsive photothermal ablation agent for Golgi apparatus activated cancer therapy, Xue F, Wen Y, Wei P, et al., 2017.25 |
Intracellular Transformation
In addition to the delivery and subsequent therapeutic efficiency, it is a prerequisite to consider several transformations of the internalized nanocarriers, which lead to the degradation pathways of nanoparticles as well as their excretion from the intracellular environment. Based on the extensive investigations, during the intracellular translocation of nanoparticles, several transformations happen within the cells and the nanoparticles, which result in the change of the generalized pathway of nanoparticles, ie, from endocytosis to main organelles. To this end, the cellular morphology, as well as organelle deformation, notably, nucleus, adapt to the transformable intracellular microenvironment.213 In addition, the intracellular circumstances can also be altered by different surface modification strategies, as is shown in the substitution of endocytosis for transcytosis,214 overcoming the barrier for drug absorption. Gu and coworkers,215 designed versatile nanostructures with a superior membrane-breaking effect, which could significantly affect after cellular internalization and excluded immediately through the cell membrane. Similarly, Baldini and coworkers216 observed that the release of nanoparticles from the cells prevailed for 72–144 h by using fluorescence polymeric nanoparticles. Further, these nanoparticles could strongly permeate tumor tissue through subsequent cell perforation approach.
Conclusions and Outlook
In summary, this review emphasized the subcellular performance and intracellular fate of the internalized nanoparticles in various main organelles such as endosomes, lysosomes, mitochondria, nucleus, ER, and Golgi apparatus. Initially, we gave a brief introduction of nanotechnology and conventional cancer therapeutics, revealing their unexpected limitations due to the adverse effects of resultant end products of diverse nanoparticles. Further, we discussed the fundamental structures, properties, and functions of the organelles referred above, highlighting the escape pathways of nanoparticles assisted with the interactions toward organelles. In addition, we discussed the typical mechanisms and application of these sophisticated approaches, along with possible mechanisms and related applications toward cancer ablation.
Despite the investigations on the intracellular fate of nanoparticles that keep making breakthroughs in recent years, these pathways need more in-depth explorations as the optimal properties of various materials and numerous biological barriers that being faced by nanomaterials, which influence the eventual pharmacokinetic profile of the therapeutic drugs. The integrated depiction from the perspective of specific behavior and physicochemical properties of nanoparticles, for instance, the effect on size, morphology, potential, and functional bond, still needs to be comprehensively explained. In addition to physicochemical properties, the performance efficacy concerning the controlled delivery and therapeutic mechanisms, as well as compatibility attributes, could be given paramount importance for their effective translation to clinics. We could able to figure out the overall function of each mechanism inside every organelle with a specific focus on the nucleus as well as mitochondria-targeted systems, but several challenges in terms of classifying the precise effects of every subcellular component still exist. Further, certain assumptions are also required to study the recycling of cells and cargos, in addition to the clearance pathways of degraded nanoparticle constituents. We are anticipated that the development of organelle-based nanoparticle exertion mechanism will undoubtedly resolve the application of an intracellular drug or gene delivery toward efficient cancer therapy.
Acknowledgments
The authors acknowledge financial support from the National Natural Science Foundation of China (NSFC, Grant Nos. 31800794, U1605225, and 81971734), the Natural Science Foundation of Fujian Province (2019J01076), the support by the Fundamental Research Funds for the Central Universities (ZQN-713), Funds for Foreign Experts from Ministry of Science and Technology, China (G20190013023), and the Program for Innovative Research Team in Science and Technology in Fujian Province University.
Disclosure
The authors report no conflicts of interest in this work.
References
1. Bouzbid S, Hamdi-Chérif M, Zaidi Z, et al. Global surveillance of trends in cancer survival 2000–14 (CONCORD-3): analysis of individual records for 37 513 025 patients diagnosed with one of 18 cancers from 322 population-based registries in 71 countries. Lancet. 2018;391(10125):1023–1075. doi:10.1016/S0140-6736(17)33326-3
2. Feng SS, Chien S. Chemotherapeutic engineering: application and further development of chemical engineering principles for chemotherapy of cancer and other diseases. Chem Eng Sci. 2003;58(18):4087–4114. doi:10.1016/S0009-2509(03)00234-3
3. Ferrell BR, Temel JS, Temin S, et al. Integration of palliative care into standard oncology care: american society of clinical oncology clinical practice guideline update. J Clin Oncol. 2017;35(1):96–112. doi:10.1200/JCO.2016.70.1474
4. Hamdy FC, Donovan JL, Lane JA, et al. 10-year outcomes after monitoring, surgery, or radiotherapy for localized prostate cancer. N Engl J Med. 2016;375(15):1415–1424. doi:10.1056/NEJMoa1606220
5. Dionne-Odom JN, Azuero A, Lyons KD, et al. Benefits of early versus delayed palliative care to informal family caregivers of patients with advanced cancer: outcomes from the ENABLE III randomized controlled trial. J Clin Oncol. 2015;33(13):1446–1452. doi:10.1200/JCO.2014.58.7824
6. Sun C, Shen S, Xu C, et al. Tumor acidity-sensitive polymeric vector for active targeted siRNA delivery. J Am Chem Soc. 2015;137(48):15217–15224. doi:10.1021/jacs.5b09602
7. Kankala RK, Liu CG, Chen AZ, et al. Overcoming multidrug resistance through the synergistic effects of hierarchical pH-Sensitive, ROS-generating nanoreactors. ACS Biomater Sci Eng. 2017;3(10). doi:10.1021/acsbiomaterials.7b00569
8. Temel JS, Greer JA, Muzikansky A, et al. Early palliative care for patients with metastatic non-small-cell lung cancer. N Engl J Med. 2010;363(8):733–742. doi:10.1056/NEJMoa1000678
9. Lowy I. “Nothing more to be done”: palliative care versus experimental therapy in advanced cancer. Sci Context. 1995;8(1):209–229. doi:10.1017/S0269889700001964
10. Timmerman R, Paulus R, Galvin J, et al. Stereotactic body radiation therapy for inoperable early stage lung cancer. JAMA. 2010;303(11):1070–1076. doi:10.1001/jama.2010.261
11. Kankala RK, Zhu K, Sun X-N, Liu C-G, Wang S-B, Chen A-Z. Cardiac tissue engineering on the nanoscale. ACS Biomater Sci Eng. 2018;4(3):800–818. doi:10.1021/acsbiomaterials.7b00913
12. Desai N. Challenges in development of nanoparticle-based therapeutics. AAPS J. 2012;14(2):282–295. doi:10.1208/s12248-012-9339-4
13. Kankala RK, Chen BQ, Liu CG, Tang HX, Wang SB, Chen AZ. Solution-enhanced dispersion by supercritical fluids: an ecofriendly nanonization approach for processing biomaterials and pharmaceutical compounds. Int J Nanomedicine. 2018;13:4227–4245. doi:10.2147/IJN.S166124
14. Kankala RK, Zhang H, Liu C-G, et al. Metal species–encapsulated mesoporous silica nanoparticles: current advancements and latest breakthroughs. Adv Funct Mater. 2019;29(43):1902652. doi:10.1002/adfm.v29.43
15. Wan ACA, Ying JY. Nanomaterials for in situ cell delivery and tissue regeneration. Adv Drug Deliv Rev. 2010;62(7):731–740. doi:10.1016/j.addr.2010.02.002
16. Wu L, Zhang J, Watanabe W. Physical and chemical stability of drug nanoparticles. Adv Drug Deliv Rev. 2011;63(6):456–469. doi:10.1016/j.addr.2011.02.001
17. Davis ME, Chen Z, Shin DM. Nanoparticle therapeutics: an emerging treatment modality for cancer. Nat Rev Drug Discov. 2008;7:771.
18. Ma Y, Huang J, Song S, Chen H, Zhang Z. Cancer-targeted nanotheranostics: recent advances and perspectives. Small. 2016;12(36):4936–4954. doi:10.1002/smll.201600635
19. Basuki JS, Qie F, Mulet X, et al. Photo-modulated therapeutic protein release from a hydrogel depot using visible light. Angew Chem Int Ed. 2016;129(4):966–971.
20. Shen T, Zhang Y, Kirillov AM, et al. Two-photon sensitized hollow Gd2O3: eu(3+)nanocomposites for real-time dual-mode imaging and monitoring of anticancer drug release. Chem Commun. 2015;52(7):1447–1450. doi:10.1039/C5CC07609A
21. Sung SE, Hwang M, Kim AY, et al. MYOD overexpressed equine adipose-derived stem cells enhanced myogenic differentiation potential. Cell Transplant. 2016;25(11):2017–2026. doi:10.3727/096368916X691015
22. Zeng X, Liu G, Tao W, et al. A drug-self-gated mesoporous antitumor nanoplatform based on pH-sensitive dynamic covalent bond. Adv Funct Mater. 2017;27(11):1605985. doi:10.1002/adfm.v27.11
23. Zou Z, Li S, He D, et al. A versatile stimulus-responsive metal–organic framework for size/morphology tunable hollow mesoporous silica and pH-triggered drug delivery. J Mater Chem B. 2017;5:11. doi:10.1039/C6TB03379B
24. Zhang K, Xu H, Jia X, et al. Ultrasound-triggered nitric oxide release platform based on energy transformation for targeted inhibition of pancreatic tumor. ACS Nano. 2016;10(12):10816. doi:10.1021/acsnano.6b04921
25. Xue F, Wen Y, Wei P, et al. A smart drug: a pH-responsive photothermal ablation agent for Golgi apparatus activated cancer therapy. Chem Commun. 2017;53(48):6424. doi:10.1039/C7CC03168H
26. Yao C, Wang W, Wang P, Zhao M, Li X, Zhang F. Near-infrared upconversion mesoporous cerium oxide hollow biophotocatalyst for concurrent pH-/H2O2-responsive O2-evolving synergetic cancer therapy. Adv Mater. 2018;30(7):1704833. doi:10.1002/adma.201704833
27. Zhao J, Zhang P, He Z, Min QH, Abdel-Halim ES, Zhu JJ. Thermal-activated nanocarriers for the manipulation of cellular uptake and photothermal therapy on command. Chem Commun. 2016;52(33):5722–5725. doi:10.1039/C6CC01162D
28. He J, Ai L, Liu X, et al. Plasmonic CuS nanodisk assemblies based composite nanocapsules for NIR-laser- driven synergistic chemo-photothermal cancer therapy. J Mater Chem B. 2018;6(7):1035–1043. doi:10.1039/C7TB02772A
29. Deng X, Li K, Cai X, et al. A hollow-structured CuS@Cu2 S@Au nanohybrid: synergistically enhanced photothermal efficiency and photoswitchable targeting effect for cancer theranostics. Adv Mater. 2017;29(36):1701266. doi:10.1002/adma.201701266
30. Zhu C, Huo D, Chen Q, Xue J, Shen S, Xia Y. A eutectic mixture of natural fatty acids can serve as the gating material for near-infrared-triggered drug release. Adv Mater. 2017;29(40):1703702. doi:10.1002/adma.201703702
31. Yu X, Gao D, Gao L, et al. Inhibiting metastasis and preventing tumor relapse by triggering host immunity with tumor-targeted photodynamic therapy using photosensitizer-loaded functional nanographenes. ACS Nano. 2017;11(10):10147.
32. Sun W, Li S, Häupler B, et al. An amphiphilic ruthenium polymetallodrug for combined photodynamic therapy and photochemotherapy in vivo. Adv Mater. 2016;29(6):1603702. doi:10.1002/adma.201603702
33. He H, Ji S, He Y, et al. Photoconversion-tunable fluorophore vesicles for wavelength-dependent photoinduced cancer therapy. Adv Mater. 2017;29(19):1606690. doi:10.1002/adma.v29.19
34. Kuai R, Ochyl LJ, Bahjat KS, Schwendeman A, Moon JJ. Designer vaccine nanodiscs for personalized cancer immunotherapy. Nat Mater. 2017;16(4):489–496. doi:10.1038/nmat4822
35. Wang N, Wang Z, Xu Z, Chen X, Zhu G. A cisplatin-loaded immuno-chemotherapeutic nanohybrid bearing immune checkpoint inhibitors for enhanced cervical cancer therapy. Angew Chem Int Ed. 2018;57(13):3426–3430. doi:10.1002/anie.201800422
36. Zhang Q, Wei W, Wang P, et al. Biomimetic magnetosomes as versatile artificial antigen-presenting cells to potentiate T-cell-based anticancer therapy. ACS Nano. 2017;11(11):10724–10732. doi:10.1021/acsnano.7b04955
37. Sun T, Zhang YS, Pang B, Hyun DC, Yang M, Xia Y. Engineered nanoparticles for drug delivery in cancer therapy. Angew Chem Int Ed. 2014;53(46):12320–12364.
38. Steichen SD, Caldoreramoore M, Peppas NA. A review of current nanoparticle and targeting moieties for the delivery of cancer therapeutics. Eur J Pharm Sci. 2013;48(3):416–427.
39. Farokhzad OC, Langer R. Impact of nanotechnology on drug delivery. ACS Nano. 2009;3(1):16–20. doi:10.1021/nn900002m
40. Huczko A. Template-based synthesis of nanomaterials. Appl Phys A. 2000;70(4):365–376. doi:10.1007/s003390051050
41. Riehemann K, Schneider SW, Luger TA, Godin B, Ferrari M, Fuchs H. Nanomedicine–challenge and perspectives. Angew Chem Int Ed. 2009;48(5):872–897. doi:10.1002/anie.200802585
42. Li L, Liu H. Biodegradable inorganic nanoparticles: an opportunity for improved cancer therapy? Nanomedicine. 2017;12(9):959–961.
43. Liu C-G, Han Y-H, Zhang J-T, Kankala RK, Wang S-B, Chen A-Z. Rerouting engineered metal-dependent shapes of mesoporous silica nanocontainers to biodegradable Janus-type (sphero-ellipsoid) nanoreactors for chemodynamic therapy. Chem Eng J. 2019;370:1188–1199.
44. Kim SE, Zhang L, Ma K, et al. Ultrasmall nanoparticles induce ferroptosis in nutrient-deprived cancer cells and suppress tumour growth. Nat Nanotechnol. 2016;11:977. doi:10.1038/nnano.2016.164
45. Shahriar S, Shahed B, Sophie L. M Laird F, Pieter S, Morteza M. Toxicity of nanomaterials. Chem Soc Rev. 2012;41(6):2323–2343. doi:10.1039/c1cs15188f
46. He Q, Zhang Z, Gao F, Li Y, Shi J. In vivo biodistribution and urinary excretion of mesoporous silica nanoparticles: effects of particle size and PEGylation. Small. 2011;7(2):271–280. doi:10.1002/smll.201001459
47. Williams DF. On the mechanisms of biocompatibility. Biomaterials. 2008;29(20):2941–2953. doi:10.1016/j.biomaterials.2008.04.023
48. Chen Y, Gao Y, Chen H, et al. Engineering inorganic nanoemulsions/nanoliposomes by fluoride-silica chemistry for efficient delivery/co-delivery of hydrophobic agents. Adv Funct Mater. 2012;22(8):1586–1597.
49. Kankala RK, Kuthati Y, Liu C-L, Lee C-H. Hierarchical coated metal hydroxide nanoconstructs as potential controlled release carriers of photosensitizer for skin melanoma. RSC Adv. 2015;5(53):42666–42680. doi:10.1039/C4RA16957C
50. Hadjidemetriou M, McAdam S, Garner G, et al. The human in vivo biomolecule corona onto PEGylated liposomes: a proof-of-concept clinical study. Adv Mater. 2019;31(4):1803335. doi:10.1002/adma.201802348
51. Tammam SN, Azzazy HM, Breitinger HG, Lamprecht A. Chitosan nanoparticles for nuclear targeting: the effect of nanoparticle size and nuclear localization sequence density. Mol Pharm. 2015;12(12):4277–4289. doi:10.1021/acs.molpharmaceut.5b00478
52. Li J, Zhang Y, Xiao Q, et al. Mitochondria as target of Quantum dots toxicity. J Hazardous Mater. 2011;194(5):440–444.
53. Salnikov V, Lukyanenko YO, Frederick CA, Lederer WJ, Lukyanenko V. Probing the outer mitochondrial membrane in cardiac mitochondria with nanoparticles. Biophys J. 2007;92(3):1058–1071. doi:10.1529/biophysj.106.094318
54. Kang JW, So PTC, Dasari RR, Lim D-K. High resolution live cell raman imaging using subcellular organelle-targeting SERS-sensitive gold nanoparticles with highly narrow intra-nanogap. Nano Lett. 2015;15(3):1766–1772. doi:10.1021/nl504444w
55. Nel AE, Madler L, Velegol D, et al. Understanding biophysicochemical interactions at the nano-bio interface. Nat Mater. 2009;8(7):543–557. doi:10.1038/nmat2442
56. Varkouhi AK, Scholte M, Storm G, Haisma HJ. Endosomal escape pathways for delivery of biologicals. J Control Release. 2011;151(3):220–228. doi:10.1016/j.jconrel.2010.11.004
57. Polo E, Collado M, Pelaz B, Del PP. Advances toward more efficient targeted delivery of nanoparticles in vivo: understanding interactions between nanoparticles and cells. ACS Nano. 2017;11(3):2397–2402. doi:10.1021/acsnano.7b01197
58. Unfried K, Albrecht C, Klotz LO, Mikecz AV, Grether-Beck S, Schins RPF. Cellular responses to nanoparticles: target structures and mechanisms. Nanotoxicology. 2007;1(1):52–71. doi:10.1080/00222930701314932
59. Steinman RM, Mellman IS, Muller WA, Cohn ZA. Endocytosis and the recycling of plasma membrane. J Cell Biol. 1983;96(1):1–27. doi:10.1083/jcb.96.1.1
60. Geng Y, Dalhaimer P, Cai S, et al. Shape effects of filaments versus spherical particles in flow and drug delivery. Nat Nanotechnol. 2007;2:249. doi:10.1038/nnano.2007.70
61. Xu S, Olenyuk BZ, Okamoto CT, Hamm-Alvarez SF. Targeting receptor-mediated endocytotic pathways with nanoparticles: rationale and advances. Adv Drug Deliv Rev. 2013;65(1):121–138. doi:10.1016/j.addr.2012.09.041
62. Kagan VE, Konduru NV, Feng W, et al. Carbon nanotubes degraded by neutrophil myeloperoxidase induce less pulmonary inflammation. Nat Nanotechnol. 2010;5:354. doi:10.1038/nnano.2010.44
63. Tian J, Ding L, Ju H, et al. A multifunctional nanomicelle for real-time targeted imaging and precise near-infrared cancer therapy. Angew Chem Int Ed. 2014;53(36):9544–9549. doi:10.1002/anie.201405490
64. Wallace DC. Mitochondria and Cancer. BioMed Central Ltd; 2007.
65. Koshkaryev A, Piroyan A, Torchilin VP. Increased apoptosis in cancer cells in vitro and in vivo by ceramides in transferrin-modified liposomes. Cancer Biol Ther. 2012;13(1):50–60. doi:10.4161/cbt.13.1.18871
66. Mocan L, Matea C, Tabaran FA, et al. Photothermal treatment of liver cancer with albumin-conjugated gold nanoparticles initiates golgi apparatus-ER dysfunction and caspase-3 apoptotic pathway activation by selective targeting of Gp60 receptor. Int J Nanomedicine. 2015;10:5435–5445. doi:10.2147/IJN.S86495
67. Ohashi Y, Okamura M, Katayama R, et al. Targeting the Golgi apparatus to overcome acquired resistance of non-small cell lung cancer cells to EGFR tyrosine kinase inhibitors. Oncotarget. 2018;9(2):1641–1655. doi:10.18632/oncotarget.v9i2
68. Liu CG, Kankala RK, Liao HY, Chen AZ, Wang SB. Engineered pH-responsive hydrazone-carboxylate complexes-encapsulated 2D matrices for cathepsin-mediated apoptosis in cancer. J Biomed Mater Res A. 2019;107A(6):1184–1194. doi:10.1002/jbm.a.36610
69. Sahin A, Eke G, Buyuksungur A, Hasirci N, Hasirci V. Nuclear targeting peptide-modified, DOX-loaded, PHBV nanoparticles enhance drug efficacy by targeting to Saos-2 cell nuclear membranes. J Biomater Sci Polym Ed. 2018;29(5):507–519. doi:10.1080/09205063.2018.1423812
70. Kulshrestha A, Katara GK, Ibrahim SA, et al. Targeting V-ATPase isoform restores cisplatin activity in resistant ovarian cancer: inhibition of autophagy, endosome function, and ERK/MEK pathway. J Oncol. 2019;2019:2343876. doi:10.1155/2019/2343876
71. Mellman I. Endocytosis and molecular sorting. Annu Rev Cell Dev Biol. 1996;12:575–625. doi:10.1146/annurev.cellbio.12.1.575
72. Stenmark H. Rab GTPases as coordinators of vesicle traffic. Nat Rev Mol Cell Biol. 2009;10(8):513–525. doi:10.1038/nrm2728
73. Yameen B, Choi WI, Vilos C, Swami A, Shi J, Farokhzad OC. Insight into nanoparticle cellular uptake and intracellular targeting. J Control Release. 2014;190:485. doi:10.1016/j.jconrel.2014.06.038
74. Sun B, Luo C, Yu H, et al. Disulfide bond-driven oxidation- and reduction-responsive prodrug nanoassemblies for cancer therapy. Nano Lett. 2018;18(6):3643–3650. doi:10.1021/acs.nanolett.8b00737
75. Teng Z, Wang S, Su X, et al. Facile synthesis of yolk–shell structured inorganic–organic hybrid spheres with ordered radial mesochannels. Adv Mater. 2014;26(22):3741–3747. doi:10.1002/adma.v26.22
76. Grant BD, Donaldson JG. Pathways and mechanisms of endocytic recycling. Nat Rev Mol Cell Biol. 2009;10(9):597–608. doi:10.1038/nrm2755
77. Zhang S, Gao H, Bao G. Physical principles of nanoparticle cellular endocytosis. ACS Nano. 2015;9(9):8655–8671. doi:10.1021/acsnano.5b03184
78. Sée V, Free P, Cesbron Y, et al. Cathepsin L digestion of nanobioconjugates upon endocytosis. ACS Nano. 2009;3(9):2461–2468. doi:10.1021/nn9006994
79. De DC. Lysosomes revisited. Eur J Biochem. 1983;137(3):391–397. doi:10.1111/j.1432-1033.1983.tb07841.x
80. Guarnieri D, Sabella S, Muscetti O, et al. Transport across the cell-membrane dictates nanoparticle fate and toxicity: a new paradigm in nanotoxicology. Nanoscale. 2014;6(17):10264–10273. doi:10.1039/C4NR02008A
81. Gao Y, Li Y, Li Y, et al. PSMA-mediated endosome escape-accelerating polymeric micelles for targeted therapy of prostate cancer and the real time tracing of their intracellular trafficking. Nanoscale. 2015;7(2):597–612. doi:10.1039/C4NR05738D
82. Kankala RK, Wang S-B, Chen A-Z, Zhang YS. Chapter 2 - self-assembled nanogels: from particles to scaffolds and membranes. In: Conde J, editor. Handbook of Nanomaterials for Cancer Theranostics. Elsevier; 2018:33–62.
83. Li H, Zhang P, Luo J, et al. Chondroitin sulfate-linked prodrug nanoparticles target the golgi apparatus for cancer metastasis treatment. ACS Nano. 2019;13(8):9386–9396. doi:10.1021/acsnano.9b04166
84. Kim D, Lee ES, Oh KT, Gao ZG, Bae YH. Doxorubicin-loaded polymeric micelle overcomes multidrug resistance of cancer by double-targeting folate receptor and early endosomal pH. Small. 2008;4(11):2043–2050. doi:10.1002/smll.v4:11
85. Shi BY, Zhang H, Bi JX, Dai S. Endosomal pH responsive polymers for efficient cancer targeted gene therapy. Colloid Surface B. 2014;119:55–65. doi:10.1016/j.colsurfb.2014.04.005
86. Kong C, Li Y, Liu ZS, et al. Targeting the oncogene KRAS mutant pancreatic cancer by synergistic blocking of lysosomal acidification and rapid drug release. ACS Nano. 2019;13(4):4049–4063. doi:10.1021/acsnano.8b08246
87. Lee CH, Cheng SH, Huang IP, et al. Intracellular pH-responsive mesoporous silica nanoparticles for the controlled release of anticancer chemotherapeutics. Angew Chem Int Ed. 2010;122(44):8390–8395. doi:10.1002/ange.201002639
88. Sun H, Guo B, Cheng R, Meng F, Liu H, Zhong Z. Biodegradable micelles with sheddable poly(ethylene glycol) shells for triggered intracellular release of doxorubicin. Biomaterials. 2009;30(31):6358–6366. doi:10.1016/j.biomaterials.2009.07.051
89. Hong H, Zhang Y, Engle JW, et al. Invivo targeting and positron emission tomography imaging of tumor vasculature with 66 Ga-labeled nano-graphene. Biomaterials. 2012;33(16):4147–4156. doi:10.1016/j.biomaterials.2012.02.031
90. Kankala RK, Tsai PY, Kuthati Y, Wei PR, Liu CL, Lee CH. Overcoming multidrug resistance through co-delivery of ROS generating Nano-machinery in cancer therapeutics. J Mater Chem B. 2017;5:7. doi:10.1039/C6TB03146C
91. Kankala RK, Liu C-G, Yang D-Y, Wang S-B, Chen A-Z. Ultrasmall platinum nanoparticles enable deep tumor penetration and synergistic therapeutic abilities through free radical species-assisted catalysis to combat cancer multidrug resistance. Chem Eng J. 2019;123138.
92. Han YH, Kankala RK, Wang SB, Chen AZ. Leveraging engineering of indocyanine green-encapsulated polymeric nanocomposites for biomedical applications. Nanomaterials-Basel. 2018;8:6.
93. Li WQ, Wang Z, Hao S, et al. Mitochondria-targeting polydopamine nanoparticles to deliver doxorubicin for overcoming drug resistance. ACS Appl Mater Interfaces. 2017;9(20):16793. doi:10.1021/acsami.7b01540
94. Kwon HJ, Cha M, Kim D, et al. Mitochondria-targeting ceria nanoparticles as antioxidants for alzheimer’s disease. ACS Nano. 2016;10(2):2860. doi:10.1021/acsnano.5b08045
95. Wang Z, Guo W, Kuang X, Hou S, Liu H. Nanopreparations for mitochondria targeting drug delivery system: current strategies and future prospective. Asian J Pharma Sci. 2017;12(6):498–508. doi:10.1016/j.ajps.2017.05.006
96. Russell MR, Nickerson DP, Odorizzi G. Molecular mechanisms of late endosome morphology, identity and sorting. Curr Opin Cell Biol. 2006;18(4):422–428. doi:10.1016/j.ceb.2006.06.002
97. Chen FY, Lee MT, Huang HW. Evidence for membrane thinning effect as the mechanism for peptide-induced pore formation. Biophys J. 2003;84(6):3751–3758. doi:10.1016/S0006-3495(03)75103-0
98. Chen FY, Lee MT, Huang HW. Sigmoidal concentration dependence of antimicrobial peptide activities: A case study on alamethicin. Biophys J. 2002;82(2):908–914. doi:10.1016/S0006-3495(02)75452-0
99. Lee MT, Hung WC, Chen FY, Huang HW. Mechanism and kinetics of pore formation in membranes by water-soluble amphipathic peptides. Proc Natl Acad Sci USA. 2008;105(13):5087–5092. doi:10.1073/pnas.0710625105
100. Meyer M, Philipp A, Oskuee R, Schmidt C, Wagner E. Breathing life into polycations: functionalization with pH-responsive endosomolytic peptides and polyethylene glycol enables siRNA delivery. J Am Chem Soc. 2008;130(11):3272–3273. doi:10.1021/ja710344v
101. Soman NR, Lanza GM, Heuser JM, Schlesinger PH, Wickline SA. Synthesis and characterization of stable fluorocarbon nanostructures as drug delivery vehicles for cytolytic peptides. Nano Lett. 2008;8(4):1131. doi:10.1021/nl073290r
102. Bei C, Bindu T, Remant KC, Peisheng X. Dual secured nano-melittin for the safe and effective eradication of cancer cells. J Mater Chem B. 2014;3(1):25–29. doi:10.1039/C4TB01401D
103. Magzoub M, Pramanik A, Gräslund A. Modeling the endosomal escape of cell-penetrating peptides: transmembrane pH gradient driven translocation across phospholipid bilayers. Biochemistry. 2005;44(45):14890. doi:10.1021/bi051356w
104. Calderóngonzalez R, Teránnavarro H, Frandecabanes E, et al. Pregnancy vaccination with gold glyco-nanoparticles carryinglisteria monocytogenesPeptides protects against listeriosis and brain- and cutaneous-associated morbidities. Nanomaterials-Basel. 2016;6(8):151. doi:10.3390/nano6080151
105. Lee JH, Kim JA, Kwon MH, Kang JY, Rhee WJ. In situ single step detection of exosome microRNA using molecular beacon. Biomaterials. 2015;54:116–125. doi:10.1016/j.biomaterials.2015.03.014
106. Huang HW, Chen FY, Lee MT. Molecular Mechanism of Peptide-Induced Pores in Membranes. Phys Rev Lett. 2004;92(19):198304. doi:10.1103/PhysRevLett.92.198304
107. Benjaminsen RV, Mattebjerg MA, Henriksen JR, Moghimi SM, Andresen TL. The possible “Proton Sponge ” effect of Polyethylenimine (PEI) does not include change in lysosomal pH. Mol Ther J Am Soc Gene Ther. 2013;21(1):149–157. doi:10.1038/mt.2012.185
108. Bucci C, Thomsen P, Nicoziani P, Mccarthy J, Bo VD. Rab7: a key to lysosome biogenesis. Mol BiolCell. 2000;11(2):467.
109. Song Q, Yao L, Huang M, et al. Mechanisms of transcellular transport of wheat germ agglutinin-functionalized polymeric nanoparticles in Caco-2 cells. Biomaterials. 2012;33(28):6769. doi:10.1016/j.biomaterials.2012.05.066
110. Chithrani BD, Ghazani AA, Chan WCW. Determining the size and shape dependence of gold nanoparticle uptake into mammalian cells. Nano Lett. 2006;6(4):662–668. doi:10.1021/nl052396o
111. Rejman J, Oberle V, Zuhorn IS, Hoekstra D. Size-dependent internalization of particles via the pathways of clathrin-and caveolae-mediated endocytosis. Biochem J. 2004;377:159–169. doi:10.1042/bj20031253
112. Muthukrishnan N, Johnson GA, Erazo-Oliveras A, Pellois JP. Synergy between cell-penetrating peptides and singlet oxygen generators leads to efficient photolysis of membranes. Photochem Photobiol. 2013;89(3):625. doi:10.1111/php.12036
113. Selbo PK, Weyergang A, Høgset A, et al. Photochemical internalization provides time- and space-controlled endolysosomal escape of therapeutic molecules. J Control Release. 2010;148(1):2–12. doi:10.1016/j.jconrel.2010.06.008
114. Maiolo JR, Ottinger EA, Ferrer M. Specific redistribution of cell-penetrating peptides from endosomes to the cytoplasm and nucleus upon laser illumination. J Am Chem Soc. 2004;126(47):15376–15377. doi:10.1021/ja044867z
115. Liu G, Hu J, Zhang G, Liu S. Rationally engineering phototherapy modules of eosin-conjugated responsive polymeric nanocarriers via intracellular endocytic pH gradients. Bioconjug Chem. 2014;26(7):1328. doi:10.1021/bc500548r
116. Perez-Hernandez M, Pino PD, Mitchell SG, et al. Dissecting the molecular mechanism of apoptosis during photothermal therapy using gold nanoprisms. ACS Nano. 2015;9(1):52. doi:10.1021/nn505468v
117. Dai L, Liu J, Luo Z, Li M, Cai K. Tumor therapy: targeted drug delivery systems. J Mater Chem B. 2016;4(42):6758–6772. doi:10.1039/C6TB01743F
118. Galluzzi L, Maiuri MC, Vitale I, et al. Cell death modalities: classification and pathophysiological implications. Cell Death Diff. 2007;14(7):1237–1243. doi:10.1038/sj.cdd.4402148
119. Galluzzi L, Vitale I, Aaronson SA, et al. Molecular mechanisms of cell death: recommendations of the nomenclature committee on cell death 2018. Cell Death Diff. 2018;8(Pt 10):90028.
120. Mizushima N. Autophagy: process and function. Genes Dev. 2007;21(22):2861–2873. doi:10.1101/gad.1599207
121. Fehrenbacher N, Gyrd-Hansen M, Poulsen B, et al. Sensitization to the lysosomal cell death pathway upon immortalization and transformation. Cancer Res. 2007;64(15):5301–5310. doi:10.1158/0008-5472.CAN-04-1427
122. Boya P, Kroemer G. Lysosomal membrane permeabilization in cell death. Oncogene. 2008;27(50):6434–6451. doi:10.1038/onc.2008.310
123. Saftig P, Sandhoff K. Cancer: killing from the inside. Nature. 2013;502(7471):312. doi:10.1038/nature12692
124. Petersen NT, Olsen O, Groth-Pedersen L, et al. Transformation-associated changes in sphingolipid metabolism sensitize cells to lysosomal cell death induced by inhibitors of acid sphingomyelinase. Cancer Cell. 2013;24(3):379–393. doi:10.1016/j.ccr.2013.08.003
125. Gulbins E, Kolesnick RN. It takes a CAD to kill a tumor cell with a LMP. Cancer Cell. 2013;24(3):279. doi:10.1016/j.ccr.2013.08.025
126. Tardy C, Codogno P, Autefage H, Levade T, Andrieu-Abadie N. Lysosomes and lysosomal proteins in cancer cell death (new players of an old struggle). Biochim Biophys Acta. 2006;1765(2):101–125. doi:10.1016/j.bbcan.2005.11.003
127. Beckmann N, Sharma D, Gulbins E, Becker KA, Edelmann B. Inhibition of acid sphingomyelinase by tricyclic antidepressants and analogons. Front Physiol. 2014;5:331. doi:10.3389/fphys.2014.00331
128. Potze L, Mullauer FB, Colak S, Kessler JH, Medema JP. Betulinic acid-induced mitochondria-dependent cell death is counterbalanced by an autophagic salvage response. Cell Death Dis. 2014;5(4):e1169. doi:10.1038/cddis.2014.139
129. Kashkar H, Wiegmann K, Yazdanpanah B, Haubert D, Kronke M. Acid sphingomyelinase is indispensable for UV light-induced Bax conformational change at the mitochondrial membrane. J Biol Chem. 2005;280(21):20804–20813. doi:10.1074/jbc.M410869200
130. Xue LY, Chiu SM, Fiebig A, Andrews DW, Oleinick NL. Photodamage to multiple Bcl-xL isoforms by photodynamic therapy with the phthalocyanine photosensitizer Pc 4. Oncogene. 2003;22(58):9197–9204. doi:10.1038/sj.onc.1207019
131. Zhitomirsky B, Assaraf YG. Lysosomal sequestration of hydrophobic weak base chemotherapeutics triggers lysosomal biogenesis and lysosome-dependent cancer multidrug resistance. Oncotarget. 2015;6(2):1143. doi:10.18632/oncotarget.v6i2
132. Shchors K, Massaras A, Hanahan D. Dual targeting of the autophagic regulatory circuitry in gliomas with repurposed drugs elicits cell-lethal autophagy and therapeutic benefit. Cancer Cell. 2015;28(4):456–471. doi:10.1016/j.ccell.2015.08.012
133. Andersson SG, Karlberg O, Canback B, Kurland CG. On the origin of mitochondria: a genomics perspective. Philos Trans R Soc Lond B. 2003;358(1429):
134. Sheahan MB, McCurdy DW, Rose RJ. Mitochondria as a connected population: ensuring continuity of the mitochondrial genome during plant cell dedifferentiation through massive mitochondrial fusion. Plant J. 2005;44(5):744–755. doi:10.1111/tpj.2005.44.issue-5
135. Gammage PA, Viscomi C, Simard ML, et al. Genome editing in mitochondria corrects a pathogenic mtDNA mutation in vivo. Nat Med. 2018;24:1691–1695. doi:10.1038/s41591-018-0165-9
136. Acin-Perez R, Salazar E, Kamenetsky M, Buck J, Levin LR, Manfredi G. Cyclic AMP produced inside mitochondria regulates oxidative phosphorylation. Cell Metab. 2009;9(3):265–276. doi:10.1016/j.cmet.2009.01.012
137. Salehi MH, Kamalidehghan B, Houshmand M, et al. Gene expression profiling of mitochondrial oxidative phosphorylation (OXPHOS) complex I in Friedreich ataxia (FRDA) patients. PLoS One. 2014;9(4):e94069. doi:10.1371/journal.pone.0094069
138. McBride HM, Neuspiel M, Wasiak S. Mitochondria: more than just a powerhouse. Curr Biol. 2006;16(14):R551–R560. doi:10.1016/j.cub.2006.06.054
139. Wallace KB, Starkov AA. Mitochondrial targets of drug toxicity. Annu Rev Pharmacol Toxicol. 2000;40(40):353. doi:10.1146/annurev.pharmtox.40.1.353
140. van der Vliet A, Janssen-Heininger YMW, Anathy V. Oxidative stress in chronic lung disease: from mitochondrial dysfunction to dysregulated redox signaling. Mol Aspects Med. 2018;63:59–69. doi:10.1016/j.mam.2018.08.001
141. Pagliarini DJ, Calvo SE, Chang B, et al. A mitochondrial protein compendium elucidates complex I disease biology. Cell. 2008;134(1):112–123. doi:10.1016/j.cell.2008.06.016
142. Sarosiek KA, Fraser C, Muthalagu N, et al. Developmental regulation of mitochondrial apoptosis by c-Myc governs age- and tissue-specific sensitivity to cancer therapeutics. Cancer Cell. 2017;31(1):142–156. doi:10.1016/j.ccell.2016.11.011
143. Guo W, Nie C, Wang L, et al. Easily prepared and stable functionalized magnetic ordered mesoporous silica for efficient uranium extraction. Sci China Chem. 2016;59(5):629–636. doi:10.1007/s11426-016-5561-8
144. Zhou G, Meng S, Li Y, Ghebre YT, Cooke JP. Optimal ROS signaling is critical for nuclear reprogramming. Cell Rep. 2016;15(5):919–925. doi:10.1016/j.celrep.2016.03.084
145. Vaseva AV, Marchenko ND, Ji K, Tsirka SE, Holzmann S, Moll UM. p53 opens the mitochondrial permeability transition pore to trigger necrosis. Cell. 2012;149(7):1536–1548. doi:10.1016/j.cell.2012.05.014
146. Joza N, Susin SA, Daugas E, et al. Essential role of the mitochondrial apoptosis-inducing factor in programmed cell death. Nature. 2011;410(6828):549–554. doi:10.1038/35069004
147. Warburg O. On the Origin of Cancer Cells. Science. 1956;123(3191):309–314. doi:10.1126/science.123.3191.309
148. Biswas S, Torchilin VP. Nanopreparations for organelle-specific delivery in cancer. Adv Drug Deliv Rev. 2014;66:26–41. doi:10.1016/j.addr.2013.11.004
149. Borm PJ, Cakmak G, Jermann E, et al. Formation of PAH-DNA adducts after in vivo and vitro exposure of rats and lung cells to different commercial carbon blacks. Toxicol Appl Pharmacol. 2005;205(2):157–167. doi:10.1016/j.taap.2004.10.020
150. Salnikov V, Lukyánenko YO, Frederick CA, Lederer WJ, Lukyánenko V. Probing the Outer Mitochondrial Membrane in Cardiac Mitochondria with Nanoparticles. Biophys J. 2007; 9(3):1058–1071. doi:10.1529/biophysj.106.094318
151. Nel A, Xia T, Mädler L, Li N. Toxic potential of materials at the nanolevel. Science. 2006;311(5761):622. doi:10.1126/science.1114397
152. Ma X, Wang X, Zhou M, Fei H. A mitochondria-targeting gold-peptide nanoassembly for enhanced cancer-cell killing. Adv Healthcare Mater. 2013;2(12):1638–1643. doi:10.1002/adhm.v2.12
153. Yamada Y, Harashima H. Mitochondrial drug delivery systems for macromolecule and their therapeutic application to mitochondrial diseases. Adv Drug Deliv Rev. 2008;60(13–14):1439–1462. doi:10.1016/j.addr.2008.04.016
154. Yamada Y, Harashima H. Enhancement in selective mitochondrial association by direct modification of a mitochondrial targeting signal peptide on a liposomal based nanocarrier. Mitochondrion. 2013;13(5):526–532. doi:10.1016/j.mito.2012.09.001
155. Lemeshko VV. Membrane superficial charge modification affects miotochondrial permeabilization by derivatives of the polycationic peptide Btm-P1. Biophys J. 2010;98(3):278a–278a. doi:10.1016/j.bpj.2009.12.1517
156. Yue C, Yang Y, Zhang C, Alfranca G, Cui D. ROS-responsive mitochondria-targeting blended nanoparticles for chemo- and photodynamic synergistic therapy for lung cancer. J Control Release. 2017;259:e15–e16. doi:10.1016/j.jconrel.2017.03.061
157. Saris NE, Teplova VV, Odinokova IV, Azarashvily TS. Interference of calmidazolium with measurement of mitochondrial membrane potential using the tetraphenylphosphonium electrode or the fluorescent probe rhodamine 123. Anal Biochem. 2004;328(2):109–112. doi:10.1016/j.ab.2004.02.045
158. Bolduc JS, Denizeau F, Jumarie C. Cadmium-induced mitochondrial membrane-potential dissipation does not necessarily require cytosolic oxidative stress: studies using rhodamine-123 fluorescence unquenching. Toxicol Sci. 2004;77(2):299–306. doi:10.1093/toxsci/kfh015
159. Schluter T, Struy H, Schonfeld P. Protection of mitochondrial integrity from oxidative stress by the triaminopyridine derivative flupirtine. FEBS Lett. 2000;481(1):42–46. doi:10.1016/S0014-5793(00)01923-2
160. Starenki D, Park JI. Selective mitochondrial uptake of MKT-077 can suppress medullary thyroid carcinoma cell survival in vitro and in vivo. Endocrinol Metab. 2015;30(4):593–603. doi:10.3803/EnM.2015.30.4.593
161. Britten CD, Rowinsky EK, Baker SD, et al. A Phase I and pharmacokinetic study of the mitochondrial-specific rhodacyanine dye analog MKT 077. Clin Cancer Res. 2000;6(1):42–49.
162. Koya K, Li Y, Wang H, et al. MKT-077, a novel rhodacyanine dye in clinical trials, exhibits anticarcinoma activity in preclinical studies based on selective mitochondrial accumulation. Cancer Res. 1996;56(3):538–543.
163. Biswas S, Dodwadkar NS, Sawant RR, Koshkaryev A, Torchilin VP. Surface modification of liposomes with rhodamine-123-conjugated polymer results in enhanced mitochondrial targeting. J Drug Target. 2011;19(7):552–561. doi:10.3109/1061186X.2010.536983
164. Jensen PK. Antimycin-insensitive oxidation of succinate and reduced nicotinamide-adenine dinucleotide in electron-transport particles. I. pH dependency and hydrogen peroxide formation. Biochim Biophys Acta. 1966;122(2):157–166. doi:10.1016/0926-6593(66)90057-9
165. Zorov DB, Juhaszova M, Sollott SJ. Mitochondrial reactive oxygen species (ROS) and ROS-induced ROS release. Physiol Rev. 2014;94(3):909. doi:10.1152/physrev.00026.2013
166. Zhang W, Hu X, Shen Q, Xing D. Mitochondria-specific drug release and reactive oxygen species burst induced by polyprodrug nanoreactors can enhance chemotherapy. Nat Commun. 2019;10(1):1704. doi:10.1038/s41467-019-09566-3
167. Antunes F, Cadenas E, Brunk UT. Apoptosis induced by exposure to a low steady-state concentration of H2O2 is a consequence of lysosomal rupture. Biochem J. 2001;356:549–555. doi:10.1042/bj3560549
168. Guicciardi ME, Deussing J, Miyoshi H, et al. Cathepsin B contributes to TNF-alpha-mediated hepatocyte apoptosis by promoting mitochondrial release of cytochrome c. J Clin Invest. 2000;106(9):1127–1137. doi:10.1172/JCI9914
169. Riboldi SA, Sampaolesi M, Neuenschwander P, Cossu G, Mantero S. Electrospun degradable polyesterurethane membranes: potential scaffolds for skeletal muscle tissue engineering. Biomaterials. 2005;26(22):4606–4615. doi:10.1016/j.biomaterials.2004.11.035
170. Zhang J, Liu Z, Lian P, et al. Selective imaging and cancer cell death: via pH switchable near-infrared fluorescence and photothermal effects. Chem Sci. 2016;7:9. doi:10.1039/C6SC00221H
171. Lv YH, Fang M, Zheng JH, et al. Low-intensity ultrasound combined with 5-aminolevulinic acid administration in the treatment of human tongue squamous carcinoma. Cell Physiol Biochem. 2012;30(2):321–333. doi:10.1159/000339067
172. Ranji-Burachaloo H, Gurr PA, Dunstan DE, Qiao GG. Cancer treatment through nanoparticle-facilitated fenton reaction. ACS Nano. 2018;12(12):11819–11837. doi:10.1021/acsnano.8b07635
173. Panduri V, Weitzman SA, Chandel N, Kamp DW. The mitochondria-regulated death pathway mediates asbestos-induced alveolar epithelial cell apoptosis. Am J Respir Cell Mol Biol. 2003;28(2):241–248. doi:10.1165/rcmb.4903
174. Fang XY, Chen W, Fan JT, et al. Plant cyclopeptide RA-V kills human breast cancer cells by inducing mitochondria-mediated apoptosis through blocking PDK1-AKT interaction. Toxicol Appl Pharmacol. 2013;267(1):95–103. doi:10.1016/j.taap.2012.12.010
175. Ahmad KA, Iskandar KB, Hirpara JL, Clement MV, Pervaiz S. Hydrogen peroxide-mediated cytosolic acidification is a signal for mitochondrial translocation of Bax during drug-induced apoptosis of tumor cells. Cancer Res. 2004;64(21):7867–7878. doi:10.1158/0008-5472.CAN-04-0648
176. Mandavilli BS, Santos JH, Van HB. Mitochondrial DNA repair and aging. Mutation Res. 2002;509(1–2):127. doi:10.1016/S0027-5107(02)00220-8
177. Berneburg M, Kamenisch Y, Krutmann J. Repair of mitochondrial DNA in aging and carcinogenesis. Photochem Photobiol Sci. 2006;5(2):190–198. doi:10.1039/B507380D
178. Mao YS, Sunwoo H, Zhang B, Spector DL. Direct Visualization of the Co-transcriptional Assembly of a Nuclear Body by Noncoding RNAs. Nat Cell Biol. 2011;13(1):95. doi:10.1038/ncb2140
179. Handwerger KE, Gall JG. Subnuclear organelles: new insights into form and function. Trends Cell Biol. 2006;16(1):19. doi:10.1016/j.tcb.2005.11.005
180. Cremer T, Cremer C. Chromosome territories, nuclear architecture and gene regulation in mammalian cells. Nat Rev Genet. 2001;2(4):292–301. doi:10.1038/35066075
181. Xu P, Van Kirk EA, Zhan Y, Murdoch WJ, Radosz M, Shen Y. Targeted charge-reversal nanoparticles for nuclear drug delivery. Angew Chem Int Ed. 2010;46(26):4999–5002. doi:10.1002/anie.200605254
182. Qiu LP, Chen T, Ocsoy I, et al. A cell-targeted, size-photocontrollable, nuclear-uptake nanodrug delivery system for drug-resistant cancer therapy. Nano Lett. 2015;15(1):457–463. doi:10.1021/nl503777s
183. Godbey WT, Wu KK, Mikos AG. Tracking the intracellular path of poly(ethylenimine)/DNA complexes for gene delivery. Proc Natl Acad Sci USA. 1999;96(9):5177. doi:10.1073/pnas.96.9.5177
184. Tanaka N, Aoyama T, Kimura S, Gonzalez FJ. Targeting nuclear receptors for the treatment of fatty liver disease. Pharmacol Therapeut. 2017;179:142–157. doi:10.1016/j.pharmthera.2017.05.011
185. Paunesku T, Vogt S, Lai B, et al. Intracellular distribution of TiO2−DNA oligonucleotide nanoconjugates directed to nucleolus and mitochondria indicates sequence specificity. Nano Lett. 2007;7(3):596–601. doi:10.1021/nl0624723
186. Ali MR, Wu Y, Ghosh D, et al. Nuclear membrane-targeted gold nanoparticles inhibit cancer cell migration and invasion. ACS Nano. 2017;11(4):3716. doi:10.1021/acsnano.6b08345
187. Austin LA, Kang B, Yen CW, Elsayed MA. Nuclear targeted silver nanospheres perturb the cancer cell cycle differently than those of nanogold. Bioconjug Chem. 2011;22(11):2324–2331. doi:10.1021/bc200386m
188. Xu W, Edwards MR, Borek DM, et al. Ebola virus VP24 targets a unique NLS binding site on karyopherin alpha 5 to selectively compete with nuclear import of phosphorylated STAT1. Cell Host Microbe. 2014;16(2):187–200. doi:10.1016/j.chom.2014.07.008
189. Tammam SN, Azzazy HME, Lamprecht A. The effect of nanoparticle size and NLS density on nuclear targeting in cancer and normal cells; impaired nuclear import and aberrant nanoparticle intracellular trafficking in glioma. J Control Release. 2017;253:30–36. doi:10.1016/j.jconrel.2017.02.029
190. Pan L, Liu J, He Q, Shi J. MSN-mediated sequential vascular-to-cell nuclear-targeted drug delivery for efficient tumor regression. Adv Mater. 2014;26(39):6742–6748. doi:10.1002/adma.v26.39
191. Peng H, Tang J, Zheng R, et al. Nuclear-targeted multifunctional magnetic nanoparticles for photothermal therapy. Adv Healthcare Mater. 2017;6(7):1601289. doi:10.1002/adhm.v6.7
192. Yu Z, Pan W, Li N, Tang B. A nuclear targeted dual-photosensitizer for drug-resistant cancer therapy with NIR activated multiple ROS. Chem Sci. 2016;7:7. doi:10.1039/C6SC00737F
193. Wang X, Zhou J, Chen B, et al. Enhanced intracellular hyperthermia efficiency by magnetic nanoparticles modified with nucleus and mitochondria targeting peptides. J Nanosci Nanotechnol. 2016;16(6):6560–6566. doi:10.1166/jnn.2016.10852
194. Jana A, Saha B, Banerjee DR, et al. Photocontrolled nuclear-targeted drug delivery by single component photoresponsive fluorescent organic nanoparticles of acridin-9-methanol. Bioconjug Chem. 2013;24(11):1828. doi:10.1021/bc400170r
195. Papageorgiou I, Yin, ZR, Ladon D, Baird D, Lewis AC, Sood A, Newson R, Learmonth ID, Case CP. Genotoxic effects of particles of surgical cobalt chrome alloy on human cells of different age in vitro. Mutat Res-Fund Mol M. 2007;619(1):45–58. doi:10.1016/j.mrfmmm.2007.01.008
196. Devereux TR, Risinger JI, Barrett JC. Mutations and altered expression of the human cancer genes: what they tell us about causes. IARC Sci Publ. 1999;146:19–42.
197. Ames BN. Identifying environmental chemicals causing mutations and cancer. Science. 1979;204(4393):587. doi:10.1126/science.373122
198. Kuchino Y, Mori F, Kasai H, et al. Misreading of DNA templates containing 8-hydroxydeoxyguanosine at the modified base and at adjacent residues. Nature. 1987;327(6117):77. doi:10.1038/327077a0
199. Greenman CD. Cancer haploinsufficient gene selection in cancer. Science. 2012;337(6090):47–48. doi:10.1126/science.1224806
200. Wu X, Ruvkun G. Cancer germ cell genes and cancer. Science. 2010;330(6012):1761–1762. doi:10.1126/science.1200772
201. Balestrieri C, Vanoni M, Hautaniemi S, Alberghina L, Chiaradonna F. Integrative transcriptional analysis between human and mouse cancer cells provides a common set of transformation associated genes. Biotechnol Adv. 2012;30(1):16–29. doi:10.1016/j.biotechadv.2011.06.013
202. Zhou BBS, Elledge SJ. The DNA damage response: putting checkpoints in perspective. Nature. 2000;408(6811):433–439. doi:10.1038/35044005
203. Mei Z, Su T, Ye J, Yang C, Zhang S, Xie C. The miR-15 family enhances the radiosensitivity of breast cancer cells by Targeting G2 checkpoints. Radiat Res. 2015;183(2):196–207. doi:10.1667/RR13784.1
204. Li X, Zhang X, Zheng L, et al. Hypericin-mediated sonodynamic therapy induces autophagy and decreases lipids in THP-1 macrophage by promoting ROS-dependent nuclear translocation of TFEB. Cell Death Dis. 2016;7(12):e2527. doi:10.1038/cddis.2016.433
205. Zhang Z, Liu C, Bai J, et al. Silver nanoparticle gated, mesoporous silica coated gold nanorods (AuNR@MS@AgNPs): low premature release and multifunctional cancer theranostic platform. ACS Appl Mater Interfaces. 2015;7(11):6211–6219. doi:10.1021/acsami.5b00368
206. Marnett LJ. Oxyradicals and DNA damage. Carcinogenesis. 2000;21(3):361–370. doi:10.1093/carcin/21.3.361
207. Schins RP. Mechanisms of genotoxicity of particles and fibers. Inhalation Toxicol. 2002;14(1):57–78. doi:10.1080/089583701753338631
208. Cubillos-Ruiz JR, Bettigole SE, Glimcher LH. Tumorigenic and immunosuppressive effects of endoplasmic reticulum stress in cancer. Cell. 2017;168(4):692–706. doi:10.1016/j.cell.2016.12.004
209. Xu CY, Bailly-Maitre B, Reed JC. Endoplasmic reticulum stress: cell life and death decisions. J Clin Invest. 2005;115(10):2656–2664. doi:10.1172/JCI26373
210. Zhang H, Fan J, Wang J, Zhang S, Dou B, Peng X. An off-on COX-2-specific fluorescent probe: targeting the Golgi apparatus of cancer cells. J Am Chem Soc. 2013;135(31):11663–11669. doi:10.1021/ja4056905
211. Rivinoja A, Pujol FM, Hassinen A, Kellokumpu S. Golgi pH, its regulation and roles in human disease. Ann Med. 2012;44(6):542. doi:10.3109/07853890.2011.579150
212. Horobin RW, Rashid-Doubell F, Pediani JD, Milligan G. Predicting small molecule fluorescent probe localization in living cells using QSAR modeling. 1. Overview and models for probes of structure, properties and function in single cells. Biotech Histochem. 2013;88(8):440–460. doi:10.3109/10520295.2013.780634
213. Zhou L, Wu Y, Meng X, et al. Dye-anchored MnO nanoparticles targeting tumor and inducing enhanced phototherapy effect via mitochondria-mediated pathway. Small. 2018;14(36):e1801008. doi:10.1002/smll.v14.36
214. Yang D, Liu D, Qin M, et al. Intestinal mucin induces more endocytosis but less transcytosis of nanoparticles across enterocytes by triggering nanoclustering and strengthening the retrograde pathway. ACS Appl Mater Interfaces. 2018;10(14):11443–11456. doi:10.1021/acsami.7b19153
215. Zhang X, Xu X, Li Y, Hu C, Zhang Z, Gu Z. Virion-like membrane-breaking nanoparticles with tumor-activated cell-and-tissue dual-penetration conquer impermeable cancer. Adv Mater. 2018;30(27):1707240. doi:10.1002/adma.201707240
216. Adinolfi B, Pellegrino M, Tombelli S, et al. Polymeric nanoparticles promote endocytosis of a survivin molecular beacon: localization and fate of nanoparticles and beacon in human A549 cells. Life Sci. 2018;215:106–112. doi:10.1016/j.lfs.2018.11.007
217. Wu CY, Wu YF, Jin Y, et al. Endosomal/lysosomal location of organically modified silica nanoparticles following caveolae-mediated endocytosis. RSC Adv. 2019;9(24):13855–13862. doi:10.1039/C9RA00404A
218. Xu CC, Haque F, Jasinski DL, Binzel DW, Shu D, Guo PX. Favorable biodistribution, specific targeting and conditional endosomal escape of RNA nanoparticles in cancer therapy. Cancer Lett. 2018;414:57–70. doi:10.1016/j.canlet.2017.09.043
219. Koide R, Nishimura SI. Antiadhesive nanosomes facilitate targeting of the lysosomal GlcNAc salvage pathway through derailed cancer endocytosis. Angew Chem Int Ed Engl. 2019;58(41):14513–14518.
220. Nosrati H, Mojtahedi A, Danafar H, Kheiri Manjili H. Enzymatic stimuli-responsive methotrexate-conjugated magnetic nanoparticles for target delivery to breast cancer cells and release study in lysosomal condition. J Biomed Mater Res A. 2018;106(6):1646–1654. doi:10.1002/jbm.a.v106.6
221. Prokhorova IV, Akulich KA, Makeeva DS, et al. Amicoumacin A induces cancer cell death by targeting the eukaryotic ribosome. Sci Rep. 2016;6. doi:10.1038/srep27720
222. Zhou Q, Hou Z, Zuo S, et al. LUCAT1 promotes colorectal cancer tumorigenesis by targeting the ribosomal protein L40-MDM2-p53 pathway through binding with UBA52. Cancer Sci. 2019;110(4):1194–1207. doi:10.1111/cas.2019.110.issue-4
223. Chida J, Araki H, Maeda Y. Specific growth suppression of human cancer cells by targeted delivery of dictyostelium mitochondrial ribosomal protein S4. Cancer Cell Int. 2014;14:56. doi:10.1186/1475-2867-14-56
 © 2020 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2020 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.