Back to Journals » International Journal of Nanomedicine » Volume 14
Stimulus-responsive vesicular polymer nano-integrators for drug and gene delivery
Authors Mu X , Gan S , Wang Y, Li H , Zhou G
Received 30 January 2019
Accepted for publication 8 May 2019
Published 18 July 2019 Volume 2019:14 Pages 5415—5434
DOI https://doi.org/10.2147/IJN.S203555
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 2
Editor who approved publication: Dr Linlin Sun
Xin Mu,1,2 Shenglong Gan,1,2 Yao Wang,1,2 Hao Li,1,2 Guofu Zhou1,2
1Guangdong Provincial Key Laboratory of Optical Information Materials and Technology & Institute of Electronic Paper Displays, South China Academy of Advanced Optoelectronics, South China Normal University, Guangzhou 510006, People’s Republic of China; 2National Center for International Research on Green Optoelectronics, South China Normal University, Guangzhou 510006, People’s Republic of China
Abstract: Over the past two decades, nano-sized biosystems have increasingly been utilized to deliver various pharmaceutical agents to a specific region, organ or tissue for controllable precision therapy. Whether solid nanohydrogel, nanosphere, nanoparticle, nanosheet, micelles and lipoproteins, or “hollow” nanobubble, liposome, nanocapsule, and nanovesicle, all of them can exhibit outstanding loading and releasing capability as a drug vehicle – in particular polymeric nanovesicle, a microscopic hollow sphere that encloses a water core with a thin polymer membrane. Besides excellent stability, toughness and liposome-like compatibility, polymeric nanovesicles offer considerable scope for tailoring properties by changing their chemical structure, block lengths, stimulus-responsiveness and even conjugation with biomolecules. In this review, we summarize the latest advances in stimulus-responsive polymeric nanovesicles for biomedical applications. Different functionalized polymers are in development to construct more complex multiple responsive nanovesicles in delivery systems, medical imaging, biosensors and so on.
Keywords: nanovesicle, stimulus-responsive, amphiphilic block copolymer, self-assembled, drug delivery
Introduction
Over the past two decades, nanoscale materials are increasingly used to deliver drugs, genes, and other agents. These new systems (eg, nanoscale polymersomes, spheres, or particles)are made of biodegradable natural and synthetic materials. Polymersomes are the vesicles, made of bilayer membrane of amphiphilic block copolymer. These vesicles are stable and robust with a 10–30 nm thick membrane,1,2 and known for their various applications in the formulation of drugs, cosmetics, paint, drug and gene carriers, and models for biological membranes.3–6 Vesicles are microscopic hollow spheres that enclose a volume with a bilayer thin membrane. The membranes are typically self-assemblies of amphiphilic molecules.7,8
Before the polymeric nanovesicles, as a kind of biological vesicle formed from amphiphilic phospholipids in dilute solution, the liposome was originally reported by Bangham in the 1960s.9 It can self-assemble to enclose an aqueous compartment with a lipid bilayer membrane. However, common liposomes have disadvantages such as low encapsulation efficiency and fast release rate in vivo due to their poor physical stability.10 Also, as previously reported,11 vesicle is capable of encapsulating hydrophilic drugs in the inner aqueous phase or lipophilic drugs in the bilayer walls. Therefore, many polymeric vesicles have been studied for many years.
In particular, stimulus-responsive polymeric nanovesicles allow physical or chemical changes to external stimulus. Their responses can be divided into two categories: the chemical response mainly includes pH, redox and molecular chain response; the physical response does temperature, light, ultrasound, magnetic, electric and gas response.12–16 These stimulus-responsive nanovesicles warrant closer study in responsive material, targeted drug release and gene carriers.17 In this review, recent advances in stimulus-responsive polymeric vesicles are systematically summarized to highlight their immense potential for medical applications.
Polymeric nanovesicles
Under appropriate preparation conditions, amphiphilic block copolymers with specific chemical composition will form vesicular assemblies, defined as polymer nanovesicle. There are some differences between liposome and polymer nanovesicle. The liposome consists of the phospholipid bimolecular and forms lamellar bilayer structures. However, for polymer, in the aqueous solution, the hydrophobic domains of the copolymer tend to associate to form the membrane while the hydrophilic domains face inner and outer solution (see Figure 1). The transition from disordered state to trilayer hydrophilic–hydrophobic–hydrophilic membrane mainly depends on the molecule weight of the copolymer, the mass ratio of each block, the effective interaction energy within polymer chains, and so on.18
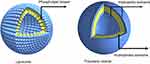 |
Figure 1 Morphological structures of liposome and polymeric nanovesicle. |
Except for the advantage of stability and toughness, polymeric vesicles have more possibilities of tailoring properties by changing the block lengths, chemical structure, and conjugation with biomolecules. Their structures can be modified to package and deliver their cargos to the desired site of action or to respond to specific physiological or external stimulus.19 It helps functionalizing polymer vesicles potential application in drug, gene, sensors, and contrast agent delivery.
Stimulus-responsive polymeric nanovesicles as drug carriers
As previously mentioned, amphiphilic block copolymers can self-assemble into nanoscale vesicle with inner aqueous core and outer trilayer membrane. It means that, different domains will interact with different agents inside polymeric nanovesicle. Typically, hydrophobic molecules can be loaded into the hydrophobic interlayer of vesicle membrane, and hydrophilic ones can be encapsulated in the aqueous core. Once specific fragments or side groups may be introduced into copolymer frame material, they will give these vesicular nanocarriers stimulus-responsive ability for controllable and precise delivery. Under the induction of the external change, eg, pH, temperature, and light, the structure of vesicles may change, disassemble, even break down to release the drug encapsulated in the vesicles.
pH-responsive polymeric nanovesicles
Compared with physiological pH values in blood and normal tissues (~7.4), both tumor and inflammatory tissues (pH ~6.8), and endosomal and lysosomal compartments of cells (pH ~5–6), provides a mildly acidic internal environment.20 Of course, this acidic microenvironment also provides a potential stimulus source and an ideal target to pH-sensitive carrier. Thus, in all the stimulus-responsive polymeric nanovesicles, pH-responsive polymeric nanovesicles have been studied most.
Hydrolysis and disassembly
Chen et al prepared a novel polymersome using diblock copolymer of poly(ethyleneglycole)-block-poly 2,4,6-trimethoxybenzylidenepentaerythritol carbonate (PEG-b-PTMBPEC) (see Figure 2).21 Its acetals can keep stable at pH 7.4, but quickly hydrolyze below pH 5.0. Naturally, the loaded hydrophilic anticancer drugs, DOX·HCl (doxorubicin hydrochloride) and PTX (hydrophobic paclitaxel), can be controllably released in a pH-dependent manner. The results showed that these polymersomes possessed high loading efficiencies (30.0–37.7% for PTX; 19.5–26.2% for DOX·HCl) and excellent pH-responsive performance. Typically, the release rates of PTX reached about 80%, 45%, and 25% at pH 4.0, 5.0 and 7.4 in 1 day, and the one of DOX·HCl reached about 65%, 45% and 32%, respectively, at the same pH conditions in 1 day.
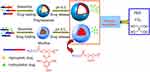 |
Figure 2 Illustration of pH-sensitive degradable polymersomes based on PEG-PTMBPEC diblock copolymer for triggered release of both hydrophilic and hydrophobic anticancer drugs. In comparison, pH-sensitive degradable micelles are typically applied for release of hydrophobic drugs only. Reprinted from Chen W, Meng F, Cheng R, Zhong Z. pH-Sensitive degradable polymersomes for triggered release of anticancer drugs: a comparative study with micelles. J Control Release. 2010;142(1):40–46. Copyright 2009, with permission from Elsevier.21 |
Similar nanocarriers based on acid-triggered copolymer hydrolysis were also reported. Taking [poly(ε-caprolactone)-block-poly[2-(methacryloyloxy) ethyl phosphorylcholine]] (PCL-b-PMPC) vesicle for an example.22 At pH 7.4, the hydrolysis rate of the PCL domains is very low, so that the permeation rate of the encapsulated DOX·HCl molecules is only controlled by the diffusion process. But at pH 5.0, the PCL membrane is destroyed by acidolysis. About 55% of DOX·HCl will be released after 12 h.
Zhu et al further prepared amphiphilic cholate-grafted poly(L-lysine) and acid-cleavable PEG-DOX conjugate to construct an multicomponent nanovesicle.23 Here, the PEG domain may detach from the vesicle surface at pH 6.5. While decreasing to pH 5.0, the complete hydrolysis of the PEG-DOX will make the polymeric vesicle destabilized. To the encapsulated calcein, the slow release appeared at pH 6.5, while a burst release occurred at lower pH 5.07.
As a classic anticancer agent, arsenic trioxide (As2O3) nanoparticles (As-NPs) was encapsulated into common poly(ethylene glycol)-block-poly(lactic acid) (PEG-b-PDLLA) vesicles to develop an effective acid-controlled delivery system.24 Due to acidolysis of PDLLA domains, about 80 wt% of As be released within 72 h at pH 5.0. In particular, the authors chose a special ligand to transmembrane glycoproteins (CD44), single chain variable fragment (scFvCD44V6) to modify As-NPs for cellular target. After treating the mice for 21 days, the tumor volumes of mice receiving scFv-As-NPs in PEG-PDLLA vesicles were significantly smaller than that of the mice only receiving As-NPs, free and the normal saline (NS) control.
Multidrug resistance (MDR) is one of the major obstacles hindering the chemotherapy of cancer. Xintao Shuai et al prepared a novel pH-sensitive nanovesicle self-assembled by methoxy-poly (ethylene glycol)–poly[(N-(6hydroxyhexyl)-g-doxorubicin-L-aspartamide)-(β-benzyl-L-aspartate)] (mPEG-P[Asp(HPA-g-DOX)-BLA)].25 Here DOX was conjugated onto the side chains via acid-liable bond. Only 14.47% of DOX was released at pH 7.4 within 24 h. By comparison, the DOX release increased to 78.74% when the pH value changed to 5.0 within 24 h owing to the acid-induced nanovesicle disassembly. It facilitates these nano-sized drug carriers to achieve precise release and therapy in the acidic lysosome of tumor cells.
Along with higher demands for precise drug delivery, triblock and even more complex copolymers have been studied to assemble smart vesicular nanocarriers with good response. We take [poly(ethyleneglycole)45-blcok-poly(diethylaminoethylmethacrylate)81] (PEG45-b-PDEAEM81) and [3,4-dimethyl maleic imidoethyl methacrylate-intergrated] (PEG45-b-PDEAEM-s-PDMIEM) polymersomes for example.26 At pH 7, the crosslinking samples density swelled stepwisely, but the non-crosslinked ones revealed a sharp drop in size, indicating an immediate disassembly. At lower pH, only small micelles with dimensions of <10 nm were present.
Another novel norbornene-derived homopolymers (NDTH) was applied for building pH-sensitive vesicles.27 Hereinto, the norbornene backbone behaved as a hydrophobic moiety, and the thiobarbiturate-functionalized unit in NDTH acted as hydrophilic head group. DOX was used as model drug inside this vesicle, featuring 70% w/w loading efficiency (see Figure 3). At pH 5.5–6, a significantly accelerated drug release was performed, compared to stable state under physiological condition. In the mammary gland cancer cell line (4T) test on mice, post treatment of NDTH-DOX at 24 hrs showed a diffused intracellular accumulation, namely a clear endosomal/lysosomal NDTH-DOX penetration.
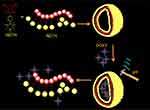 |
Figure 3 Cartoon representation of NDTH polymersomes and the release process of pH-responsive nanovesicles encapsulated DOX. stimulus-responsive. Reprinted with permission from Mane SR, Rao NV, Chaterjee K, Dinda H, Nag S, Kishore A, Das Sarma J, Shunmugam R. Amphiphilic Homopolymer Vesicles as Unique Nano-Carriers for Cancer Therapy. Macromolecules. 2012;45(19):8037–8042. Copyright 2012, American Chemical Society.27 |
Protonation and deprotonation of copolymer
Under the different values of pH, some molecules and groups can be protonated in acidic condition and be deprotonated in alkaline condition. Thus, their hydrophilicity will undergo sharp reversal to change the structure and morphology of their assemblies. Typically, a copolymer of methoxy PEG and [poly(β-amino ester)] (PAE) -grafted PDLLA was designed as the frame material of protonated vesicles.28 As the pH value changes, so the size of nano-sized polymersome will change under the induction of PAE protonation. Once PAE is thoroughly ionized, these vesicles will not swell. The results of calcein leakage experiment showed that the encapsulated calcein behaved with an obvious release from pH 7.4 to 4.5, and completely escaped out within 15 min at pH 4.5. Similarly, PAE-grafted PEG-b-PCL was also used to form DOX∙HCl-loaded polymersomes (loading efficiency: ~29%).29 In the drug release test, the release of DOX∙HCl was significantly accelerated at pH 6.4 because of the PAE ionization. It can enhance the cellular uptake of DOX∙HCl. Another PAE-grafted mPEG-b-PLA polymersome was also prepared for DOX controlled release.30
Besides PAE, other copolymer vesicles with protonated characters were also reported. For example, triblock [poly (ethylene glycol) methyl ether acrylate-block-poly(L-lysine)-block-poly(L-histidine)] (p(PEGA)30-b-p(Lys)25-b-p(His)n) (n=25, 50, 75, and 100) were synthesized for tumoral intracellular release of DOX.31 Under the acidic conditions inside tumor, the ionization degree of the p(His)n blocks will rise. So, these vesicles become unstable to diminish hydrophobic interactions, resulting in rapid DOX release. At pH 5.5, the vesicle size increased noticeably, and >50% of the initial DOX load was released within 24 h, as well as 80% within 72 h.
To identify pyridine, hydrogen proton will be introduced to protonate its nitrogen atom, following by the resulting electrostatic repulsion. It may contribute to induce the vesicle membranes separated till a distinct bilayer form. Typically, an amphiphilic homopolymer, poly(2-(4-vinylphenyl) pyridine) (PVPPy), was developed into a sensitive vesicle.32 At pH 3, 7.4 and 10, the release rates of fluorescein isothiocyanate (FITC) were 57%, 45% and 38% within the first 25 min, respectively; the pyrene release from the polymersome reached 49%, 63%, and 66% within the first 25 min, respectively. These findings suggest that this vesicle is a good candidate for carrying drugs.
Poly(2-(methacryloyloxy) ethylphosphorylcholine)-block-poly(2-(diisopropylamino) ethyl methacrylate) (PMPC-b-PDPA) vesicle was also studied.33 Here, 2-diisopropylamino group is a pH-responsive point with an appropriate pKa value (~6.3). Depending on pH value, the drug could be partially adsorbed into the polymeric membrane through electrostatic interactions, and vice versa.
As a homopolymer with terminal functionalized group, NH2-PEG2000-4-aminobenzoic acid diethylaminoethyl ester (DEAAB), namely PDEP, was introduced into a self-assembled vesicular system.34 In the acidic circumstance, the tertiary amino groups might be protonated to weaken the hydrophobicity of DEAAB in the PDEP. Naturally, the PDEP vesicles inhibited the DOX·HCl release at pH 7.4 while enabling fast release at pH 5.5.
Polyethyleneimine (PEI) is also a pH-responsive polymer based on three protonation levels of amine groups: fully deprotonated at high pH (>10), primarily protonated in neutral (~7), and most protonated at low pH (<4). In recent, PEI was employed to co-assemble with Ag(I) nanoclusters and (NH4)6[Ag6(mna)6](H2mna=2-mercaptonicotinicacid, Ag6-NCs) for building pH-responsive nanovesicles. These vesicles can be well-established candidates in controlled drug delivery, biomarker and sensors in aqueous solution.35
To improve the vivo delivery of chemotherapeutics, the cholesteryl hemisuccinate (CholHS) was inserted into the polyphosphazene block of poly[(PEG)x[1H-benzo[d]imidazol-2-yl) methanamine)yphosphazene]n (PEBP) for forming sensitive nanovesicles.36 Particularly, the weakly acidic microenvironment in tumor cells can induce the protonation of benzimidazole domains to take the hydrophobic-hydrophilic transformation with fast drug release. At pH 7.4, the release ratio of DOX·HCl reached 59% after 24 h; and when the pH decreased to 6.5 and 5.5, the release ratio increased to 75 and 90%, respectively.
Of course, protonation and deprotonation also exist in triblock and more complex polymeric vesicles, ie, poly[2-(methacryloyloxy)ethyl methacrylate]-block-poly[2-(dimethylamino) ethyl methacrylate]-block-poly[2-(diisopropylamino) ethyl methacrylate] (PMPC-b-PDMA-b-PDPA) and PMPC-b-PDPA-b-PDMA copolymer vesicles.37 Obviously, PDPA can be protonated to appear hydrophilic at lower pH and be deprotonated to recover hydrophobic at higher pH. The vesicles can release the cargo controllably by PDPA protonation. After 24 h, the DOX release content was nearly 25% at pH 5.0, much higher than at pH 7.4.
Totally different from conventional strategy, supramolecular system is available for controllable drug delivery.38 Wang et al reported a facile, one-pot spontaneous assembly to fabricate pH-responsive hydrogen-bonded polymersome.39 Here, PVP [poly(N-vinylpyrrolidone)], PSEMA [poly(2-succinyloxyethyl methacrylate)] and PMEMA [poly (2-maleyloxyethyl methacrylate)] can form vesicular self-assembly by interdiffusion and hydrogen-bond between PVP and PSEMA or PMEMA droplet. The feasibility of such a scenario was illustrated by the reversible trapping of Nile red within the vesicles. Once the pH value rose to 8, the encapsulated Nile red was fully released owing to carrier dissolution.
Temperature-responsive polymeric nanovesicle
In the twentieth century, thermo-sensitive materials with temperature-response properties were extensively studied as potential candidates for applications in nanomedicines.40–43 These copolymers can undergo conformational changes or phase transitions in solution, to change partial hydrophilicity, even break down. These specific behaviors make heat-controlling drug release possible.
Change of size and structure
As a classic thermoresponsive polymer, poly(N-isopropylacrylamide) (PNIPAM) can undergo the transition from hydrophilic to hydrophobic sate over lower critical solution temperature (LCST; ~31 °C),44 which contributes to change the size and structure of their assembly. For example, poly(2-(dimethylamino)ethyl methacrylate) (PDMAEMA)-block-coumarin methacrylate (CMA)-b-PNIPAM was reported.45 As the ambient temperature drops to below LCST, the hydrophobic shell-forming PNIPAM blocks become soluble in water until the crosslinked corona or shell of the vesicles becomes fully hydrated and expands to release the cargo.
Differently, poly(2-vinylpyridine)66-block-poly(ethylene oxide)46 (P2VP66-b-PEO46) polymersomes have specific size distribution at different temperatures.46 With increasing temperature, the solubility of PEO in water decreases, and then the volume of the water-swollen PEO layer also decreases. Thereby the interfacial area at the hydrophilic/hydrophobic interface reduces. An intermediate incubation at 16 °C for 24 h resulted in slightly larger particles before further warming to 25 °C. It is helpful to regulate permeation of the encapsulated drug in this polymersome.
Another change of vesicular structure is much more dramatic than size changes. Typically, by heat induction, the wormlike micelles split into a mixture of wormlike micelles and a few smaller vesicles, then develop into a mixture of closed and open vesicles, and finally convert into to exclusively closed vesicles.43 As polystyrene-block-polyacrylicacid (PS139-b-PAA17) previously was reported, the sharp changes on its solubility cause the vesicle-to-micelle transition, while their nano-integrator is heated from room temperature to 45 °C.47–49
Sabrina Hocine et al took advantage of the critical dehydration temperature of PEG corona, and thermotropic liquid crystal (LC), to tune the chemical structure and chain mobility of the hydrophobic block in PEG-polybutylacrylate (PBA)/polystyrene (PS)/LC systems.50 Different ambient temperatures allow for controllable vesicular self-assembly whose membrane thickness and morphological change are irreversible. As calcein is encapsulated in the inner aqueous compartment of these LC polymersomes, there are no changes below 55 °C, and notable increases in fluorescence intensity at 55 °C. At this time, 19% and 33% of fluorescence intensity are recovered for nematic and smectic polymersomes, respectively. At 75 °C, the fluorescence percentage further reached 40% and 64% for nematic and smectic polymersomes, respectively.
When both thermo-sensitive composites are introduced into a copolymer, eg mPEO-b-PCL-b-PNIPAM, it will undergo self-assembly in aqueous solutions forming stable nanovesicles.51 The corresponding LCST of the triblock copolymers is adjustable by varying the PNIAM segment length as well as the PCL segment length. Here their LCST covered a range from 33.9 to 41.0 °C in water. Obviously, the diameters of nanovesicles are different below and above LCST. Below LCST, a hydrophobic anticancer drug, IMC was stably entrapped within the hydrophobic PCL membrane via hydrogen bonding. Above LCST, PNIPAM chains changed from being hydrophilic to hydrophobic,52,53 and then collapsed onto the PCL membrane. So, a faster release rate of IMC occurred at 40 °C (above LCST), indicating that the nanovesicles has a good response to the thermo-stimulus (see Figure 4).
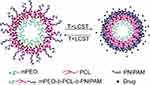 |
Figure 4 Self-assembly (SA) of the copolymer and the thermally induced drug release of resulting micelles in aqueous solution. Reprinted from Cao X, Chen Y, Chai W, Zhang W, Wang Y, Fu PF. Thermoresponsive self-assembled nanovesicles based on amphiphilic triblock copolymers and their potential applications as smart drug release carriers. Journal of Applied Polymer Science. 2015;132(4):41361–41372. Copyright 2014, with permission from John Wiley and Sons.51 |
Disassembly and self-assembly
Nowadays, polymer nano-assemblies from double hydrophilic block copolymers are growing into a novel and powerful carrier for drug delivery, owing to their thermo-controlling self-assembly.54–57 Take a reversibly crosslinked PEG-b-poly(acrylic acid) (PAA)-b-PNIPAM nanovesicle, for example.58 This triblock copolymer can dissolve readily in aqueous solution at room temperature, and quickly self-assemble into nanovesicles at body temperature (~37 °C). In drug experiment, FITC–dextran (MWCO: 4 kDa) was chosen as a model protein inside the vesicle, featuring a very high loading content of 85–140 wt%. The results showed that, only 20% of FITC–dextran 4 kDa was released from the crosslinked polymersomes in 6 h at 20 °C, but 45% and 61% of FITC–dextran was released within the first 1 h and 6 h at 37 °C, respectively.
In recent years, other polymersomes that can self-assemble and disassemble by heating were also studied.59,60 As shown in Figure 5, poly(N-vinylcaprolactam)n-block-poly(N-vinylpyrrolidone)m (PVCLn-b-PVPONm) diblock copolymers can interact with tannic acid (TA) via hydrogen bonding to form an interlocking structure.61 Above the copolymer’s LCST, PVCLn-b-PVPONm can self-assemble into the polymersomes due to dehydration of the PVCL blocks. Once cooled to room temperature, these vesicles will disassemble into free polymer chains. Naturally, DOX encapsulated in TA-locked polymersome was controllably released in the nuclei of human alveolar adenocarcinoma tumor cells after 6-h incubation via biodegradation of the TA shell.
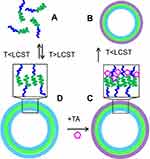 |
Figure 5 PVCLn-b-PVPONm diblock copolymers (A) assembled into polymersomal nanocapsules at T>LCST (B) can be locked with TA (C) via hydrogen bonds with PVPON, which results in PVCLn-PVPONm nanocapsules stable at T<LCST (D). Reprinted with permission from Kozlovskaya V, Liu F, Xue B, Ahmad F, Alford A, Saeed M Kharlampieva E. Polyphenolic Polymersomes of Temperature-Sensitive Poly(N-vinylcaprolactam)-block-Poly(N-vinylpyrrolidone) for Anticancer Therapy. Biomacromolecules. 2017;18(8):2552–2563. Copyright 2017, American Chemical Society.61 |
Novelly, nonconjugated PEGylated poly(amide-imide) (PAI) (PEG-PAI4) with isolated benzene rings was applied to self-assemble thermoresponsive nanovesicle owing to its LCST behavior.62 Below LCST, PEG500 shielding self-assembled PEG-PAI4 into nanovesicles; above LCST, nanovesicles collapsed and occurred phase separation. Accompanied by the excellent biostability and photostability, PEGylated poly(amide-imide) shows potential as a candidate for cell imaging.
Photoresponsive polymeric nanovesicles
Light has been widely considered to be the most promising operation mode because of the unique advantages in remote control, clean and instant input/remocal, wavelength variation and biocompatibility.63 While the photoresponsive group is imported into frame copolymer and the vesicular integrator, light can precisely change vesicle structure to modulate drug release and delivery.
Structure change
A photo-cleavable linker, O-nitrobenzyl (ONB), was placed between two polymer blocks to obtain poly(methyl caprolactone) (PMCL)-ONB-PAA (PMCL-ONB-PAA).64 Clearly, ultraviolet light (UV) can induce a successful cleavage of the diblock copolymer chains. About 40% of the encapsulated fluorescein was released within 200 min by UV irradiation with an intensity of 20 mW cm−2, or within 20 min at a higher UV intensity of 700 mW cm−2.
A potent photosensitizer, Al(III) phthalocyanine chloride disulfonic acid (AlPcS2a), was chosen to incorporate with PEG-block-poly(α,β-aspartic acid) (PAsp) (PEG-b-PAsp) vesicle (11 wt%), namely PICsome.65 Under photoirradiation, the photochemical damage of the PIC membranes can trigger the quick release of AlPcS2a. Typically, the red fluorescence of AlPcS2a originated from the PICsomes significantly increased within the lysosomes after 7 min of photoirradiation, and gradually diffused from the lysosomal membranes into the cytoplasm.
Disassembly and assembly
A common organic chromophore, azobenzene can undergo conformational transition by trans-cis photoisomerization.66 In many self-assembly, azobenzene is employed as photoswitch to trigger self-assembly forming or breaking down. Take a supramolecular vesicle containing PEG43-b-PAA153 and azobenzene surfactant (AzoC10) for example.67 Here the photoisomerization of azobenzene moieties can tune the AzoC10 amphiphilicity reversibly, to induce the vesicle assembly and disassembly. The results showed that, these vesicles swelled to several hundred nanometers under UV irradiation. Thereby most of the encapsulated model fluorescent probes were released rapidly from the vesicles, owing to the swelling-induced high permeation.
Differently, 2-nitrobenzyl with the photolabile nature was inset into PEO-poly(2-nitrobenzyloxycarbonylaminoethyl methacrylate) (PNBOC) (PEO-PNBOC) to develop a novel photosensitive polymersomes.68 UV light can decompose 2-nitrobenzyl to make NBOC converted into 2-aminoethyl methacrylate (AEMA), following by the release of 2-nitrobenzaldehyde and CO2 (see Figure 6). Here a hydrophilic anticancer drug, DOXHCl, and a hydrophobic model drug, Nile red, were co-loaded into vesicles by self-assembly. To Nile red, the crosslinking and permeabilization of hydrophobic interlayer is synchronized during UV-induced reconstruction of the vesicle membrane, leading to immediate release of the loaded Nile red. To DOX·HCl, photo-triggered permeabilization also facilitates its release from the inner aqueous core. Typically, within 8 h, 40%, 70%, and 95% of DOX·HCl was escaped upon irradiation for 5, 10, and 20 min, respectively.
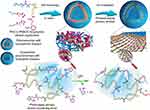 |
Figure 6 Design of BCP vesicles exhibiting concurrent phototriggered “traceless” crosslinking and vesicle membrane permeabilization. PEO-b-PNBOC amphiphilic BCPs self-assemble into polymersomes with the hydrophobic bilayer containing carbamate-caged primary amine moieties. UV irradiation triggers decaging reactions and the release of primary amine functionalities, prominent amidation reaction then occurs because of a suppressed amine pKa within the hydrophobic vesicle membrane, resulting in vesicle crosslinking instead of vesicle-to-unimer disassembly. 1) Enhanced amidation within the hydrophobic microenvironment. 2) Unreactive primary amines because of protonation. Reprinted from Wang X, Liu G, Hu J, Zhang G, Liu S. Concurrent block copolymer polymersome stabilization and bilayer permeabilization by stimuli-regulated “traceless” crosslinking. Angew Chem Int Ed Engl. 2014;53(12):3138–3142. Copyright 2014, with permission from John Wiley and Sons.68 |
Other stimulus-responsive polymeric nanovesicles
Besides the above mentioned, there are some other stimulus-responsive polymeric nanovesicles, eg, polypeptide-responsive, redox-responsive, voltage-responsive, ultrasound-responsive, CO2-responsive vesicles and so on.
Given its high specificity and efficiency, polypeptide-responsive technology has been viewed as a promising strategy for precise drug delivery. Rodriguez et al reported enzyme-sensitive polypeptide vesicles comprising of water-soluble methionine sulfoxide, MO, and diblock polypeptide, poly(L-methionine)65-b-poly(L-leucine0.5-stat-L-phenylalanine0.5)20.69 As the substrate of reductase, MO segments can be regenerated into hydrophobic M segments by enzyme, resulting in its morphology changing from sphere to crumpled sheet. It favors vesicle disruption and cargo release very much. Only <10% release completed over 200 h without enzymes, but with enzyme treatment these vesicles reached >60% release during the same period.
Similarly, an amphiphilic copolymer of poly(trimethylene carbonate) (PTMC) and matrix metalloproteinase (MMP-2)-sensitive peptide, βAla-Pro-Val-Gly-LeuIle-Gly-βAla-Cys (PVGLIG), namely PTMCn-b-PVGLIG, was synthesized to construct enzyme-responsive polymersomes.70 Here MMP-2 can specifically cleave PVGLIG between the glycine and leucine residues. Once treated by MMP-2 active enzyme, PVGLIG breakage in the vesicle membrane made the vesicular nanostructures fully degraded over a period of 48 h.
MLN8237 (alisertib), a known inhibitor of aurora kinases, has poor solubility and transport across the cell membrane. Inchanalkar et al developed dextran polysaccharide (DEX-PDP) nanovesicels with enzyme-response for MLN8237 delivery (loading content: 0.40%; loading efficiencies: 56%).71 The hydrophobic PDP units can be cleaved in the enzyme-rich intracellular lysosomal compartment. Naturally, the release of MLN8237 was stable with <25% over 25 h at pH 7.4 and 37 °C, and rose to >85% in the presence of 10 U of esterase enzyme. Similarly, Yang et al designed a drug-delivery system (DDS) with esterase-esponsive release behavior by self-assembling polyphenols and PEG block polymers.72 The polyphenol blocks can be hydrolyzed by esterase, so that the formed nanoparticles (PPNP) will degrade responsively in the colitis microenvironment. An anti-inflammatory corticosteroid, dexamethasone (DEX), was further encapsulated in PPNP (loading efficiency: 35 wt%; encapsulation efficiency: 22.7 wt%) for oral delivery. About 30% of DEX was finally released during the whole simulated transit process, but 62% of DEX did this within 4 h inside the colitis environment increased with 30 U/mL esterase. And PPNP=DEX degraded ~36% in the presence of esterase, versus only 12% in the absence of esterase.
A redox-responsive vesicle was developed to regulate drug release via glutathione (GSH), using a novel triblock copolymer, PEG and poly(ε-benzyloxycarbonyl-L-lysine) (PzLL) linked by disulfide bond (PzLL-SS-PEG-SS-PzLL) (see Figure 7).73 Here GSH can trigger disulfide bond cleavage to make PzLL-SS-PEG-SS-PzLL vesicles disassembled. Specifically, DOX·HCl release was greatly accelerated from only 30% to 90% within 48 h in the presence of GSH.
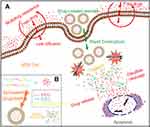 |
Figure 7 (A) Schematic illustration of redox-responsive polymeric vesicles for overcoming the multidrug resistance of cancer cells and (B) Schematic outline of the predicted self-assembly behavior of PzLL-SSPEG-SS-PzLL triblock copolymers and their drug-release behavior. Reprinted with permission from Ren T, Wu W, Jia M, Dong H, Li Y, Ou Z. Reduction-cleavable polymeric vesicles with efficient glutathione-mediated drug release behavior for reversing drug resistance. ACS Appl Mater Interfaces. 2013;5(21):10,721–10,730. Copyright 2013, American Chemical Society.73 |
In 2010, a voltage-responsive supramolecular vesicle was built by dripping equal amount of PEG-ferrocene (PEO-Fc) aqueous solution to PS-β-cyclodextrin (PS-β-CD) via self-assembly (see Figure 8).74 Hereinto, the encapsulated molecules showed a slow full release (450 min) at 1.0 V, and a slightly fast full release (120 min) at 2.0 V. Moreover, the release time could be precisely tuned through the external potential strength.
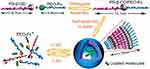 |
Figure 8 Structure of PS-β-CD and PEO-Fc and schematic of the voltage-responsive controlled assembly and disassembly of PS-β-CD/PEO-Fc supramolecular vesicles. Reprinted with permission from Yan Q, Yuan J, Cai Z, Xin Y, Kang Y, Yin Y. Voltage-Responsive Vesicles Based on Orthogonal Assembly of Two Homopolymers. J Am Chem Soc. 2010;132(27):9268–9270. Copyright 2010, American Chemical Society.74 |
A low frequency ultrasound-responsive vesicle was developed from PEG-b-polybutadiene (PEO-b-PBD) copolymer.75 Under the action of ultrasonic cavitation, the vesicle membrane can be torn off to turn into smaller vesicular or micellar structures, or perforate temporarily until termination of ultrasonic exposure.
Furthermore, gas-sensitive polymersomes have to be mentioned. Especially a specific amidine-containing block copolymer, PEO-block-poly(N-amidino dodecyl acrylamide) (PEO-b-PAD) was used to fabricate CO2-responsive vesicles with a biomimetic “breathing” feature (see Figure 9A).76–78 As a type of acidic gas-switchable molecule, amidine group can transform from an uncharged hydrophobic into a charged hydrophilic amidinium species by reaction with CO2. Importantly, this reaction is reversible upon exposure to argon. Here fluorescent rhodamine B (Rh-B) was chosen as a model agent loaded in the aqueous core of the vesicle.79 The Rh-B-loaded vesicles displayed a low-level free release of <35% over 10 h without stimulus. Once CO2 flux was performed, the release quantity had a sudden increment to 25% within 1 h and then remained fairly constant, indicating the outstanding responsiveness of these vesicles.
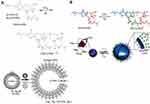 |
Figure 9 (A) Gas-switchable structural change of amidine-containing diblock copolymer PEO-b-PAD (top) and schematic representation of its self-assembly into vesicles and their reversible gas-responsive “breathing” in aqueous media (bottom). (B) Gas-switchable chemical structural change of the PEG-b-PAD block copolymer. The self-assembly of the copolymer into polymersomes and reversible gas-controlled breathing behavior in aqueous media. Reprinted from Yan Q, Zhou R, Fu C, Zhang H, Yin Y, Yuan J. CO2-responsive polymeric vesicles that breathe. Angew Chem Int Ed Engl. 2011:50(21):4923–4927. Copyright 2011, with permission from John Wiley and Sons.79 Reprinted from Yan Q, Wang J, Yin Y, Yuan J. Breathing polymersomes: CO2-tuning membrane permeability for size-selective release, separation, and reaction. Angew Chem Int Ed Engl. 2013;52(19):5070–5073. Copyright 2013, with permission from John Wiley and Sons.80 |
In 2013, CO2-responsive capability of PEO-b-PAD was utilized to tune the permeability of the self-assembled polymersome membranes (see Figure 9B).80 Using CO2 levels to control the size of nanopores in the membrane, these nanocontainers can attain the goal of releasing and separating globular nanoparticles of different sizes. To this end, pyrene-decorated (PEI-5; 5000 Da, ~4 nm), and rhodamine B end-capping hyperbranched poly (ethylene imine) (PEI) nanoparticles (PEI-25; 25,000 Da, ~10 nm), were loaded in these vesicles. By means of step-controlled CO2 levels, the overall the separation selectivity of PEI-5 (5000 Da, ~4 nm) and PEI-25 (25,000 Da, ~10 nm) were up to 96.8% and 91.5%, respectively.
In terms of chemoradiation, X-ray irradiation-triggered instant drug release was built via oxidation-responsive poly (propylene sulfide)-block-poly (ethylene glycol) (PPS-b-PEG) nanovesicles containing Au nanoparticles tethered with irradiation labile linoleic acid hydroperoxide (LAHP) molecules.81 DOX was co-encapsulated inside with the loading content of 14.70 wt%. Under X-ray irradiation, the LAHP molecules can generate the hydroxyl radicals (·OH) to trigger the internal oxidation of PPS converted from being hydrophobic to hydrophilic, following by vesicle degradation. During 30 min of irradiation, Au-LAHP-DOX presented a burst DOX release of 46.70% or 52.20% in PBS and H2O2 solutions, respectively So a remarkable efficacy (99.70%) of Au-LAHP-vDOX (+) was also testified by the quantitative percentage (%) of tumor volume inhibition at 20 days post treatment for the mouse group.
Dual stimulus-responsive polymeric nanovesicles
Only single responsiveness to ambient or external stimulus fail to meet the increasingly stringent criteria for precise release and targeting of drug carrier, especially for early-stage cancer therapy. So dual and multiple stimulus-responsive nanovesicles are constantly emerging.
In 2011, Naik et al applied poly(propylene oxide)-block-poly(L-lysine) (PPO-b-PK) to self-assemble temperature and pH-responsive polymersome.82 Given PPO with LCST of about 10 °C, PPO-b-PK will be in the double hydrophilic state below 10 °C. As a pH-sensitive polypeptide, PK can undergo a reversible helix-coil transition due to ionization of side amine groups. The loading and passive release of DOX·HCl was investigated using PPO44-b-PK62 vesicles (DOX·HCl loading efficiency: 35%) and PPO44-b-PK217 micelles (DOX·HCl loading efficiency: 8%). The DOX·HCl release profile appears to reach an equilibrium elution of ~32% after 300 min.
Chiang et al prepared PNIPAAm-grafted, and both PNIPAAm and mPEG-grafted, copolymers comprising acrylic acid (AAc) and 2-methacryloylethyl acrylate (MEA) units, to construct dual stimulus-responsive nanovesicles serving as an efficient intracellular drug delivery platform.83 At pH 3.0 and room temperature, the initial vesicle diameter was 170 nm. With ambient temperature increasing and pH value decreasing, the vesicle size had a significant reduction. Over a period of 3 h, the cumulative DOX release of 50% at pH 5.0 and 37 °C is much higher than that of 20% at pH 7.4 and 37 °C. A higher 3-h unloading of DOX was obtained from these contractive particles at pH 5.0 and 4 °C, owing to the hydrophilic recovery of PNIPAAm.
Similarly, a AnBm-b-ApCq block copolymer was synthesized, where A, B, C are N-propylacrylamide (nPA), 2-(diethylamino)ethyl methacrylate (DEAEMA), and N-ethylacrylamide (EA), respectively.84 Both PA and EA are thermo-sensitive, and the tertiary amine group of hydrophobic DEAEMA may be protonated into hydrophilic moiety at low pH value. So, at pH 7.0 and 37 °C, this copolymer formed polymeric nanovesicles with the diameter of ~148 nm via self-assembly. At 25 °C, both the coronas and core of the vesicle membrane were reversed with poly(nPA0.8-co-DEAEMA0.2) blocks to form the new inverted nanovesicles with a diameter of ~60 nm in an alkaline medium (pH 10; see Figure 10).
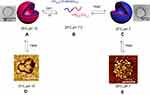 |
Figure 10 Self-assembling process of poly(nPA0.8-co-DEAEMA0.2)-block-poly(nPA0.8-co-EA0.2) into vesicles, and then aggregates upon heating. Reprinted with permission from Savoji MT, Strandman S, Zhu XX. Switchable vesicles formed by diblock random copolymers with tunable pH- and thermo-responsiveness. Langmuir. 2013;29(23):6823–6832. Copyright 2013, American Chemical Society.84 |
In 2012, Mane et al reported a pH and lipid-sensitive vesicle originated from an amphiphilic, norbornene-derived thiobarbiturate homopolymer (NTBH).85 Here both hydrophilic doxorubicin and hydrophobic Nile red can be simultaneously encapsulated in the reversible nature of the NTBH vesicles. In their sensitivity test, there was no significant release of the Nile red from the vesicles at pH 7.4, but the breakage of hydrogen bond between the barbiturate functionality may facilitate a fast release in the acidic environment.
Another photo- and pH-responsive vesicle was prepared using amphiphilic block copolymer (PEO-b-PAP) composed of hydrophilic PEO and hydrophobic polymethacrylate with chromatic azopyridine side groups (PAP).86 After UV irradiation of 70 s, this self-assembled system reached the photostationary state and terminate the release of the loaded Nile red. Along with pH value decreasing, the turbidity of the solution decreased rapidly, indicating that the vesicles began to dissociate. When the pH value dropped to 2.5, these vesicles were dissociated completely. Similar photo-crosslinked polymersome was also formed by covalent and non-covalent approaches, using PEG-b-poly[2-(diethylamino)ethyl methacrylate-stat-2-hydroxy-4-(methacryloyloxy)benzophenone] (BC2) (see Figure 11).87 Here nitroveratryloxycarbonyl-protected amine molecules (NVOC) as light-responsive moieties were embedded into the polymersomes. Obviously, the average membrane thickness increased from 18.6 ± 1.7 to 26.8 ± 3.8 nm when changing pH value from 9.0 to 5.0. At pH 5.0 within 140 h, ~53% of the encapsulated DOX was released from PS1C-DOX polymersomes without optical processing, and ~51% did so from the PS1D-DOX ones with optical processing. Although applied UV irradiation favored PS1D-DOX membrane being more compact by photo cleavage, the polymer chains with same charges under the acidic condition appeared repelled to generate the membrane pores, which is more than adequate for efficient drug release. Moreover, the closed membrane greatly decreased the undesired DOX release at pH 7.4: only ~20% from PS1C-DOX, and 26% from PS1D-DOX within 140 h.
 |
Figure 11 (A) Schematic overview of the DOX-encapsulated PS1C and PS1D formation. In vitro release of doxorubicin from PS1C-DOX polymersomes.(B) PS1D-DOX polymersomes (C) at 37 °C in different pH media. Reprinted with permission from Iyisan B, Kluge J, Formanek P, Voit B, Appelhans D. Multifunctional and Dual-Responsive Polymersomes as Robust Nanocontainers: Design, Formation by Sequential Post-Conjugations, and pH-Controlled Drug Release. Chemistry of Materials. 2016;28(5):1513–1525. Copyright 2016, American Chemical Society.87 |
Wang et al designed and prepared a novel pH and redox-responsive polymeric lipid vesicle with PEG-decorated corona (PPLV).88 As treated with buffered solution (pH 5.0) or GSH aqueous solution (10 mM) for 3 h, the vesicle size increased from 109.7 to ~380 nm, and the release of the encapsulated DOX was significantly accelerated. Within 12 h, 47.2% of DOX was released, likely due to pH-induced hydrolysis of hydrazone bonds at pH 5.0. Within same treatment period, 71.3% and 80.8% of DOX were rapidly released in a reductive GSH media (10 mM) at pH 7.4 and 5.0, respectively.
For breast cancer therapy, pH- and enzyme-sensitive polysaccharide nanovesicles were developed, in which dextran was suitably modified with a renewable 3-pentadecyl phenol unit.89 Here this modification was completed through imine and aliphatic ester chemical linkages that response to pH value and esterase, respectively. In vitro studies revealed that ~70–80% of the DOX·HCl cargo was retained in the vesicle at pH 7.4 and 37 °C. But at low pH (5.0~6.0) and in the presence of esterase, both imine and ester linkages were broken down instantaneously to thoroughly release all the loaded DOX·HCl.
In order to accomplish receptor-mediated endocytosis in cancer cells, Deshpande et al designed a biotin-tagged and multiresponsive polysaccharide vesicle with DOX·HCl loading.90 To the frame polymer (DEX-SS-PDP-Biotin), renewable hydrophobic units were connected to the hydrophilic dextran backbone via redox-degradable disulfide bonds (S−S) and enzyme-degradable aliphatic ester linkages disulfide (S−S) and aliphatic ester chemical linkages. In the tumor microenvironment, glutathione (GSH) can redox-trigger the cleavage of −S−S− bond to disrupt the vesicular assembly. Similarly, the esterase enzyme can degrade aliphatic ester linkages. The results showed that only 20% drug leaching was observed in the absence of GSH, and the DOX·HCl release ratio significantly increased to 60% in the presence of GSH. In addition, it was observed that the drug release was much higher for esterase enzyme exposure. About 90% DOX·HCl was released in 12 h. Particularly, through the combined effects of GSH and esterase-enzyme, the release ratio reached 100%. Similar polysaccharide nanovesicle was studied using amphiphilic dextran derivatives (DEX-IM-5).91 Clearly, acid can hydrolyze imine linkage to generate subsequent leakage of the loaded Rh-B content. The control release studies showed that ~90% of Rh-B burst from DEX-IM-5 vesicles in 48 h at low pH.
Liu et al prepared PNIPAM-b-PCL-b-poly(N,N-dimethylaminoethyl methacrylate) (PDMAEMA) to self-assemble into CO2 and temperature-responsive vesicles (see Figure 12).92 As treated with CO2 for 10 min (flow rate: ∼1 mL/s), these vesicles greatly expanded from 139.6 to 299.9 nm. It was affirmed that tertiary amine groups were protonated by CO2 bubbles to form charged ammonium bicarbonates. While heated from 40 to 80 °C, these vesicles obviously shrank into small spherical micelles.
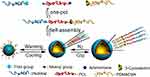 |
Figure 12 Illustration for synthesis and self-assembly of the supramolecular triblock copolymer PNIPAM-b-PCL-b-PDMAEMA, as well as their CO2−temperature dual stimulus-responsive process. Reprinted with permission from Liu B, Zhou H, Zhou S, et al. Synthesis and Self-Assembly of CO2–Temperature Dual Stimulus-responsive Triblock Copolymers. Macromolecules. 2014;47(9):2938–2946. Copyright 2014, American Chemical Society.92 |
The same dual-responsive vesicle was also reported by Zou et al93. In a determined CO2 flow, the size of poly(N-amidino)dodecyl acrylamide) (PADS)-b-PDMAEMA nanovesicles in water increased from 500 to 1,000 nm. As heated to 45 °C above LCST of PDMAEMA segments, the contained vesicles in the solution transformed into micelle aggregates by the hydrophilicity transition of PDMAEMA. Typically, the release of DOX cargo only reached 15.5% at 25 °C within 40 h, but increased to 23.0% after bubbling CO2 into the vesicle solution for 5 min. At 45 °C, the cumulative release rose rapidly to 34.7% within 5 h and 55.9% after 30 h, respectively. Furthermore at 45 °C, the DOX release with bubbling CO2 for 5 min was lower than the one without bubbling CO2.
Multiresponsive polymeric nanovesicles
In 2014, block copolymer comprised with thermo-sensitive PNIPAM, and pH and voltage-sensitive tetraaniline (TA) blocks (TA-b-PNIPAM), was employed to form multiresponsive polymersomes.94 Here, block length, block ratio, organic solvent, solvent ratio, pH, temperature, and voltage can affect the morphology and properties of the polymersomes. Typically, ambient pH value can weaken or strengthen the internal hydrogen bond, and voltage can control the oxidation state based on the excellent electroactive of aniline tetramer. The vesicle size increased while the pH value decreased from 7.0 to 3.0, or the ambient temperature rose from 26 to 30 °C. Particularly, these TA-b-PNIPAM vesicles had two oxidation potentials in its CV curve (0.27 and 0.60 V), corresponding to its two oxidized state transitions. As the neutral vesicle solution electrochemically oxidized at 0.60 V, the total release of the encapsulated DOX was ~39%, faster than that in the control group.
Differently, a disulfide bond-linked and perylen-3-yl, 2-nitrobenzyl-funtionalized copolymer containing poly(benzyl carbamate) PBC and poly(N,N-dimethylacrylamide) (PDMA) blocks, PDMA28-SS-PBC14-b-PDMA28, was developed to assemble into a self-immolative polymersome.95 After UV irradiation or entrapment into intracellular reductive milieu, the self-immolative depolymerization occurred, and then destroyed the resulting vesicles. Only ∼4% of hydrophilic DOX and ∼19% of hydrophobic camptothecin (CPT) encapsulated inside the polymersomes was released within 20 h in the absence of GSH, but ∼86% DOX and ∼82% CPT were quickly released within the same timescale in the presence of GSH (10 mM).
By controlling the structural transformation of frame copolymer, a novel polypeptide-b-PEO vesicle was formed, capable of responding to multiple physiological and clinic-related stimuli (see Figure 13).96 Hereinto, the polypetide (PLC) was composed of light-sensitive o-nitrobenzyl groups, oxidizable thioether linkers, photo-caged redox-sensitive thiol groups. After UV irradiation, the vesicles transformed into spherical micelles. Owing to the present of thiol groups, the decaged PLC56 within PLC56-b-PEO114 enabled the reassembled micelles becoming redox-sensitive, and then these micelles automatically aggregated from 180 ± 10 to 410 ± 58 nm. Likewise, under UV irradiation, the released DOX reached 91% within 12 h.
 |
Figure 13 Schematic description for the multiresponsive transformation of the polypeptidosome in an aqueous solution. Reprinted from Liu G, Zhou L, Guan Y, Su Y, Dong CM. Multi-responsive polypeptidosome: characterization, morphology transformation, and triggered drug delivery. Macromol Rapid Commun. 2014;35(19):1673–1678. Copyright 2014, with permission from John Wiley and Sons.96 |
Polymeric nanovesicles as gene carriers
Recently, siRNA has been developing to an effective tool for profiling protein function and a potential therapeutic strategy for diseases. They are typically about 23 bp in length, and can utilize a cellular RNA interference pathway to catalyze the degradation of targeted mRNA. The encapsulation of siRNA and antisense oligonucleotides into polymersomes has been studied for years.
For example, correctly proportioned PEG-PCL and PEG-PLA copolymers self-assembled into “OCL” and “OLA” polymersomes that can release encapsulants by the polyester degradation, respectively.97 Further 30% of fluorescent siRNA and at least 20% of fluorescent antisense oligonucleotides (AON) were encapsulated by OLA polymersomes and OCL polymersomes, respectively. According to dynamic light scattering measurements, these initial vesicles with the size of 100 nm were indeed sufficiently small for extended circulation as well as efficient cell entry, but after 5–6 h at 37 °C, their average hydrodynamic radius decreased to 60 nm, and appeared in a mixture of vesicles and micelles. After 18–48 h, their sizes decreased to ~40–50 nm again, indicating that they undergo a transformation to small, surfactant-like micelles to presumably release their oligos.
PRB peptide-functionalized poly(1,2-butadiene)-b-PEO polymersomes was also applied for siRNA delivery.98 Hereinto, both the average encapsulation efficiencies of two siRNA molecules siRNA were 50.2 ± 9.5%. Particularly, commercial siRNA transfection agent (RNAiMAX) achieved 50−60% knockdown of target Orai3, with no statistically significant difference in the knockdown levels between MCF10A cells and T47D.
As well-known, cationic poly(amino acid) vesicles have also received considerable attention for co-delivery of siRNA and chemotherapeutic drugs.99 As the ambient pH value increased from 5.5 to 7.4, partial deprotonation of the cationic vesicles induced the particle size decreased from 540 ± 10.0 to 201 ± 6.6 nm., indicating good pH-responsive ability. Once DOX·HCl was encapsulated into these vesicles, its release reached to about 40.9%, 50.0% and 100% at pH 5.5, 6.8, and 7.4 in 50 h, respectively.
Similarly, a novel poly(amino acid) derivative with hydrophobic poly(L-glutamate) (PLG) backbone and pH-responsive hydrophilic poly (2-aminoethyl methacrylate hydrochloride) (PAMA) side chains (PLG-g-PAMA), was utilized to construct another cationic vesicles of gene delivery.100 The gel retardation assay revealed that, PLG-g-PAMA could efficiently bind with DNA. Here the loading content and efficiency of DNA were 6.5% and 34.8%, respectively. In detail, about 60.0%, 75.3% and 92.2% of the initial loaded DNA were eventually released from the vesicle within 50 h at pH 5.5, 6.8 and 7.4, respectively. In addition, Sun et al synthesized an arginine-leucine block copolypeptide (R60L20) to self-assemble into novel cationic vesicle.101
For active oligonucleotide delivery, a pH-sensitive vesicle was built using triblock copolymer PEG-b-pImHeMA-b-pGMA that comprises two terminal hydrophilic blocks, PEG and polyglycerolmethacrylate (poly-GMA), and a central weakly basic block, polyimidazole-hexyl methacrylate (poly-ImHeMA).102 Here pH-sensitive imidazolic side chains were partially functionalized by folic acid as a targeting moiety. At pH 7.4, PEG-pImHeMA-pGMA copolymers self-assembled into polymersomes, but also undergo structural transformation and dissociation at pH 6.5–5.0. This kind of vesicle can complex with oligonucleotides via pH variations. The overall results showed that, the polymersomes were suitable for encapsulating significant amounts of oligonucleotides (MW: ~13.3 kDa; encapsulation efficiency: 5.4 mol%). These oligonucleotides were tightly associated to the carrier, which enabled their efficient delivery to cancer cells by folate-mediated active targeting.
Polymeric nanovesicles as multifunctional carriers
Multifunctional carriers can combine with targeted delivery of therapeutic and diagnostic agents into one nano-sized “cage” for synergistic disease theranostics. It may greatly enhance the therapy efficacy and the diagnosis/prognosis accuracy. In recent years, many polymeric nanovesicles have been multifunctionalized for multimodal medical applications, ie, drug delivery, antibacterial activities,103 magnetic resonance imaging (MRI),104–106 potential boron neutron capture therapy (BNCT), and other diagnostic imaging.107
In 2010, a multifunctional and tumor-targeting wormlike polymersome was first reported, simultaneously loading superparamagnetic iron oxide (SPIO) nanoparticles (NPs) as MRI contrast agent.108 Wormlike polymer vesicles were formed by triblock copolymers R (R=methoxy or folate (FA))-PEG-PLA-PEG-acrylate. DOX was loaded into the hydrophobic membrane of the vesicles (loading content: 9.0 wt%), and a cluster of hydrophilic SPIO NPs was encapsulated into the aqueous cores (loading content: 48.0 wt%; see Figure 14). Apparently, this wormlike vesical can serve as both ultrasensitive T2 contrast agent and drug nanocarrier. At the same time, another multifunctional polymer vesicle was developed by this group.104 The copolymer was similar to the above, R(R=folate (FA) or methoxy)-poly(ethylene glycol)-poly(glutamate hydrozone doxorubicin)-poly(ethylene glycol)-acrylate (R(R=FA or methoxy)-PEG-P(Glu-Hyd-DOX)-PEG-acrylate). Different from the above platform, DOX was conjugated onto the polyglutamate segment to form the hydrophobic membrane of the vesicles, using a pH-sensitive hydrazone bond. SPIO NPs was also simultaneously encapsulated for MRI detection. Similarly, chitosan and poly (γ-glutamicacid-co-γ-glutamyl oxysuccinimide)-g-PEG-FA were deposited in sequence into the vesicular self-assembly.109 The loading efficiency and content of SPIONs within this polymersomes were estimated to be 40.5% and 10.7 wt%, respectively. And the SPION/DOX-loaded vesicles possessed higher drug loading efficiency (73.3%) and content (9.3 wt.%) via stable electrostatic interaction between DOX and side groups.
 |
Figure 14 Illustration of the stable and tumor-targeting multifunctional wormlike polymer vesicles formed by triblock copolymers for targeted cancer chemotherapy and imaging. Reprinted from Biomaterials, 31(43), Yang X, Grailer JJ, Rowland IJ, et al, Multifunctional SPIO/DOX-loaded wormlike polymer vesicles for cancer therapy and MR imaging, 9065–9073, Copyright 2010, with permission from Elsevier.108 |
Another diblock copolymer of PEG and 2-(diisopropylamino) ethanol-grafted poly(L-aspartic acid) was self-assembled into pH-sensitive nanovesicles for hydrophilic SPIO NPs and DOX delivery.110 Their pH-sensitive behavior was considered to enhance the control release of DOX and MRI. In PEO-block-poly (tert-butyl acrylate-stat-acrylic acid) [PEO-b-P(AA-stat-tBA)] vesicle, hydrophobic Fe3O4 NPs were chosen to conjugate in situ within the pH-sensitive membrane.111 Particularly, the vesicle size can decrease with the pH decreasing. The overall results indicated that 2.4% and 4.8 wt% of SPIO NPs, and approximately 22.5% and 26.9% of drug, were encapsulated into PEO-b-P(AA-stat-tBA) 2 and 3 vesicles with different blocks ratio, respectively.
Differently, modified maghemite (γ-Fe2O3) nanoparticles were encapsulated within poly(trimethylene carbonate)-block-poly(L-glutamic acid) (PTMC-b-PGA) vesicles for DOX delivery and MRI.112 This formation method gave simple access to a very high loading efficiency of magnetic nanoparticles (MNPs; 70 wt%). When heated from 5 to 45 °C, the release of the co-encapsulated DOX rose from 5% to 85%.
Moreover, perfluorooctyl bromide (PFOB)-loaded nanovesicles with a size of ~500 nm was prepared by self-assembly of mphiphilic PEG-PDLLA for blood pool ultrasound imaging and other potential drug delivery.113 Recently, a 4-armed porphyrin-PLA (PPLA) porphysomes was employed as a versatile theranostic agent for efficient photodynamic therapy (PDT) and contrast-enhanced ultrasonic imaging.114 Their unique hollow structure enabled the porphysomes to act as ultrasound contrast agent. And PPLA porphysomes may provide a robust and highly stable platform for1 O2 encapsulation to kill cancer cells in vitro.
Summary and outlook
In this review, we introduced the newest advances in drug and gene delivery of pH-responsive, thermos-responsive, photoresponsive, and other stimuli-responsive nanovesicles, as well as dual and multiresponsive nanovesicles. As an effective carrier, these polymeric vesicles can simultaneously encapsulate hydrophilic, hydrophobic and amphiphilic agent in different parts. Their size, structure, and morphology can be tuned by change of external stimulus, to complete controlled release and targeted delivery of drug or gene cargo. This key advantage may be attributed to the ingenious design of amphiphilic block copolymer as frame materials, including its chemical structure, stimulus-responsiveness, and self-assembled configuration.
However, polymersome places some harsh requirements on block ratio, molecular weight, block hydrophilicity or hydrophobicity, chain flexibility, and so on, for frame polymer. It makes the design and synthesis of vesicle-constructed material very complicated, even tricky. Of course, it also makes their preparation difficult to control, with the result of low reproducibility. Only PEG-b-PDLLA, a biocompatible and easily degradable polymer, is anticipated to be developed into a promising material candidate for mass production of polymeric vesicles.
Meanwhile, novel stimulus-responsive polymeric nanovesicles continue to be developed for advanced functions and wide applications. We believe that vesicular polymer nano-integrators offer considerable promise for future therapy.
Acknowledgments
This work was financially supported by the Natural Science Foundation of China (No. 51773069), Guangdong Provincial Key Laboratory of Optical Information Materials and Technology (Grant No. 2017B030301007), MOE International Laboratory for Optical Information Technologies and the 111 Project.
Disclosure
The authors report no conflicts of interest in this work.
References
1. Discher DE, Eisenberg A. Polymer vesicles. Science. 2002;297:967–973. doi:10.1126/science.1074972
2. Discher BM, Won YY, Ege DS, et al. Polymersomes: tough vesicles made from diblock copolymes. Science. 1999;284(5417):1143–1146.
3. Yan, L., Higbee E, Tsourkas A, Cheng, Z. A simple method for the synthesis of porous polymeric vesicles and their application as MR contrast agents. J Mater Chem B. 2015;3(48):9277–9284. doi:10.1039/C5TB02067K
4. P P G, Frail PR, Susumu K, et al. Near-infrared-emissive polymersomes self-assembled soft matter for in vivo optical imaging. Pans. 2005;102:2922–2927. doi:10.1073/pnas.0409394102
5. Lee JS, Feijen J. Polymersomes for drug delivery: design, formation and characterization. J Control Release. 2012;161(2):473–483. doi:10.1016/j.jconrel.2011.10.005
6. Torchilin VP. Recent advances with liposomes as pharmaceutical carriers. Nat Rev Drug Discov. 2005;4(2):145–160. doi:10.1038/nrd1632
7. Rapoport N. Physical stimulus-responsive polymeric micelles for anti-cancer drug delivery. Prog Polym Sci. 2007;32(8–9):962–990. doi:10.1016/j.progpolymsci.2007.05.009
8. Liu M,GL, Chen L, Chen L, et al. Supramolecular core-shell nanosilica@liposome nanocapsules for drug delivery. Langmuir. 2012;28(29):10725–10732. doi:10.1021/la3021645
9. Antunes FE, Marques EF, Miguel MG, Lindman B. Polymer-vesicle association. Adv Colloid Interface Sci. 2009;147–148:18–35. doi:10.1016/j.cis.2008.10.001
10. Horne ADBARW. Negative staining of Phospholipids and their structural modification by surface-active agents as observed in the electron microscope. J Mol Biol. 1963;8:9.
11. Antonietti M, Vesicles FS. Liposomes: a self-assembly principle beyond lipids. Adv Mater. 2003;15(16):1323–1333. doi:10.1002/adma.200300010
12. Meng F,ZZ, Feijen J. Stimulus-responsive polymersomes for programmed drug delivery. Biomacromolecules. 2008;10:197–209. doi:10.1021/bm801127d
13. Du J, O’Reilly RK. Advances and challenges in smart and functional polymer vesicles. Soft Matter. 2009;5(19):3544–3561. doi:10.1039/b905635a
14. Li M-H, Keller P. Stimulus-responsive polymer vesicles. Soft Matter. 2009;5(5):927–937. doi:10.1039/b815725a
15. Kim KT, Meeuwissen SA, Nolte RJM, van Hest JCM. Smart nanocontainers and nanoreactors. Nanoscale. 2010;2(6):844–858. doi:10.1039/b9nr00409b
16. Deshpande PP, Biswas S, Torchilin VP. Current trends in the use of liposomes for tumor targeting. Nanomedicine (Lond). 2013;8(9):1509–1528. doi:10.2217/nnm.13.118
17. Anja Rank SH, Hauschild S, Förster S, Schubert R. Preparation of monodisperse block copolymer vesicles via a thermotropic cylinder-vesicle transition. Langmuir. 2009;25:1337–1344. doi:10.1021/la802709v
18. Eisenberg DEDAA. Polymer vesicles. Science. 2002;297:967–973. doi:10.1126/science.1074972
19. Elsabahy M, Wooley KL. Design of polymeric nanoparticles for biomedical delivery applications. Chem Soc Rev. 2012;41(7):2545–2561. doi:10.1039/c2cs15327k
20. Engin K, Leeper DB, Cater JR, Thistlethwaite AJ, Tupchong L, McFarlane JD. Extracellular pH distribution in human tumours. Int J Hyperthermia. 1995;11(2):211–216. doi:10.3109/02656739509022457
21. Chen W, Meng F, Cheng R, Zhong Z. pH-Sensitive degradable polymersomes for triggered release of anticancer drugs: a comparative study with micelles. J Control Release. 2010;142(1):40–46. doi:10.1016/j.jconrel.2009.09.023
22. Sun L, Du J. Revisiting the time for removing the unloaded drug by dialysis method based on a biocompatible and biodegradable polymer vesicle. Polymer. 2012;53(10):2068–2073. doi:10.1016/j.polymer.2012.03.016
23. Zhu L, Zhao L, Qu X, Yang Z. pH-sensitive polymeric vesicles from coassembly of amphiphilic cholate grafted poly(L-lysine) and acid-cleavable polymer-drug conjugate. Langmuir. 2012;28(33):11988–11996. doi:10.1021/la3015767
24. Qian C, Wang Y, Chen Y, et al. Suppression of pancreatic tumor growth by targeted arsenic delivery with anti-CD44v6 single chain antibody conjugated nanoparticles. Biomaterials. 2013;34(26):6175–6184. doi:10.1016/j.biomaterials.2013.04.056
25. Xiao H., He J,Li X, et al. Polymeric nanovesicles as simultaneous delivery platforms with doxorubicin conjugation and elacridar encapsulation for enhanced treatment of multidrug-resistant breast cancer. J Mater Chem B. 2018;6(45):7521–7529. doi:10.1039/C8TB01829D
26. Gaitzsch J., Appelhans D, Gräfe D, Schwille P, Voit B. Photo-crosslinked and pH sensitive polymersomes for triggering the loading and release of cargo. Chem Commun (Camb). 2011;47(12):3466–3468. doi:10.1039/c0cc05355d
27. Mane SR, Rao NV, Chaterjee K, et al. Amphiphilic homopolymer vesicles as unique nano-carriers for cancer therapy. Macromolecules. 2012;45(19):8037–8042. doi:10.1021/ma301644m
28. Kim MS, Lee DS. Biodegradable and pH-sensitive polymersome with tuning permeable membrane for drug delivery carrier. Chem Commun (Camb). 2010;46(25):4481–4483. doi:10.1039/c001500h
29. Kang SW, Li Y, Park JH, Lee DS. pH-triggered unimer/vesicle-transformable and biodegradable polymersomes based on PEG-b-PCL–grafted poly(β-amino ester) for anti-cancer drug delivery. Polymer. 2013;54(1):102–110. doi:10.1016/j.polymer.2012.10.055
30. Jeong IK, Gao GH, Li Y, Kang SW, Lee DS. A biodegradable polymersome with pH-tuning on-off membrane based on poly(beta-amino ester) for drug delivery. Macromol Biosci. 2013;13(7):946–953. doi:10.1002/mabi.201200468
31. Johnson RP, Uthaman S, John JV, et al. Poly(PEGA)-b-poly(L-lysine)-b-poly(L-histidine) hybrid vesicles for tumoral pH-triggered intracellular delivery of doxorubicin hydrochloride. ACS Appl Mater Interfaces. 2015;7(39):21770–21779. doi:10.1021/acsami.5b05338
32. Changez M, Kang N-G, Lee CH, Lee J-S. Reversible and pH-sensitive vesicles from amphiphilic homopolymer poly(2-(4-vinylphenyl)pyridine). Small. 2010;6(1):63–68. doi:10.1002/smll.200901670
33. Pegoraro C, Cecchin D, Gracia LS, et al. Enhanced drug delivery to melanoma cells using PMPC-PDPA polymersomes. Cancer Lett. 2013;334(2):328–337. doi:10.1016/j.canlet.2013.02.007
34. Fu J, Qiu L. Optimizing hydrophobic groups in amphiphiles to induce gold nanoparticle complex vesicles for stability regulation. Langmuir. 2017;33(43):12291–12299. doi:10.1021/acs.langmuir.7b02745
35. Shen J, Wang Z, Sun D, et al. pH-responsive nanovesicles with enhanced emission co-assembled by Ag(I) nanoclusters and polyethyleneimine as a superior sensor for Al(3). ACS Appl Mater Interfaces. 2018;10(4):3955–3963. doi:10.1021/acsami.7b16316
36. Liang L, Qiu L, Fu J. Design of pH-sensitive nanovesicles via cholesterol analogue incorporation for improving in vivo delivery of chemotherapeutics. ACS Appl Mater Interfaces. 2018;10(6):5213–5226. doi:10.1021/acsami.7b16891
37. Du J, Fan L, Liu Q. pH-Sensitive block copolymer vesicles with variable trigger points for drug delivery. Macromolecules. 2012;45(20):8275–8283. doi:10.1021/ma3015728
38. Pluen A, Boucher Y, Ramanujan S, et al. Role of tumor–host interactions in interstitial diffusion of macromolecules: cranial vs. subcutaneous tumors. Pans. 2001;98:
39. Wang Y, Chou T, Sukhishvili SA. Spontaneous, one-pot assembly of pH-responsive hydrogen-bonded polymer capsules. ACS Macro Lett. 2015;5(1):35–39. doi:10.1021/acsmacrolett.5b00716
40. Genc R, Murphy D, Fragoso A, Ortiz M, O’Sullivan CK. Signal-enhancing thermosensitive liposomes for highly sensitive immunosensor development. Anal Chem. 2011;83(2):563–570. doi:10.1021/ac1023765
41. Orsinger GV, Williams JD, Romanowski M. Intracellular light-induced release of signaling molecules from gold-coated liposomes. Proc SPIE. 2014;8955:
42. Li J. Block Copolymers Consisting of Biobased Polyesters and Their Self-Assembly Nanostructures for Biomaterials Applications. American Chemical Society; Abstracts of Papers of the American Chemical Society. Vol 244. NW, Washington DC, USA: Amer Chemical Soc;2012.
43. Zhu J-L, Liu KL, Li J. Block copolymers consisting of biobased polyesters and their self-assembly nanostructures. Polym Prepr (Am Chem Soc , Div Polym Chem). 2012;53(2):330–331.
44. Hayashi H, Kono K, Takagishi T. Temperature-controlled release property of phospholipid vesicles bearing a thermo-sensitive polymer. Biochim Biophys Acta. 1996;1280(1):127–134.
45. He, J., Tong X, Tremblay L, Zhao Y. Corona-cross-linked polymer vesicles displaying a large and reversible temperature-responsive volume transition. Macromolecules. 2009;42(19):7267–7270. doi:10.1021/ma901817k
46. Rank A, Hauschild S, FöRster S, Förster S, Schubert R. Preparation of monodisperse block copolymer vesicles via a thermotropic cylinder-vesicle transition. Langmuir. 2009;25:1337–1344. doi:10.1021/la802709v
47. Bhargava P, Tu Y, Zheng JX, Xiong H, Quirk RP, Cheng SZD. Temperature-induced reversible morphological changes of polystyrene-block-poly(ethylene oxide) micelles in solution. J Am Chem Soc. 2007;129(5):1113–1121. doi:10.1021/ja0653019
48. Zhou Y, Yan D, Dong W, Tian Y. Temperature-responsive phase transition of polymer vesicles: real-time morphology observation and molecular mechanism. J Phyl Chem B. 2007;111(6):1262–1270. doi:10.1021/jp0673563
49. Larue I, Adam M, Pitsikalis M, Hadjichristidis N, Rubinstein M, Sheiko SS. Reversible morphological transitions of polystyrene-b-polyisoprene micelles. Macromolecules. 2014;39(1):309–314. doi:10.1021/ma051548z
50. Hocine S, Di C, Rager MN, et al. Polymersomes with PEG corona: structural changes and controlled release induced by temperature variation. Langmuir. 2013;29(5):1356–1369. doi:10.1021/la304199z
51. Cao X, Chen Y, Chai W, et al. Thermoresponsive self-assembled nanovesicles based on amphiphilic triblock copolymers and their potential applications as smart drug release carriers. J Appl Polym Sci. 2015;132(4):41361–41372. doi:10.1002/app.41361
52. Achiha K, Ojima R, Kasuya Y, Fujimoto K, Kawaguchi H. Interactions between temperature-sensitive hydrogel microspheres and granulocytes. Polym Adv Technol. 1995;6(7):534–540. doi:10.1002/pat.1995.220060715
53. Kawaguchi H. Functions of monodisperse, thermosensitive hydrogel microspheres. Front Biomed Biotechnol. 1996;3:157–168. (Biomedical Functions and Biotechnology of Natural and Artificial Polymers).
54. Akimoto J, Nakayama M, Okano T. Temperature-responsive polymeric micelles for optimizing drug targeting to solid tumors. J Controll Release. 2014;193:2–8. doi:10.1016/j.jconrel.2014.06.062
55. Larson N, Ghandehari H. Polymeric conjugates for drug delivery. Chem Mater. 2012;24(5):840–853. doi:10.1021/cm2031569
56. Adair JH, Parette MP, Altinoğlu EI, Kester M. Nanoparticulate alternatives for drug delivery. ACS Nano. 2010;4(9):4967–4970. doi:10.1021/nn102324e
57. Lavasanifar A, Samuel J, Kwon GS. Poly (ethylene oxide)-block-poly (L-amino acid) micelles for drug delivery. Adv Drug Deliv Rev. 2002;54(2):169–190.
58. Xu H, Meng F, Zhong Z. Reversibly crosslinked temperature-responsive nano-sized polymersomes: synthesis and triggered drug release. J Mater Chem. 2009;19(24):4183–4190. doi:10.1039/b901141b
59. Bixner O, Kurzhals S, Virk M, Reimhult E. Triggered release from thermoresponsive polymersomes with superparamagnetic membranes. Materials. 2016;9(1):
60. Li A, Pazzi J, Xu M, Subramaniam AB. Cellulose abetted assembly and temporally decoupled loading of cargo into vesicles synthesized from functionally diverse lamellar phase forming amphiphiles. Biomacromolecules. 2018;19(3):849–859. doi:10.1021/acs.biomac.7b01645
61. Kozlovskaya V, Liu F, Xue B, et al. Polyphenolic polymersomes of temperature-sensitive poly(N-vinylcaprolactam)-block-poly(N-vinylpyrrolidone) for anticancer therapy. Biomacromolecules. 2017;18(8):2552–2563. doi:10.1021/acs.biomac.7b00687
62. Yan J-J, Wang X-Y, Wang M-Z, et al. Self-assembling nonconjugated poly(amide-imide) into thermoresponsive nanovesicles with unexpected red fluorescence for bioimaging. Biomacromolecules. 2019;20(3):1455–1463. doi:10.1021/acs.biomac.9b00051
63. Balzani V, Bergamini G, Ceroni P. Light: a very peculiar reactant and product. Angew Chem Int Ed Engl. 2015;54(39):11320–11337. doi:10.1002/anie.201502325
64. Cabane E, Malinova V, Menon S, Menon S, Palivan CG, Meier W. Photoresponsive polymersomes as smart, triggerable nanocarriers. Soft Matter. 2011;7(19):9167–9176. doi:10.1039/c1sm05880k
65. Photosensitizer, P.C.V.f.P.I.D.o.A. Polyion complex vesicles for photoinduced intracellular delivery of amphiphilic photosensitizer. J Am Chem Soc. 2014;136(1):157–163. doi:10.1021/ja406992w
66. Blasco E, Schmidt Bernhard VKJ, Barner-Kowollik C, et al. A novel photoresponsive azobenzene-containing miktoarm star polymer: self-assembly and photoresponse properties. Macromolecules. 2014;47(11):3693–3700. doi:10.1021/ma500254p
67. Wang Y, Han P, Xu H, et al. Photocontrolled self-assembly and disassembly of block ionomer complex vesicles: a facile approach toward supramolecular polymer nanocontainers. Langmuir. 2010;26(2):709–715. doi:10.1021/la9023844
68. Wang X, Liu G, Hu J, Zhang G, Liu S. Concurrent block copolymer polymersome stabilization and bilayer permeabilization by stimuli-regulated “traceless” crosslinking. Angew Chem Int Ed Engl. 2014;53(12):3138–3142. doi:10.1002/anie.201310589
69. Rodriguez AR, Kramer JR, Deming TJ. Enzyme-triggered cargo release from methionine sulfoxide containing copolypeptide vesicles. Biomacromolecules. 2013;14(10):3610–3614. doi:10.1021/bm400971p
70. Bacinello D, Garanger E, Taton D, Taton D, Tam KC, Lecommandoux S. Enzyme-degradable self-assembled nanostructures from polymer-peptide hybrids. Biomacromolecules. 2014;15(5):1882–1888. doi:10.1021/bm500296n
71. Inchanalkar S, Deshpande NU, Kasherwal V, Jayakannan M, Balasubramanian N. Polymer nanovesicle-mediated delivery of MLN8237 preferentially inhibits Aurora kinase a to target RalA and anchorage-independent growth in breast cancer cells. Mol Pharmaceutics. 2018;15(8):3046–3059. doi:10.1021/acs.molpharmaceut.8b00163
72. Wang X, Yan J-J, Wang L, et al. Rational design of polyphenol-poloxamer nanovesicles for targeting inflammatory bowel disease therapy. Chem Mater. 2018;30(12):4073–4080. doi:10.1021/acs.chemmater.8b01173
73. Ren T,WW, Jia M, Jia M, Dong H, Li Y, Ou Z. Reduction-cleavable polymeric vesicles with efficient glutathione-mediated drug release behavior for reversing drug resistance. ACS Appl Mater Interfaces. 2013;5(21):10721–10730. doi:10.1021/am402860v
74. Yan Q YJ, Cai Z, Cai Z, Xin Y, Kang Y, Yin Y. Voltage-responsive vesicles based on orthogonal assembly of two homopolymers. J Am Chem Soc. 2010;132:9268–9270. doi:10.1021/ja1027502
75. Pangu GD, Davis KP, Bates FS, Hammer DA. Ultrasonically induced release from nanosized polymer vesicles. Macromol Biosci. 2010;10(5):546–554. doi:10.1002/mabi.201000081
76. Jessop PG, Phan L, Carrier A, Robinson S, Dürr CJ, Harjani JR. A solvent having switchable hydrophilicity. Green Chem. 2010;12(5):809–814. doi:10.1039/b926885e
77. Ding Y, Chen S, Xu H, et al. Reversible dispersion of single-walled carbon nanotubes based on a CO2-responsive dispersant. Langmuir. 2010;26(22):16667–16671. doi:10.1021/la103519t
78. Liu Y, Jessop PG, Cunningham M, Eckert CA, Liotta CL. Switchable surfactants. Science. 2006;313(5789):958–960. doi:10.1126/science.1128142
79. Yan Q, Zhou R, Fu C, Zhang H, Yin Y, Yuan J. CO2-responsive polymeric vesicles that breathe. Angew Chem Int Ed Engl. 2011;50(21):4923–4927. doi:10.1002/anie.201100708
80. Yan Q, Wang J, Yin Y, Yuan J. Breathing polymersomes: CO2-tuning membrane permeability for size-selective release, separation, and reaction. Angew Chem Int Ed Engl. 2013;52(19):5070–5073. doi:10.1002/anie.201300397
81. Zhou, Z., Chan A, Wang Z, et al. Synchronous chemoradiation nanovesicles by X-Ray Triggered Cascade of Drug Release. Angew Chem Int Ed. 2018;57(28):8463–8467. doi:10.1002/anie.201802351
82. Naik SS, Ray JG, Savin DA. Temperature- and pH-responsive self-assembly of poly(propylene oxide)-b-poly(lysine) block copolymers in aqueous solution. Langmuir. 2011;27(11):7231–7740. doi:10.1021/la200882f
83. Chiang WH,HVT, Huang WC, Huang W-C, Huang Y-F, Chern C-S, Chiu H-C. Dual stimulus-responsive polymeric hollow nanogels designed as carriers for intracellular triggered drug release. Langmuir. 2012;28(42):15056–15064. doi:10.1021/la302903v
84. Savoji MT, Strandman S, Zhu XX. Switchable vesicles formed by diblock random copolymers with tunable pH- and thermo-responsiveness. Langmuir. 2013;29(23):6823–6832. doi:10.1021/la4009625
85. Mane SR, Rao N, Shunmugam R. Reversible pH- and lipid-sensitive vesicles from amphiphilic norbornene-derived thiobarbiturate homopolymers. ACS Macro Lett. 2012;1(4):482–488. doi:10.1021/mz2002092
86. Wei J,YZ, Lin L, Lin L, Gu J, Feng Z, Yu Y. Photo/pH dual-responsive behavior of azopyridine-containing copolymer vesicles. React Funct Polym. 2013;73(8):1009–1014. doi:10.1016/j.reactfunctpolym.2013.05.009
87. Iyisan B,KJ, Formanek P, Formanek P, Voit B, Appelhans D. Multifunctional and dual-responsive polymersomes as robust nanocontainers: design, formation by sequential post-conjugations, and pH-controlled drug release. Chem Mater. 2016;28(5):1513–1525. doi:10.1021/acs.chemmater.5b05016
88. Wang S,ZS, Liu J, Liu J, et al. pH- and reduction-responsive polymeric lipid vesicles for enhanced tumor cellular internalization and triggered drug release. ACS Appl Mater Interfaces. 2014;6(13):10706–10713. doi:10.1021/am502579e
89. Pramod PS, Shah R, Jayakannan M. Dual stimuli polysaccharide nanovesicles for conjugated and physically loaded doxorubicin delivery in breast cancer cells. Nanoscale. 2015;7(15):6636–6652. doi:10.1039/c5nr00799b
90. Deshpande NU, Jayakannan M. Biotin-tagged polysaccharide vesicular nanocarriers for receptor-mediated anticancer drug delivery in cancer cells. Biomacromolecules. 2018;19:3575–3585. doi:10.1021/acs.biomac.8b00833
91. Pramod PS, Deshpande NU, Jayakannan M. Real-time drug release analysis of enzyme and pH responsive polysaccharide nanovesicles. J Phys Chem B. 2015;119(33):10511–10523. doi:10.1021/acs.jpcb.5b05795
92. Liu B, Zhou H, Zhou S, et al. Synthesis and self-assembly of CO2–temperature Dual Stimulus-responsive Triblock Copolymers. Macromolecules. 2014;47(9):2938–2946. doi:10.1021/ma5001404
93. Zou H, Yuan W. CO2- and thermo-responsive vesicles: from expansion–contraction transformation to vesicles-micelles transition. Polym Chem. 2015;6(13):2457–2465. doi:10.1039/C5PY00024F
94. Wu Y, Liu S, Tao Y, et al. New strategy for controlled release of drugs. Potential pinpoint targeting with multiresponsive tetraaniline diblock polymer vesicles: site-directed burst release with voltage. ACS Appl Mater Interfaces. 2014;6(3):1470–1480. doi:10.1021/am404696u
95. Liu G, Wang X, Hu J, Zhang G, Liu S. Self-immolative polymersomes for high-efficiency triggered release and programmed enzymatic reactions. J Am Chem Soc. 2014;136(20):7492–7497. doi:10.1021/ja5030832
96. Liu G, Zhou L, Guan Y, Su Y, Dong C-M. Multi-responsive polypeptidosome: characterization, morphology transformation, and triggered drug delivery. Macromol Rapid Commun. 2014;35(19):1673–1678. doi:10.1002/marc.201400343
97. Kim Y, Tewari M, Pajerowski JD, et al. Polymersome delivery of siRNA and antisense oligonucleotides. J Control Release. 2009;134(2):132–140. doi:10.1016/j.jconrel.2008.10.020
98. Pangburn TO, Georgiou K, Bates FS, Kokkoli E. Targeted polymersome delivery of siRNA induces cell death of breast cancer cells dependent upon Orai3 protein expression. Langmuir. 2012;28(35):12816–12830. doi:10.1021/la300874z
99. Ding J, Xiao C, He C, et al. Facile preparation of a cationic poly(amino acid) vesicle for potential drug and gene co-delivery. Nanotechnology. 2011;22(49):494012. doi:10.1088/0957-4484/22/49/494012
100. Ding J, Xiao C, Zhuang X, Zhuang X, He C, Chen X. Direct formation of cationic polypeptide vesicle as potential carrier for drug and gene. Mater Lett. 2012;73:17–20. doi:10.1016/j.matlet.2011.12.092
101. Sun VZ, U-J C, Rodriguez AR, et al. Transfection of mammalian cells using block copolypeptide vesicles. Macromol Biosci. 2013;13(5):539–550. doi:10.1002/mabi.201200383
102. Gallon E,MT, Matini T, Sasso L, et al. Triblock copolymer nanovesicles for pH-responsive targeted delivery and controlled release of siRNA to cancer cells. Biomacromolecules. 2015;16(7):1924–1937. doi:10.1021/acs.biomac.5b00286
103. Wang M, Zhou C, Chen J, Xiao Y, Du J. Multifunctional biocompatible and biodegradable folic acid conjugated poly(epsilon-caprolactone)-polypeptide copolymer vesicles with excellent antibacterial activities. Bioconjug Chem. 2015;26(4):725–734. doi:10.1021/acs.bioconjchem.5b00061
104. Yang X, Grailer JJ, Rowland IJ, et al. Multifunctional stable and pH responsive polymer vesicles formed by heterofunctional triblock copolymer for targeted anticancer drug delivery and ultrasensitive MR IMAGING. ACS Nano. 2010;4:6805–6817. doi:10.1021/nn101670k
105. Liu Q, Zhu H, Qin J, Dong H, Du J. Theranostic vesicles based on bovine serum albumin and poly(ethylene glycol)-block-poly(L-lactic-co-glycolic acid) for magnetic resonance imaging and anticancer drug delivery. Biomacromolecules. 2014;15(5):1586–1592. doi:10.1021/bm500438x
106. Feng ST, Li H, Luo Y, et al. Molecular targeted magnetic resonance imaging of human colorectal carcinoma (LoVo) cells using novel superparamagnetic iron oxide- loaded nanovesicles: in vitro and in vivo studies. Curr Cancer Drug Targets. 2016;16(6):551–560.
107. Chen G, Yang J, Lu G, et al. One stone kills three birds: novel boron-containing vesicles for potential BNCT, controlled drug release, and diagnostic imaging. Mol Pharm. 2014;11(10):3291–3299. doi:10.1021/mp400641u
108. Yang X, Grailer JJ, Rowland IJ, et al. Multifunctional SPIO/DOX-loaded wormlike polymer vesicles for cancer therapy and MR imaging. Biomaterials. 2010;31(34):9065–9073. doi:10.1016/j.biomaterials.2010.08.039
109. Chiang WH, W-C H, Chang CW, et al. Functionalized polymersomes with outlayered polyelectrolyte gels for potential tumor-targeted delivery of multimodal therapies and MR imaging. J Control Release. 2013;168(3):280–288. doi:10.1016/j.jconrel.2013.03.029
110. Sun Q, Du C, Yu X, et al. A pH-sensitive polymeric nanovesicle based on biodegradable poly(ethylene glycol)-b-poly(2-(diisopropylamino)ethyl aspartate) as a MRI-visible drug delivery system. J Mater Chem. 2011;21(39):15316–15326. doi:10.1039/c1jm12404h
111. Ren T, Liu Q, Lu H, et al. Multifunctional polymer vesicles for ultrasensitive magnetic resonance imaging and drug delivery. J Mater Chem. 2012;22(24):12329–12338. doi:10.1039/c2jm31891a
112. Sanson C, Diou O, Thevenot J, et al. Doxorubicin loaded magnetic polymersomes. ACS Nano. 2011;5:1122–1140. doi:10.1021/nn102762f
113. Li H, Wang P, Wang X, et al. Perfluorooctyl bromide traces self-assembled with polymeric nanovesicles for blood pool ultrasound imaging. Biomater Sci. 2016;4(6):979–988. doi:10.1039/c6bm00080k
114. Hsu CY, Nieh MP, Lai PS. Facile self-assembly of porphyrin-embedded polymeric vesicles for theranostic applications. Chem Commun (Camb). 2012;48(75):9343–9345. doi:10.1039/c2cc33851c
 © 2019 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2019 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
