Back to Journals » Neuropsychiatric Disease and Treatment » Volume 15
Stigmasterol Exerts Neuro-Protective Effect Against Ischemic/Reperfusion Injury Through Reduction Of Oxidative Stress And Inactivation Of Autophagy
Authors Sun J, Li X, Liu J, Pan X, Zhao Q
Received 20 June 2019
Accepted for publication 9 September 2019
Published 18 October 2019 Volume 2019:15 Pages 2991—3001
DOI https://doi.org/10.2147/NDT.S220224
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 2
Editor who approved publication: Dr Yuping Ning
Jiadong Sun, Xuemei Li, Junling Liu, Xin Pan, Qianqian Zhao
Department of Neurology, Affiliated Hospital of Weifang Medical University, Weifang City, Shandong Province 262100, People’s Republic of China
Correspondence: Jiadong Sun
Department of Neurology, Affiliated Hospital of Weifang Medical University, No. 2428 Yuhe Road, Weifang City, Shandong Province 262100, People’s Republic of China
Tel +86 536 3081268
Email [email protected]
Purpose: Stroke remains the primary cause of pain, suffering, and death in patients. One of the major thrusts in stroke therapy is to find an effective prevention strategy. Objectives of this study are to testify the neuro-protection effect of stigmasterol in ischemic/reperfusion injury model.
Methods: The dosage-dependent effects (20, 40, and 80 mg/kg) of stigmasterol on physiological behaviors and oxidative stress biomarkers were investigated. Expression and phosphorylation of beclin1, microtubule-associated protein 1 light chain 3 (LC3), adenosine monophosphate-activated protein kinase (AMPK), mTOR, and N-terminal kinase (JNK) were detected.
Results: The results showed that stigmasterol was able to effectively reduce neurological deficits and infarct damage induced by the ischemic/reperfusion injury, improve histopathology changes, and restore the levels of the endogenous antioxidant defense system in a dose–response mode. Stigmasterol effectively depressed the expression level of beclin1, and the conversion of LC3 I to LC3 II, while promoted the phosphorylation of mTOR, and remarkably inhibited the phosphorylation of AMPK and JNK, as well as the expression of JNK induced by 24 hrs of reperfusion.
Conclusion: These findings reveal that stigmasterol has neuro-protective effect against the ischemic/reperfusion injury, possibly associated with reduction of oxidative stress and inactivation of autophagy via AMPK/mTOR and JNK pathways.
Keywords: stigmasterol, ischemia/reperfusion injury, oxidative stress, autophagy, AMPK pathway, mTOR pathway, JNK pathway
Introduction
The global burden of disease reports that stroke is becoming a crisis of unimaginable proportion due to ongoing demographic changes.1,2 Because of narrow therapeutic window, risk of hemorrhage and late hospitalization, stroke is characterized with high morbidity, disability, and mortality.3 Ischemic stroke is one type of stroke with higher incidence rate comparable to hemorrhagic stroke and transient ischemic attack.4 Moreover, it always results in neurological function loss and neuronal death, which are considered to be closely related to the excessive production of ROS and reactive nitrogen species.5 Given the inadequacies of existing strategies for stroke, finding an effective neuro-protective agent offers new hope for better treatment.6
Elimination of oxidative stress is an alternative to increase tolerance of brain tissue to ischemia stroke as the ischemic/reperfusion injury induces an overproduction of ROS. Scientists and clinicians are concentrating their efforts on finding free radical scavengers. Edaravone and gabapentin have been marketed and proved to possess the neuro-protective effects.7 Recently, various researchers have begun to focus on the development of natural-derived radical scavengers as neuro-protective agents due to their properties of abundance and low drug resistance.8,9 For example, evidences from Zhu et al indicated that Paeoniae Radix Rubra, the dried root of Paeonia lactiflora Pall. and Paeonia veitchii Lynch, could significantly upregulate the levels of SOD1, SOD2, and catalase, and decrease neuronal injuries induced by the ischemic/reperfusion injury.10
Stigmasterol (C29H48O), a naturally occurring steroid derivative, is found in many plants, such as Cabbage, Gypsophila oldhamiana, Arabidopsis, Aralia cordata, Eucalyptus globules, Physcomitrella patens, etc.11,12 Chemical structure of stigmasterol is shown in Figure 1A. It has been widely used in traditional Chinese medicine and food industry due to its various important bioactivities.13,14 For example, literature have well demonstrated that stigmasterol can act as the precursors of corticoids-1, progesterone, androgens, estrogens and vitamin D3, and easily pass blood–brain barrier.15,16 Kangsamaksin et al proposed stigmasterol as a candidate in cancer treatment. They found that stigmasterol could prevent the tumor growth by disrupting tumor angiogenesis, which mainly functioned by selectively suppressing the viability, migration, and morphogenesis of human umbilical vein endothelial cells via downregulation of tumor necrosis factor-α and VEGFR-2.17 Yenn et al proved that stigmasterol could serve as an antibiotic adjuvant to improve the effect of ampicillin against infections caused by β-lactamase producing pathogens.18 Antwi et al found that 50 mg/kg and 100 mg/kg of stigmasterol would be helpful to inhibit lipopolysaccharide-induced innate immune responses and improve survival up to 40% in murine models.19
Importantly, the double bond structure of stigmasterol makes it easy to be oxidized and this supports stigmasterol as the anti-peroxidative agent to effectively reduce oxidative stress.20 In this regard, here, we focused on testifying the feasibility of stigmasterol against ischemic stroke and understanding the possible mechanism. The ischemic/reperfusion injury model was constructed in Wistar rats, following with a dosage-dependent treatment using stigmasterol. The changes of physiological behaviors, oxidative stress biomarkers levels, and autophagy behaviors suggested the potential of stigmasterol at neuro-protection against ischemic stroke.
Methods And Materials
Animals Study Ethics
Male Wistar rats (250±300 g) were purchased from Animal Center of Shandong University and were housed for 1 week in a facility accredited by the committee for Assessment and Accreditation of Laboratory Animal Care. All animal procedures were approved by the Animal Ethics Committee of Affiliated Hospital of Weifang Medical University in accordance with the guidelines for the use of laboratory animals in People’s Republic of China.
Rat Model
The focal cerebral ischemic/reperfusion injury procedure was performed for inducing ischemia stroke in rats.4 In brief, 10% (w/v) chloral hydrate was administered (350 mg/kg, intraperitoneally (i.p.)) to anesthetize rats and a thermostatic heater was used to maintain the body temperature at 37°C during the whole experiment. Midline neck was incised in order to isolate the left common carotid artery (CCA) and the external carotid artery, which were further exposed and clipped with artery clamp. From the CCA, a nylon monofilament (Diameter: 0.25–0.28 mm, Beijing Sunbio Biotech Ltd. Co., Beijing, People’s Republic of China) was inserted into the internal carotid artery until a mild resistance. Laser-Doppler flowmetry (LDF, PeriFlux 5000 Perimed Co., People’s Republic of China) was performed to confirm the successful occlusion, characterizing with a <20% decline of the baseline in the regional cerebral blood flow (CBF). After 2 hrs of middle cerebral artery occlusion (MCAO), the monofilament was withdrawn and the reperfusion was accomplished. The sham group carried out all surgical procedures except the insertion process of nylon monofilament.
Stigmasterol Intervention
A total of 252 animals were randomly divided into six groups (n=42): 1) sham group; 2) I/R group; 3) stigmasterol group at a dose of 20 mg/kg; 4) stigmasterol group at a dose of 40 mg/kg; 5) stigmasterol group at a dose of 80 mg/kg; and 6) 3-MA group. Stigmasterol (purity ≥99%, Sigma) or 3-MA (purity ≥99%, Sigma) was dissolved in a mixture solution containing 0.1% dimethyl sulfoxide and 1% hydroxylethyl cellulose, respectively. In the pilot experiments (data not shown), we tested the effect of stigmasterol at different doses (5–160 mg/kg) and the whole experimental procedure is shown in Figure 1B. Stigmasterol treatment at three doses was i.p. administered immediately after 2 hrs ischemia and 300 μg/kg 3-MA was i.p. administered at 30 mins before ischemia.21 Meanwhile, the mixture solution without any drug was i.p. administered in sham and ischemic/reperfusion groups. The results revealed that stigmasterol shows a dose-dependent effect on I/R-induced brain injury in rats under this administration condition. Therefore, the dosage of stigmasterol (20, 40, and 80 mg/kg) was chosen in the present study and the same administration condition was applied.
Changes Of Neurobehavioral Parameters
Neurological Deficit Grade
An observer blinded to experimental groups was invited to grade neurological deficits after 24 hrs reperfusion (n=6):22 Grade 0 means that there was no neurological deficits and rats behave normally; Grade 1 means that rat completely failed to stretch their left front legs; Grade 2 means that rats turned around into a circle; Grade 3 means rats failed down to the left side; Grade 4 means rats could not move by themselves and lose their consciousness.
Quantification Of Brain Infarct Volume
Rats in 80 mg/kg stigmasterol treatment group and ischemic/reperfusion group were sacrificed at 2, 12, 24, and 48 hrs after reperfusion and the whole brains were isolated (n=6). Rats in other groups were sacrificed after 24 hrs post-surgery (n=6). Slices of brain tissues with 2 mm thickness were made and then stained with 2% 2,3,5-triphenyltetrazolium chloride (TTC, Sigma Cruz, California, USA) in PBS for 15 mins at 37°C. Following that, an overnight-fixation with 4% paraformaldehyde was performed. Infarct zone was analyzed using ImageJ® software on the basis of coronal slices photographs and the infarct volume was calculated as in the following:
Infarct volume (%)=(contralateral hemisphere area–healthy area of ipsilateral hemisphere)×thickness of slice
Brain Water Content Determination
Brain water contents were determined after 24 hrs reperfusion (n=6). The wet weight of infarct brain hemispheres was quantified after removing the surface water. After that, it was dried overnight at 105°C and the dry weight was obtained. Brain water content was obtained according to the formula: [(wet weight–dry weight)/wet weight]×100%.23
Measurement Of CBF
CBF was determined on a laser Doppler flowmetry and the images were acquired during MCAO and after 24 hrs of reperfusion (n=6). Here, the exposed cortex was irradiated by low-power laser beam with the aid of a computer-controlled optical scanner. The scanning probe was placed parallel to cerebral cortex and the distance was set as 20 cm. A video monitor was used to display a color-coded image aiming at denoting specific relative perfusion levels.
Histological Assessment
After reperfusion, brain tissues were collected immediately from rats under deep anesthetization and fixed for 12 hrs using 10% formalin in 0.1 M PBS (n=6). Then, a routine process of paraffin embedding was made. The sections with 5 μm thickness were obtained and stained with H&E staining for histological assessment by a pathologist blinded to the treatment groups.
Quantification Of Oxidative Stress Biomarkers
A centrifugation operation was carried out to isolate the supernatant from the blood plasma after 24-hr reperfusion (n=6). The brain hemispheres were homogenized in 10% (w/v) physiological saline and the precipitation was removed by centrifugation at 3500 rpm for 10 mins (n=6).
Quantification Of Nitric Oxide (NO) Level
Blood supernatant was supplemented with 100 μL of the Griess solution. NaNO2 was used as the standard. The reaction system was maintained at room temperature for 15 mins and its OD value was read at 540 nm to measure the NO level.
Quantification Of MDA Level
The supernatant from brain tissue homogenate was incubated with thiobarbituric acid in boiling water for 15 mins. The malondialdehyde (MDA) level was determined at 532 nm according to the manufacturer’s instructions (Nanjing Jiancheng Bioengineering Institute, Nanjing, People’s Republic of China) and expressed as μmol/mg protein.
Quantification Of GSH And GSSG
Total glutathione (GSH) level was measured using the 5,5′-dithiobis-(2-nitrobenzoic acid) (DTNB)-GSSG reductase according to the previous study.24 As for quantification of the GSSG level, 2-vinylpyridine solution was firstly supplemented to eliminate the reduced GSH. Following that, the same method was applied.
Quantification Of The SOD And Its Isoforms Activity
Enzyme activities were analyzed using assay kits (Beyotime Institute of Biotechnology, Haimen, People’s Republic of China). Oxyamine was used as the substrate to measure the activity of T-SOD in xanthine–xanthine oxidase system, here, T-SOD could prevent the oxidation of oxyamine. The Cu/Zn-SOD inhibitor was used to assess the Mn-SOD activity and the difference between T-SOD and Mn-SOD was used for calculation of the Cu/Zn-SOD activity. The enzyme consumption at inhibiting 50% of WST1 was defined as one unit of SOD activity.
Quantification Of GSH-PX Activity
The measurement of GSH-PX activity was performed at 25°C in a reaction system, which included tripho-sphopyridinenucleotide, reduced GSH, t-Bu-OOH and GSH reductase. The calculation was exerted according to the consumption that was required for oxidization of 1 μmol NADPH into NADP+ in 1 min at 25°C.
Western Blot Analysis
The isolation of brain tissues of rats was performed under anesthesia and −80°C refrigerator was used to temporarily keep samples. Samples were gently homogenized using a lysis buffer (10 mM KCl, 1 mM EDTA, 1.5 mM MgCl2, 1 mM EGTA, 20 mM HEPES, 250 mM sucrose, 1 mM dithiothreitol, 0.1 mM phenylmethylsulfonyl fluoride, and proteinase inhibitor cocktail; pH 7.9) (n=6). The homogenate supernatant was obtained by centrifugation at 14,000 rpm and at 4°C for 15 mins. Protein concentration was quantified using the Bradford assay. Proteins were separated using 10% SDS-PAGE. The electric current was set as 400 mA in order to transfer protein to a PVDF membrane in 35 mins (Immobilon-P, Millipore, Bedford, MA, USA). The membrane was further blocked with 5% skim milk at 4°C for 3 hrs and immersed into primary antibodies solution (anti-beclin1 (1:1000, sigma Cruz), anti-LC3B (1:1000, Sigma), anti-p-AMPKα at Thr 172 (1:1000, CST, Boston, USA), anti-AMPKα (1:1000, CST), anti-p-mTOR (1:1000, CST), anti-mTOR (1:1000, CST), anti-p-JNK (1:1000, CST), anti-JNK (1:1000, CST), and anti-GAPDH (1:5000, Beijing Zhongshan Golden Bridge Biotechnology Co. Ltd., Beijing, People’s Republic of China)) at 4°C overnight. After washing with TBST, the membrane was immersed into secondary antibodies linked to horseradish peroxidase solution for 1 hr at room temperature. The blue X-ray film was used to detect the luminescence band after incubation for 5 mins in an ECL substrate solution. Finally, the gray density was quantified using ImageJ® software.
Statistical Analysis
All data were presented as the mean± standard deviation. Comparisons between experimental and control groups were performed by one-way factorial ANOVA and followed by Tukey’s test. Significant effects were defined when p<0.05.
Results
Stigmasterol Improved Neurobehavioral Function After Cerebral Ischemic/Reperfusion Injury
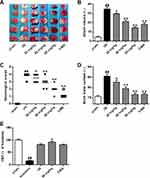
Figure 2A shows brain infarct volume in different groups after TTC staining and Figure 2B shows a quantitative analysis results. The infarct volume was significantly enhanced in the ischemic/reperfusion group (34.83%±1.71%) by comparison with the sham group (4.35%±0.65%) (p<0.01). With the application of stigmasterol, the infarct volume caused by the ischemic/reperfusion injury was significantly reduced and exhibited in a dose-dependent mode (p<0.05, p<0.01). The highest inhibition rate appeared in 80 mg/kg stigmasterol treatment group and got to 58.25%, which was better than that in the 3-MA treatment group (p<0.01).
Figure 2C and D shows neurological scores and brain water content in the ischemic/reperfusion injury model and protection effect of stigmasterol. It could be seen that the ischemic/reperfusion injury significantly resulted in the increase of neurological scores (3.83±0.37) and brain water content (85.53%±0.80%) (p<0.01). It was significantly attenuated by stigmasterol treatment and negative correlation with the concentration (Figure 2C and D) (p<0.05, p<0.01).
Figure 2E shows CBF results from a laser Doppler perfusion image system. CBF was significantly decreased after ischemia and restored after reperfusion in all groups. Administration of 80 mg/kg stigmasterol caused a significant enhancement of CBF after 24-hr reperfusion (91.75%±2.67%) (p <0.05), which had no significance compared with that in the 3-MA group.
Protection Of Stigmasterol On Neuronal Cell After Cerebral Ischemic/Reperfusion Injury
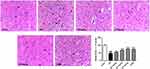
H&E staining indicated that the neurons in the sham group exhibited normal characters with uniformly distribution and a complete organization structure (Figure 3). However, brain tissue in the ischemic/reperfusion group exhibited liquefied changes and looked like polynesic sponginess. Glial cells swelled and neurons showed a confusing structure. Moreover, neural lost, nuclei appeared atrophic and stained dark. Both stigmasterol and 3-MA treatments could significantly relieve the abnormalities (p<0.05, p<0.01).25
Reduction Of Stigmasterol On Oxidative Stress After Cerebral Ischemic/Reperfusion Injury
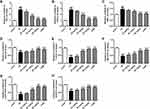
Figure 4 shows the level changes of oxidative stress parameters. It could be seen the ischemic/reperfusion injury induced higher levels of NO and GSSG, and produced more MDA by comparison with the sham group (p<0.01). However, treatment with stigmasterol or 3-MA could effectively decrease them and a dose-dependent effect was observed (p<0.05, p<0.01) (Figure 4A–C). There were no significant difference in the levels of NO, GSSG, and MDA between 3-MA treatment group and 80 mg/kg stigmasterol treatment group.
Simultaneously, the analysis on antioxidase revealed that the ischemic/reperfusion injury caused dramatic decrease in the levels of GSH, GSH-PX, T-SOD, Cu/Zn-SOD, and Mn-SOD compared with the sham group (p<0.01). The application of stigmasterol could improve the situation, and the levels of antioxidase almost could restore when 80 mg/kg stigmasterol was administered (p<0.05, p<0.01) (Figure 4D–H).
Inhibition Effects Of Stigmasterol On Autophagy Following Cerebral Ischemic/Reperfusion Injury
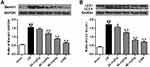
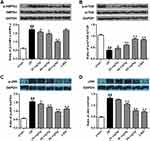
Both beclin1 and LC3 are the autophagic biomarkers. We found that the ischemic/reperfusion injury significantly promoted the expression of beclin1 by comparison with the sham group and the ratio of LC3 II/I was also enhanced (p<0.01) (Figure 5A–B). With the application of stigmasterol and 3-MA at 24 hrs of reperfusion, the expression of beclin1 and LC3 was significantly inhibited (p<0.05, p < 0.01).
Intervention Mechanism Of Stigmasterol On Autophagy
As autophagy plays important roles in protecting rats against cerebral ischemic/reperfusion injury, we also explored the intervention mechanism of stigmasterol on autophagy activation by Western blot analysis. The ischemic/reperfusion injury significantly induced a higher expression of JNK, and phosphorylation of p-AMPKα and p-JNK by comparison with that in the sham group, but contrary to the phosphorylation of p-mTOR (p<0.01) (Figure 6A–D). Stigmasterol could significantly reverse these changes and a dose-dependent effect could be observed (p<0.05, p<0.01). 3-MA also could function at decreasing JNK phosphorylation and increasing mTOR phosphorylation (p<0.01), but cannot affect p-AMPKα. These results reveal stigmasterol was able to intervene the occurrence of autophagy caused by cerebral ischemic/reperfusion injury via inhibiting the AMPK/mTOR and JNK signal pathways.
Discussion
Ischemic stroke is a result of cerebral blood vessel blockage and always leads to dysfunction and cell death, linked to high fatality and disability rates.26 The early neurological function deficits are considered to be important predictors, and neurological deficit grading and brain infarct volume analysis can be applied to assess the progression of stroke.27,28 In this study, we confirmed that stigmasterol intervention successfully reversed the adverse effects that the ischemic/reperfusion injury caused the increases at infarct volume and neurological grade (Figure 2). Additionally, H&E analysis further revealed that stigmasterol improved the distribution, arrangement, and morphological structure of nerve cells (Figure 3).25 Especially, the effect of 80 mg/kg stigmasterol treatment was similar to that treated by 3-MA, suggesting that stigmasterol could alleviate brain injury induced by ischemic stroke and might be a novel therapeutic agent for ischemic stroke.
Furthermore, we explored the possible mechanism of stigmasterol against ischemic stroke. As researchers develop a greater understanding of the biology underlying drug-function relationships, they find that the injury and treatment mechanism of the ischemic/reperfusion involve oxidative stress, inflammatory responses, apoptosis, blood–brain barrier disruption, ionic imbalance, etc.29 It has been well documented that oxidative stress are most closely related with ischemic neuronal injury.30 Considerable groups have found that the oxidation of lipids, proteins, and DNA during the ischemic/reperfusion injury and confirmed the adverse effects of the accumulation of ROS, such as cellular damage and apoptosis.31,32 The ROS products will further aggravate the damage on neuron by attacking macromolecules within glial cells. Therefore, the endogenous antioxidant defense system plays an important role at neuro-protection against ischemia/reperfusion injury. Several groups, including us, have proved that the ischemic/reperfusion injury induced excessively ROS that could not be promptly removed by the endogenous defense system (Figure 4A–C). Thus, many attempts have been made and testified that antioxidants can weaken the neuronal damage after cerebral ischemic/reperfusion injury. In this study, the levels of endogenous antioxidant defense, including GSH, GSH-PX, T-SOD, Cu/Zn-SOD, and Mn-SOD, were significantly enhanced after treatment with stigmasterol by comparison with those in the ischemic/reperfusion injury group (Figure 4D–H). According to the molecular structure of stigmasterol, the double bond is the structure basis for its anti-peroxidative function (Figure 1A). It could be concluded that stigmasterol induces the increase of endogenous antioxidant enzymes and decreases the accumulation of ROS, which will contribute to the improvement of neuronal damage caused by the ischemic/reperfusion injury (Figures 2–4).
Along with oxidative stress, recently, researchers demonstrate that autophagy does exist in cerebral ischemia and is another important target to protect brain damage during the ischemic/reperfusion injury.33–35 Proper activation of autophagy is helpful for cells to maintain energy and cellular homeostasis, and further protect cells avoiding death in response to nutrient starvation or metabolic stress. As a double-edged sword, however, excessive or prolonged autophagy will facilitate the necrotic and apoptotic cascades.34 Although the exact effect of autophagy during the ischemic/reperfusion injury is not clear, increasing evidences display that the ischemic/reperfusion injury causes a higher level activation of autophagy.21,36 Several groups report that both beclin1 and LC3 are the important autophagy-associated protein and used as reliable biomarkers of autophagosomes.21,37,38 LC3-I conversion towards LC3-II marks the beginning of autophagy and the upregulation of beclin1 expression is also necessary for autophagic processing.39,40 On the contrary, silencing of beclin1 and reduction of LC3-I conversion towards LC3-II will helpful to prevent autophagosome processing.41,42 As expected, we also confirmed the upregulation of autophagy activation after the ischemic/reperfusion injury, supported by the content increase of beclin1 and LC3 II (Figure 5). To our knowledge, this was the first study demonstrating the function of stigmasterol at inactivating autophagy. Moreover, changes in beclin1 and LC3 II/I levels were negatively correlated with the dosage of stigmasterol.
The signal regulation of autophagy activation is complex and involves several pathways, including PI3K-Akt, beclin1-Bcl2, JNK, AMPK-mTOR signal pathways.42–45 In response to stress, autophagy can generate nutrients and energy to promote the cells adaptation. AMPK can balance hypothalamic energy as an intracellular energy sensor and mTOR functions by maintaining nutrient homeostasis as a nutrient sensor. Thus, we investigated whether stigmasterol exerted protective effects on the expression and phosphorylation of AMPK and mTOR caused by the ischemic/reperfusion injury. Our results demonstrated that the ischemic/reperfusion injury could not intervene the protein expression of AMPK and mTOR, but significantly promoted the phosphorylation of AMPK while reducing the phosphorylation of mTOR (Figure 6A and B). Furthermore, the protein expression and phosphorylation of JNK were simultaneously upregulated (Figure 6C and D). All these changes could be attenuated via stigmasterol or 3-MA treatment. These data suggested that stigmasterol has an impact on autophagy partly during the cerebral ischemic/reperfusion injury via AMPK-mTOR and JNK signaling pathway.
Conclusion
Summarily, this study demonstrates that stigmasterol exhibits neuro-protective effect against the cerebral ischemia/reperfusion injury. The main mechanism is related with enhancing the endogenous antioxidant defense system and inhibiting autophagy activation via AMPK-mTOR and JNK pathways. These findings support the potential of stigmasterol at intervening ischemic stroke-induced neuropathology.
Acknowledgment
We thank the financial support from Natural Science Foundation of Shandong Province (ZR2014HL106).
Disclosure
The authors report no conflicts of interest in this work.
References
1. Murray CJL, Lopez AD. Measuring the global burden of disease. New Engl J Med. 2013;369(5):448–457. doi:10.1056/NEJMra1201534
2. Mensah GA, Norrving B, Feigin VL. The global burden of stroke. Neuroepidemiology. 2015;45(3):143–145. doi:10.1159/000441082
3. Feigin VL, Norrving B, George MG, Foltz JL, Roth GA, Mensah GA. Prevention of stroke: a strategic global imperative. Nat Rev Neurol. 2016;12(9):501–512. doi:10.1038/nrneurol.2016.107
4. Zhang B, Zhang HX, Shi ST, et al. Interleukin-11 treatment protected against cerebral ischemia/reperfusion injury. Biomed Pharmacother. 2019;115:108816. doi:10.1016/j.biopha.2019.108816
5. Wong KS, Caplan LR, Kim JS. Stroke mechanisms. Front Neurol Neurosci. 2016;40:58–71. doi:10.1159/000448302
6. Singh D, Reeta KH, Sharma U, Jagannathan NR, Dinda AK, Gupta YK. Neuro-protective effect of monomethyl fumarate on ischemia reperfusion injury in rats: role of Nrf2/HO1 pathway in peri-infarct region. Neurochem Int. 2019;126:96–108. doi:10.1016/j.neuint.2019.03.010
7. Wu S, Sena E, Egan K, Macleod M, Mead G. Edaravone improves functional and structural outcomes in animal models of focal cerebral ischemia: a systematic review. Int J Stroke. 2014;9(1):101–106. doi:10.1111/ijs.12163
8. Hua S, Wang B, Chen R, et al. Neuroprotective effect of dichloromethane extraction from piper nigrum L. and piper longum L. on permanent focal cerebral ischemia injury in rats. J Stroke Cerebrovasc Dis. 2019;28(3):751–760. doi:10.1016/j.jstrokecerebrovasdis.2018.11.018
9. Kwon H, Jung JW, Lee YC, Ryu JH, Kim DH. Neuroprotective effect of the ethanol extract of Artemisia capillaris on transient forebrain ischemia in mice via nicotinic cholinergic receptor. Chin J Nat Med. 2018;16(6):428–435. doi:10.1016/S1875-5364(18)30076-1
10. Zhu XL, Yan BC, Tang C, et al. Neuroprotective effect of Paeoniae Radix Rubra on hippocampal CA1 region of mice induced by transient focal cerebral ischemia via anti-gliosis and anti-oxidant activity. Chin Herb Med. 2019;11(1):86–91. doi:10.1016/j.chmed.2018.10.005
11. Ramu R, Shirahatti PS, Nayakavadi S, et al. The effect of a plant extract enriched in stigmasterol and beta-sitosterol on glycaemic status and glucose metabolism in alloxan-induced diabetic rats. Food Funct. 2016;7(9):3999–4011. doi:10.1039/C6FO00343E
12. Aboobucker SI, Suza WP. Why do plants convert sitosterol to stigmasterol? Front Plant Sci. 2019;10:354. doi:10.3389/fpls.2019.00354
13. Kaur N, Chaudhary J, Jain A, Kishore L. Stigmasterol: a comprehensive review. Int J Pharm Sci Res. 2011;2(9):2259–2265.
14. Ras RT, Koppenol WP, Garczarek U, et al. Increases in plasma plant sterols stabilize within four weeks of plant sterol intake and are independent of cholesterol metabolism. Nutr Metab Cardiovasc. 2016;26(4):302–309. doi:10.1016/j.numecd.2015.11.007
15. Kametani T, Furuyama H. Synthesis of vitamin D3 and related compounds. Med Res Rev. 1987;7(2):147–171.
16. Newill H, Loske R, Wagner J, Johannes C, Lorenz RL, Lehmann L. Oxidation products of stigmasterol interfere with the action of the female sex hormone 17beta-estradiol in cultured human breast and endometrium cell lines. Mol Nutr Food Res. 2007;51(7):888–898. doi:10.1002/mnfr.200700025
17. Kangsamaksin T, Chaithongyot S, Wootthichairangsan C, Hanchaina R, Tangshewinsirikul C, Svasti J. Lupeol and stigmasterol suppress tumor angiogenesis and inhibit cholangiocarcinoma growth in mice via downregulation of tumor necrosis factor-alpha. PLoS One. 2017;12(12):e0189628. doi:10.1371/journal.pone.0189628
18. Yenn TW, Arslan Khan M, Amiera Syuhada N, Chean Ring L, Ibrahim D, Tan WN. Stigmasterol: an adjuvant for beta lactam antibiotics against beta-lactamase positive clinical isolates. Steroids. 2017;128:68–71. doi:10.1016/j.steroids.2017.10.016
19. Antwi AO, Obiri DD, Osafo N, Forkuo AD, Essel LB. Stigmasterol inhibits lipopolysaccharide-induced innate immune responses in murine models. Int Immunopharmacol. 2017;53:105–113. doi:10.1016/j.intimp.2017.10.018
20. Antwi AO, Obiri DD, Osafo N. Stigmasterol modulates allergic airway inflammation in guinea pig model of ovalbumin-induced asthma. Mediators Inflamm. 2017;2017:2953930. doi:10.1155/2017/2953930
21. Guo Z, Cao G, Yang H, et al. A combination of four active compounds alleviates cerebral ischemia-reperfusion injury in correlation with inhibition of autophagy and modulation of AMPK/mTOR and JNK pathways. J Neurosci Res. 2014;92(10):1295–1306. doi:10.1002/jnr.23400
22. Longa EZ, Weinstein PR, Carlson S, Cummins R. Reversible middle cerebral artery occlusion without craniectomy in rats. Stroke. 1989;20:84–91. doi:10.1161/01.STR.20.1.84
23. Mdzinarishvili A, Kiewert C, Kumar V, Hillert M, Klein J. Bilobalide prevents ischemia-induced edema formation in vitro and in vivo. Neuroscience. 2007;144(1):217–222. doi:10.1016/j.neuroscience.2006.08.037
24. Abegg MA, Alabarse PVG, Schuller AK, Benfato MS. Glutathione levels in and total antioxidant capacity of Candida sp cells exposed to oxidative stress caused by hydrogen peroxide. Rev Soc Bras Med Tro. 2012;45(5):620–626. doi:10.1590/S0037-86822012000500015
25. Zhao Q, Zhang C, Wang X, Chen L, Ji H, Zhang Y. (S)-ZJM-289, a nitric oxide-releasing derivative of 3-n-butylphthalide, protects against ischemic neuronal injury by attenuating mitochondrial dysfunction and associated cell death. Neurochem Int. 2012;60(2):134–144. doi:10.1016/j.neuint.2011.11.013
26. Ozaki T, Nakamura H, Kishima H. Therapeutic strategy against ischemic stroke with the concept of neurovascular unit. Neurochem Int. 2019;126:246–251. doi:10.1016/j.neuint.2019.03.022
27. Kasner SE. Clinical interpretation and use of stroke scales. Lancet Neurol. 2006;5(7):603–612. doi:10.1016/S1474-4422(06)70495-1
28. Chelluboina B, Klopfenstein JD, Gujrati M, Rao JS, Veeravalli KK. Temporal regulation of apoptotic and anti-apoptotic molecules after middle cerebral artery occlusion followed by reperfusion. Mol Neurobiol. 2014;49(1):50–65. doi:10.1007/s12035-013-8486-7
29. Yang Y, Lv SY, Lyu SK, Wu D, Chen Q. The protective effect of apelin on ischemia/reperfusion injury. Peptides. 2015;63:43–46. doi:10.1016/j.peptides.2014.11.001
30. Gao X, Chen W, Li J, et al. The protective effect of alpha-lipoic acid against brain ischemia and reperfusion injury via mTOR signaling pathway in rats. Neurosci Lett. 2018;671:108–113. doi:10.1016/j.neulet.2018.02.012
31. Liu NN, Dong ZL, Han LL. MicroRNA-410 inhibition of the TIMP2-dependent MAPK pathway confers neuroprotection against oxidative stress-induced apoptosis after ischemic stroke in mice. Brain Res Bull. 2018;143:45–57. doi:10.1016/j.brainresbull.2018.09.009
32. Kotur-Stevuljevic J, Bogavac-Stanojevic N, Jelic-Ivanovic Z, et al. Oxidative stress and paraoxonase 1 status in acute ischemic stroke patients. Atherosclerosis. 2015;241(1):192–198. doi:10.1016/j.atherosclerosis.2015.05.016
33. Wei K, Wang P, Miao CY. A double-edged sword with therapeutic potential: an updated role of autophagy in ischemic cerebral injury. CNS Neurosci Ther. 2012;18(11):879–886. doi:10.1111/cns.12005
34. Wang P, Shao BZ, Deng Z, Chen S, Yue Z, Miao CY. Autophagy in ischemic stroke. Prog Neurobiol. 2018;163–164:98–117. doi:10.1016/j.pneurobio.2018.01.001
35. Hou K, Xu D, Li F, Chen S, Li Y. The progress of neuronal autophagy in cerebral ischemia stroke: mechanisms, roles and research methods. J Neurol Sci. 2019;400:72–82. doi:10.1016/j.jns.2019.03.015
36. Zhang DM, Zhang T, Wang MM, et al. TIGAR alleviates ischemia/reperfusion-induced autophagy and ischemic brain injury. Free Radic Biol Med. 2019;137:13–23. doi:10.1016/j.freeradbiomed.2019.04.002
37. Tanida I, Ueno T, Kominami E. LC3 and Autophagy. Methods Mol Biol. 2008;445:77–88. doi:10.1007/978-1-59745-157-4_4
38. Wirawan E, Lippens S, Vanden Berghe T, et al. Beclin1: a role in membrane dynamics and beyond. Autophagy. 2012;8(1):6–17. doi:10.4161/auto.8.1.16645
39. Luo C, Ouyang MW, Fang YY, et al. Dexmedetomidine protects mouse brain from ischemia-reperfusion injury via inhibiting neuronal autophagy through up-regulating HIF-1alpha. Front Cell Neurosci. 2017;11:197. doi:10.3389/fncel.2017.00197
40. Yao X, Yao R, Huang F, Yi J. LncRNA SNHG12 as a potent autophagy inducer exerts neuroprotective effects against cerebral ischemia/reperfusion injury. Biochem Biophys Res Commun. 2019;514:490–496. doi:10.1016/j.bbrc.2019.04.158
41. Wang X, Sun D, Hu Y, et al. The roles of oxidative stress and Beclin-1 in the autophagosome clearance impairment triggered by cardiac arrest. Free Radic Biol Med. 2019;136:87–95. doi:10.1016/j.freeradbiomed.2018.12.039
42. He H, Zeng Q, Huang G, et al. Bone marrow mesenchymal stem cell transplantation exerts neuroprotective effects following cerebral ischemia/reperfusion injury by inhibiting autophagy via the PI3K/Akt pathway. Brain Res. 2019;1707:124–132. doi:10.1016/j.brainres.2018.11.018
43. Liang J, Shao SH, Xu ZX, et al. The energy sensing LKB1-AMPK pathway regulates p27(kip1) phosphorylation mediating the decision to enter autophagy or apoptosis. Nat Cell Biol. 2007;9(2):218–224. doi:10.1038/ncb1537
44. Mehrpour M, Esclatine A, Beau I, Codogno P. Autophagy in health and disease. 1. Regulation and significance of autophagy: an overview. Am J Physiol Cell Physiol. 2010;298(4):C776–C785. doi:10.1152/ajpcell.00507.2009
45. Wang P, Guan YF, Du H, Zhai QW, Su DF, Miao CY. Induction of autophagy contributes to the neuroprotection of nicotinamide phosphoribosyltransferase in cerebral ischemia. Autophagy. 2012;8(1):77–87. doi:10.4161/auto.8.1.18274
 © 2019 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2019 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.