Back to Journals » Drug Design, Development and Therapy » Volume 10
Stability studies of lincomycin hydrochloride in aqueous solution and intravenous infusion fluids
Authors Czarniak P , Boddy M, Sunderland B , Hughes J
Received 18 August 2015
Accepted for publication 4 December 2015
Published 7 March 2016 Volume 2016:10 Pages 1029—1034
DOI https://doi.org/10.2147/DDDT.S94710
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 2
Editor who approved publication: Prof. Dr. Wei Duan
Petra Czarniak, Michael Boddy, Bruce Sunderland, Jeff D Hughes
School of Pharmacy, Curtin University, Perth, WA, Australia
Purpose: The purpose of this study was to evaluate the chemical stability of Lincocin® (lincomycin hydrochloride) in commonly used intravenous fluids at room temperature (25°C), at accelerated-degradation temperatures and in selected buffer solutions.
Materials and methods: The stability of Lincocin® injection (containing lincomycin 600 mg/2 mL as the hydrochloride) stored at 25°C±0.1°C in sodium lactate (Hartmann’s), 0.9% sodium chloride, 5% glucose, and 10% glucose solutions was investigated over 31 days. Forced degradation of Lincocin® in hydrochloric acid, sodium hydroxide, and hydrogen peroxide was performed at 60°C. The effect of pH on the degradation rate of lincomycin hydrochloride stored at 80°C was determined.
Results: Lincomycin hydrochloride was found to maintain its shelf life at 25°C in sodium lactate (Hartmann’s) solution, 0.9% sodium chloride solution, 5% glucose solution, and 10% glucose solution, with less than 5% lincomycin degradation occurring in all intravenous solutions over a 31-day period. Lincomycin hydrochloride showed less rapid degradation at 60°C in acid than in basic solution, but degraded rapidly in hydrogen peroxide. At all pH values tested, lincomycin followed first-order kinetics. It had the greatest stability near pH 4 when stored at 80°C (calculated shelf life of 4.59 days), and was least stable at pH 2 (calculated shelf life of 0.38 days).
Conclusion: Lincocin® injection was chemically found to have a shelf life of at least 31 days at 25°C when added to sodium lactate (Hartmann’s) solution, 0.9% sodium chloride solution, 5% glucose solution, and 10% glucose solution. Solutions prepared at approximately pH 4 are likely to have optimum stability.
Keywords: lincomycin, stability, pH, intravenous fluids, IV additives
Introduction
Lincomycin is a naturally occurring lincosamide antibiotic obtained as a fermentation product of Streptomyces lincolnensis var. lincolnensis. It has a spectrum of activity against Gram-positive bacteria and most anaerobes, but not Gram-negative aerobes.1 Its action may be bactericidal or bacteriostatic, dependent on the concentration of the drug attained at the site of infection and the susceptibility of the infecting organism.2 It is indicated for the treatment of serious infections due to susceptible strains of Gram-positive aerobes, such as staphylococci, streptococci, and pneumococci, and is generally reserved for patients who are allergic to penicillin.3
In Australia, lincomycin is only available as a solution for injection (600 mg in 2 mL), and is the only parenteral lincosamide where the cost is subsidized by the Pharmaceutical Benefits Scheme.4 Intravenous (IV) doses are administered on the basis of 1 g Lincocin® diluted in not less than 100 mL of an appropriate solution and infused over a period of not less than 1 hour to avoid severe cardiopulmonary reactions.3 Infusion solutions reported to be physically compatible with Lincocin® include glucose 5% solution, glucose 10% solution, sodium chloride 0.9% + glucose 5% solution, sodium chloride 0.9% + glucose 10% solution, compound sodium lactate solution, sodium lactate 1/6 molar solution, and dextran 70 solution.3 However, according to the Monthly Index of Medical Specialities, “compatibility determinations of lincomycin in these IV fluids are physical observations only and not chemical determinations. Adequate clinical evaluation of the safety and efficacy of these combinations has not been performed”,2 although stability for 24 hours in dextrose 5% solution, dextrose 10% solution, dextrose 5% in sodium chloride 0.9% solution, and sodium chloride 0.9% solution has been reported by the American Society of Health-System Pharmacists.5 A dose of 600 mg in a 500 mL infusion solution provides therapeutic levels for 14 hours.3
Few studies on the chemical stability of lincomycin are available in the literature.6,7 However, there may be instances where prolonged storage of lincomycin reconstituted in an IV solution is required. For example, in cases of rural or remote administration sites, several days’ supply of reconstituted lincomycin in IV solutions may be needed, or in the “hospital in the home” setting, it may be necessary to store reconstituted lincomycin in IV solution for several days.
The purpose of this study was to evaluate the chemical stability of Lincocin® (lincomycin hydrochloride) over a 1-month period in commonly used IV fluids, including glucose 5% solution, glucose 10% solution, sodium lactate (Hartmann’s) solution, and sodium chloride 0.9% solution at room temperature (25°C) and in various buffer solutions at 80°C.
Materials and methods
Materials
Lincocin® injection solution containing lincomycin 600 mg/2 mL as the hydrochloride and benzyl alcohol 9.45 mg/mL (batch number G47185, expiry November 2013; Pfizer Inc, New York, NY, USA), 0.9% sodium chloride solution (batch number S58R4, expiry June 2015; Baxter International Inc, Deerfield, IL, USA), glucose monohydrate 5% solution (batch number 12186410, expiry April 2015; B Braun Melsungen AG, Melsungen, Germany), glucose monohydrate 10% solution (batch number 14DC7301, expiry March 2015; Fresenius SE and Co KGaA, Bad Homburg, Germany), and sodium lactate (Hartmann’s solution, batch number 122358143, expiry May 2015; B Braun) were obtained commercially. Pure analytical grade lincomycin hydrochloride monohydrate – Vetranal™ analytical standard (lot SZB8329XV, expiry November 24, 2014; Sigma-Aldrich Co, St Louis, MO, USA) – was also obtained commercially. High-performance liquid chromatography (HPLC) mobile-phase components, including acetonitrile, were HPLC grade. Water was obtained from a Milli-Q ultrapure water system, (EMD Millipore, Billerica, MA, USA) consisting of a four-bowl ultrapure cartridge kit with a conductivity of 0.05 μS. Measurements of pH were carried out at room temperature using a digital pH meter (model HI8519N; Hanna Instruments Inc, Woonsocket, RI, USA), which was calibrated with standard buffer solutions.
A pH 2 solution was prepared from 1 M hydrochloric acid, pH 3.1 buffer solution was prepared from anhydrous citric acid and sodium hydroxide, pH 4 acetate-buffer solution was prepared from acetic acid and sodium hydroxide, and pH 6.1 and 8 phosphate-buffer solutions were prepared from orthophosphoric acid and sodium hydroxide. All were adjusted to an ionic strength of 0.5 using sodium chloride.
Preparation of solutions
With an aseptic technique, 0.4 mL Lincocin® (lincomycin hydrochloride) was transferred with sterile plastic syringes and stainless steel needles from the original vial to clear-glass volumetric flasks sealed with polypropylene stoppers and brought to 200 mL with either sodium lactate (Hartmann’s) solution, 0.9% sodium chloride solution, 5% glucose solution, or 10% glucose solution. The flasks were stored at 25°C±0.1°C in a controlled-temperature water bath under standard laboratory lighting. After agitation, two 5 mL samples were immediately removed from each volumetric flask and evaluated at time 0 (control), then on day 1 (ie, after 24 hours), 4, 7, 11, 14, 24, and 31. Each sample was analyzed three times by HPLC and the overall mean determined. In addition, one 5 mL sample was used neat to test pH.
To determine the effect of pH on the degradation rate of lincomycin hydrochloride, 0.6 mg/mL lincomycin hydrochloride was prepared at pH 2, 3.1, 4, 6.1, and 8, and stored at 80°C (water bath ±0.2°C). Prior to HPLC analysis, each pH measurement was noted precisely after 10 minutes using a stopwatch.
Assay of lincomycin hydrochloride
Lincomycin hydrochloride concentrations were determined using chromatographic variables adapted from a reverse-phase HPLC assay from Catena et al.8 HPLC analysis was performed using an HPLC pump (501; Waters Corporation, Milford, MA, USA) connected to a Rheodyne® model 7125 syringe loading sample injector with 20 μL sample loop (Sigma-Aldrich), an ultraviolet detector (484 tunable absorbance detector; Waters), and a Hewlett-Packard HP 3396 series II integrator/printer. An Apollo C18 (150×4.6 mm, particle size 5 μm) reverse-phase HPLC column was used in conjunction with a reverse-phase guard column. All operations were carried out under ambient conditions.
Samples were eluted isocratically with a mobile phase consisting of 8% acetonitrile in 50 mM aqueous phosphoric acid adjusted to pH 3 and a flow rate of 1.5 mL/min. Detection was performed at 220 nm. The retention time for lincomycin hydrochloride was approximately 11.7 minutes.
The method used to assay lincomycin hydrochloride was validated using standards of Lincocin® solution of 0.1, 0.2, 0.3, 0.4, 0.5, 0.6, 0.8, and 1 mg/mL to produce a linear relationship (area [millions] = [20.4263x] +0.3166; R2=0.9993). The linearity of the detector response for a range of pure analytical grade lincomycin hydrochloride monohydrate concentrations (0.1, 0.2, 0.4, 0.6, 0.8, and 1 mg/mL) was determined (area [millions] =17.832x +0.2457; R2=0.9999) to validate the reverse-phase HPLC method and to determine the amount of lincomycin in a freshly prepared 0.6 mg/mL lincomycin hydrochloride sample using Lincocin® injection. For each of the standards, samples were injected into the HPLC system three times and the mean value determined. Intraday and interday coefficients of variation of the 0.6 mg/mL lincomycin hydrochloride solution were 0.63%.
Forced degradation of 0.6 mg/mL Lincocin® was performed by heating solutions at 60°C in a water bath each in 0.1 M hydrochloric acid and 0.1 M sodium hydroxide for 7 days, and also by exposing the drug solution to 3% hydrogen peroxide for 60 minutes. Initial lincomycin hydrochloride concentrations (t=0) in the samples were defined as 100%, and all subsequent sample concentrations were expressed as a percentage of the initial concentration. Lincomycin hydrochloride was defined as stable if not less than 90% of the initial drug concentration remained in the prepared samples. The British Pharmacopoeia and US Pharmacopoeia specifications for the content of lincomycin in lincomycin injection solution are 92.5%–107.5% and 90%–120%, respectively.9,10
Results
Forced degradation
There was no interference of any degradation-product peaks with the peak for intact lincomycin. Lincomycin hydrochloride showed less rapid degradation in acid than in base solutions, with 48.8% lincomycin remaining in the acid solution after 7 days compared to 8% remaining in the basic solution after the same time. Lincomycin hydrochloride was found to degrade rapidly in 3% hydrogen peroxide over 60 minutes. No peak remained at the retention time for intact lincomycin. When the logarithm of the peak area was plotted against time, the following linear relationship was produced:
Log10 peak area = −(0.03968× hours) +7.0225 | (1) |
with a coefficient of determination (R2) of 0.98333.
HPLC assay
The calibration curve of pure analytical grade lincomycin hydrochloride monohydrate was used to determine the amount of lincomycin in the 0.6 mg/mL lincomycin hydrochloride sample prepared from Lincocin®, and was found to contain 0.629 mg/mL lincomycin. This was equivalent to 104.9% of the labeled amount. Therefore, the calculated quantity of lincomycin in Lincocin® injections (104.9%) met the specifications of the British Pharmacopoeia 2014.9
HPLC analysis for lincomycin in the presence of degradation products was achieved with 8% acetonitrile and 92% water, with a retention time for lincomycin of 11.7 minutes, approximately 5.2 and 8.3 minutes for degradation products, and 11 minutes for lincomycin B (Figure 1).
Intravenous additive solutions
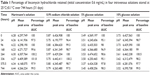
One-month stability testing of lincomycin hydrochloride at 25°C found that it was stable in sodium lactate (Hartmann’s) solution, 0.9% sodium chloride solution, 5% glucose solution, and 10% glucose solution, with less than 5% lincomycin degradation in all IV solutions over a 31-day period and thus a shelf life of at least 31 days (Table 1).
Buffer solutions
The results for the accelerated stability testing of lincomycin in buffer solutions at pH 2, 3.1, 4, 6.1, and 8 showed that lincomycin followed first-order kinetics at all pH values tested. It had the greatest stability near pH 4 when stored at 80°C, with a calculated shelf life of 4.59 days. It was least stable at pH 2, with a calculated shelf life of 0.38 days. The calculated first-order rate constants and shelf-life values of lincomycin at each pH value tested are summarized in Table 2. All kinetic runs were examined to achieve a 15% or greater loss of lincomycin.
Figure 2, which shows a logk: pH profile of lincomycin, also indicates that lincomycin was most stable near pH 4 with respect to the pH values tested. Since lincomycin is a basic compound in which the tertiary amino group has a pKa of 7.6 at low pH (ie, pH 2–3),11 it undergoes acid-catalyzed degradation of the protonated form. At higher pH values, from pH 6.1–8, there is some hydroxyl ion catalysis possibly limited by the slow reaction of the unionized species occurring in the higher pH range.
  | Figure 2 Logk: pH profile of lincomycin 0.6 mg/mL as lincomycin hydrochloride at 80°C in selected buffers (I =0.5). |
Discussion
This study has provided important practical chemical stability data to facilitate the administration of lincomycin in commonly used IV fluids. The data show lincomycin to be a relatively stable antibiotic, with a shelf life of at least 31 days at room temperature (25°C) in all IV solutions tested. These solutions were stored in sealed clear-glass flasks. Stability in IV bags could be dependent on the specific bag selected. This would enable the aseptic preparation of solutions for storage in hospital or home settings. Although the shelf life was assigned on the initial loss of 10% lincomycin, it is also notable that all solutions conformed with the British Pharmacopoeia specification of 92.5%–107.5% and the US Pharmacopoeia requirement of 90%–120% content of lincomycin as the hydrochloride. It is stated that Lincocin® (which contains lincomycin hydrochloride) can be administered by direct intramuscular injection every 12–24 hours at a dose of 10 mg/kg/day. Alternatively, IV doses can be administered on the basis of 1 g Lincocin® diluted in not less than 100 mL of appropriate IV solution, including sodium lactate solution, 0.9% sodium chloride solution, 5% glucose solution, and 10% glucose solution, and infused over at least 1 hour.3 Current stability information on lincomycin in these IV solutions is limited to the compatibility of lincomycin in these IV fluids, which are physical determinations rather than chemical determinations.3 Physical compatibility has been reported only for 24 hours at room temperature.2
An initial investigation was also carried out to ensure that the Lincocin® sample used in the study met the British Pharmacopoeia 2014 specifications for the content of lincomycin in the lincomycin hydrochloride injection. The amount of lincomycin base in a 0.6 mg/mL lincomycin sample prepared from Lincocin® was found to contain 0.629 mg/mL lincomycin, equivalent to 104.9% of the stated amount. Therefore, the calculated quantity of lincomycin in Lincocin® injection (104.9%) met the specifications of both the British Pharmacopoeia and US Pharmacopoeia.9,10
Forced-degradation studies examined the degradation of 0.6 mg/mL lincomycin in 0.1 M hydrochloric acid solution, 0.1 M sodium hydroxide solution, and 3% hydrogen peroxide solution at 60°C. These showed that lincomycin degradation occurred most rapidly in hydrogen peroxide, suggesting that lincomycin hydrochloride readily undergoes oxidation. Less rapid degradation was observed in strong acid and base solutions after 7 days. A study investigating the stability of 0.4% lincomycin hydrochloride in 0.1 N hydrochloric acid solution at 37°C and 70°C reported that lincomycin showed no degradation for at least 48 hours at 37°C and slow degradation (half-life 39 hours) at 70°C.6
In a study investigating the stability of the closely structurally related clindamycin in 5% dextrose and 0.9% sodium chloride at 4°C and room temperature (23°C) over 21 days, researchers reported that the degradation of clindamycin was slow, with less than 5% loss occurring at various concentrations of clindamycin in each diluent and at each temperature. This is similar to the findings for lincomycin in the current study, although a single concentration (0.6 mg/mL) was used in each IV solution.7
Degradation studies of lincomycin hydrochloride at 80°C at pH 2, 3, 4, 6.1, and 8 followed first-order kinetics and the rate constant was lowest near pH 4, with a calculated shelf life of 4.59 days. It was least stable at pH 2, with a calculated shelf life of 0.38 days. The pH-rate profile shows specific acid catalysis of the protonated species at low pH values; however, the hydroxide ion catalyses of lincomycin showed a slowing in reaction rate as the molecular species increased in concentration.
This finding would indicate that infusions prepared at approximately pH 4 would have the greatest potential stability, making the 5% or 10% glucose solutions having optimum pH values regarding long-term stability. Benzyl alcohol was included in Lincocin®; however, in the preparation of the infusions for this study, it was diluted to 0.0189 mg/mL. It would not be expected that its inclusion as a preservative in small-volume solutions would influence the stability data reported in this study.
Conclusion
Lincocin® injection solution containing lincomycin hydrochloride 300 mg/mL and benzyl alcohol 9.45 mg/mL was found to retain its shelf life at 25°C when added to sodium lactate (Hartmann’s) solution, 0.9% sodium chloride solution, 5% glucose solution, and 10% glucose solutions for at least 31 days.
Degradation studies of lincomycin hydrochloride at 80°C at various pH values showed that lincomycin was most stable near pH 4, with a calculated shelf life of 4.59 days, and was least stable at pH 2, with a calculated shelf life of 0.38 days. These data would suggest solutions prepared at approximately pH 4 are likely to have optimum stability.
Disclosure
The authors report no conflicts of interest in this work.
References
Greenwood D. Lincosamines. In: Finch RG, Greenwood D, Norrby SR, Whitley RJ, editors. Antibiotic and Chemotherapy: Anti-infective Agents and Their Use in Therapy. Edinburgh: Saunders Elsevier; 2010:272–275. | ||
Lincocin® [product information]. Australian Government Department of Health. Therapeutics Goods Administration. Available from: https://www.ebs.tga.gov.au/ebs/picmi/picmirepository.nsf/pdf?OpenAgent=&id=CP-2010-PI-05409-3&d=2016020516114622412. Accessed February 5, 2016. | ||
Monthly Index of Medical Specialities (eMIMS). Lincocin. 2015. Available from: https://www.mimsonline.com.au.dbgw.lis.curtin.edu.au/Search/Search.aspx. Accessed December 2, 2015. | ||
Australian Government Department of Health. Schedule of Pharmaceutical Benefits: Effective 1 June–30 June 2015. Canberra: Commonwealth of Australia; 2015. Available from: http://www.pbs.gov.au/publication/schedule/2015/06/2015-06-01-general-schedule.pdf. Accessed December 2, 2015. | ||
American Society of Health-System Pharmacists. Handbook on Injectable Drugs. 18th ed. Bethesda (MD): ASHP; 2014. | ||
Forist AA, Brown LW, Royer ME. Acid stability of lincomycin. J Pharm Sci. 1965;54(3):476–477. | ||
Walker SE, Iazzetta J, Law S, Biniecki K. Stability of commonly used antibiotic solutions in an elastomeric infusion device. Can J Hosp Pharm. 2010;63(3):212–224. | ||
Catena E, Perez G, Sadaba B, Azanza JR, Campanero MA. A fast reverse-phase high performance liquid chromatographic tandem mass spectrometry assay for the quantification of clindamycin in plasma and saliva using a rapid resolution package. J Pharm Biomed Anal. 2009;50(4):649–654. | ||
Medicines and Healthcare Products Regulatory Agency. British Pharmacopoeia 2014. London: Stationery Office; 2013. | ||
US Pharmacopeial Convention. US Pharmacopeia National Formulary (USP37–NF32). Rockville (MD): US Pharmacopeia National Formulary; 2014. | ||
Herr RR, Slomp G. Lincomycin. II. Characterization and gross structure. J Am Chem Soc. 1967;89(10):2444–2447. |
 © 2016 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2016 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.