Back to Journals » Drug Design, Development and Therapy » Volume 10
Stability of apomorphine in solutions containing selected antioxidant agents
Authors Ang ZY, Boddy M, Liu Y, Sunderland B
Received 9 July 2016
Accepted for publication 9 August 2016
Published 3 October 2016 Volume 2016:10 Pages 3253—3265
DOI https://doi.org/10.2147/DDDT.S116848
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 2
Editor who approved publication: Professor Frank Boeckler
Video abstract presented by Zen Yang Ang.
Views: 497
Zen Yang Ang, Michael Boddy, Yandi Liu, Bruce Sunderland
School of Pharmacy, Faculty of Health Sciences, Curtin University, Bentley, WA, Australia
Abstract: Apomorphine in solution undergoes rapid autoxidation, producing greenish colored solutions, making it difficult to formulate as a stable pharmaceutical solution. To identify the optimum antioxidant agent/combination for apomorphine solution, a high performance liquid chromatography assay was used to study the stability of 50 µg/mL apomorphine HCl in 0.1% L-ascorbic acid (AA), 0.1% sodium metabisulfite (SMB), 0.1% EDTA, and in selected combinations at 25°C, 32°C, and 37°C over a period of 14 days. The stability of apomorphine HCl (10 mg/mL) in 0.1% AA solution and in 0.1% EDTA solution at 25°C and 37°C was also evaluated. Apomorphine HCl solution (50 µg/mL) in 0.1% AA plus 0.1% SMB solution retained 99.7% (at 25°C) and 95.9% (at 37°C) of the initial concentration, as 0.1% AA plus SMB solution minimized the reactive oxygen content in solution which, in turn, reduced the oxidation rate of apomorphine HCl, and there was no green coloration perceptible. Conversely, apomorphine HCl solution (50 µg/mL) in 0.1% SMB solution was unstable as only 0.53% (at 25°C) and 0.06% (at 37°C) of the initial concentration was retained after 14 days. All 10 mg/mL apomorphine HCl samples were stable in both studies. The initial concentration of apomorphine HCl solution markedly affected its rate of oxidation and discoloration. The addition of 0.1% AA to a current formulation of apomorphine HCl injection (Apomine®), which contains SMB as an antioxidant, was recommended as providing the most stable solution.
Keywords: apomorphine HCl, oxidation, ascorbic acid, EDTA, sodium metabisulfite, HPLC
Introduction
Apomorphine is a basic derivative of morphine formed by its acid-catalyzed rearrangement.1,2 The chemical structure of apomorphine was reported3 more than a century after its first synthesis.1 It is a nonselective dopamine D1 and D2 receptor agonist, which is utilized as rescue medication in late stage Parkinson’s disease.4 Nevertheless, the compound is well-known for its instability in aqueous solution. In the presence of light and air, it spontaneously forms oxoapomorphine producing a green coloration. Garrido et al5 studied the effect of pH on the anodic oxidation of apomorphine reporting a complex mechanism. In highly acidic media oxidation of catechol occurred, whereas above pH 3 oxidation of the tertiary amine occurred. Above pH 6, apomorphine in equilibrium with oxoapomorphine was oxidized. Recently, Udvardy et al1 suggested that below pH 7 oxoapomorphine was the main degradation product with a quinone formed in alkaline media. At pH 6.4, they identified oxoapomorphine as the major degradation product with a phenanthrene as a minor (7%) additional product, which was brownish yellow in color. An earlier study of the kinetics of this reaction showed a marked increase in the rate of oxidation in solution from pH 5.2 to 6.8.6 Oxidative instability has become a barrier to the formulation of apomorphine, particularly in aqueous solution.1
Although the mechanism of autoxidation has been studied extensively in recent years, there are only a few studies that have investigated formulation factors aimed to improve the stability of apomorphine in aqueous solution.7–9 For example, Wilcox et al9 identified that the addition of ascorbic acid or sodium metabisulfite (SMB) to apomorphine solution, at room temperature, could only significantly delay oxidation of apomorphine in solution for less than 1–3 days. The author also established that a higher concentration of antioxidant and lower storage temperature could also significantly reduce the rate of oxidation of apomorphine. However, the study had an information gap where the sample solutions were not buffered and color changes of the sample solutions were not reported. The initial pH of the sample solution could affect the rate of degradation of apomorphine, and a color change in solution was an important indication of instability of the solution. Similarly, Ng Ying Kin et al7 found that 1 mg/mL apomorphine solution including 0.125% SMB was stable for more than 6 months at 4°C. In addition, they reported that apomorphine solution was less stable at a lower concentration (0.1 mg/mL), despite the addition of SMB and storage at low temperature (ie, 4°C), suggesting that SMB, which is a common antioxidant added to stabilize apomorphine injection formulation available on the market, might not be effective in preventing the degradation of apomorphine in solution.
Owing to limited data available from previous studies, a greater understanding of the oxidative degradation of apomorphine in the presence of antioxidants is necessary because it would contribute to improved formulations of apomorphine, which in turn increases the utilization and safety of this drug. This study aims to evaluate the stability of apomorphine HCl at standard and diluted concentrations in selected antioxidant systems in aqueous solution over a specific time period in order to identify the most effective antioxidant agent or antioxidant combination to minimize oxidation without any color change.
Materials and methods
Chemicals and reagents
Apomorphine HCl, purchased from Professional Compounding Center of America ([purity – minimum 99%] Houston, TX, USA), was used throughout the study. L-ascorbic acid (AA) was supplied by Thermo Fisher Scientific (Waltham, MA, USA), EDTA was obtained from Sigma-Aldrich Co. (St Louis, MO, USA), and SMB was purchased from Mallinckrodt Inc (Phillipsburg, NJ, USA). High performance liquid chromatography (HPLC) grade acetonitrile and methanol were obtained from Sigma-Aldrich Co. Water used throughout the study was prepared using a Milli-Q water purification system (EMD Millipore, Billerica, MA, USA).
Synthesis and extraction of 6-methyl-5, 6-dihydro-4H-dibenzo[de,g]quinoline-10, 11-dione (oxoapomorphine)
The procedures described by Abarca et al10 were adapted for the production and extraction of oxoapomorphine. Briefly, 300 mg of apomorphine HCl was dissolved in 50 mL of water and mixed with 5 mL of glacial acetic acid before the mixture was cooled in an ice bath for 2 hours. Subsequently, 40 mL of 0.1 M aqueous solution of potassium dichromate was added and the mixture formed a dark green/blackish color. Dichloromethane was added to extract the mixture and the organic layer was bluish in color. The organic layer was neutralized with adequate sodium bicarbonate, dried with anhydrous sodium sulfate, filtered and evaporated with a Rotavapor® R-210 (Büchi Labortechnik AG, Flawil, Switzerland) before it was purified using thin layer chromatography (TLC) (refer to the section “TLC for Compound 1 extraction” for the details of TLC procedures), giving a blue solid Compound 1. Compound 1 was then subjected to liquid chromatography–mass spectrometry (LC–MS) (refer to the section “LC–MS for apomorphine HCl and Compound 1 analysis” for the details of LC–MS procedures) in order to identify its molecular weight.
Instrumentation
HPLC for assay validation and stability studies
The HPLC system consisted of a Agilent HPLC 1100 Series isocratic LC system (Agilent Technologies, Santa Clara, CA, USA) with diode array detector employed for assay validation and stability studies of apomorphine HCl solution. Separation of analytes was performed using an Apollo HPLC column (octadecylsilane-C18; 5 μM; pore size: 100 Å; length: 150 mm; internal diameter 4.6 mm; W.R. Grace & Co., Columbia, MD, USA). The mobile phase consisted of 20% (v/v) acetonitrile and 80% aqueous solution of 50 mM orthophosphoric acid adjusted to pH 3.5 with 5 M sodium hydroxide. The HPLC was run with a flow rate of 1.0 mL/min and UV detection wavelength of 280 nm. The sample injection volume was 20 μL.
TLC for Compound 1 extraction
Alugram® TLC sheets (200×200×0.2 mm; aluminum foil, silica gel 60 layer, with fluorescence indicator UV254) and ethyl acetate:hexane (9:1) mixture (mobile phase) were employed for the separation of apomorphine HCl and its degradants, Compound 1 (blue solid) was scraped off the TLC plates and dissolved in dichloromethane before analysis by LC–MS.
LC–MS for apomorphine HCl and Compound 1 analysis
LC–MS 2020, using a single quad mass spectrometer (Shimadzu, Kyoto, Japan) assay was developed for apomorphine HCl and Compound 1 at ambient temperature. Optimized mass spectra were acquired with an interface voltage of 4.5 kV, a detector voltage of 1 kV, a heat block temperature of 400°C, and a desolvation gas temperature of 250°C. Nitrogen was used as nebulizer gas at a flow rate of 1.5 L/min and dry gas flow rate of 10 L/min.
Assay validation
Specificity
The specificity/stability indicating assay was modified from the procedures of earlier studies by Priston and Sewell11 and Ingram et al.12 There were two sets of samples consisting of 2.5 mL of (100 μg/mL) apomorphine HCl solutions which included: 1) 1 mL of 0.1 M NaOH; 2) 1 mL of 0.1 M HCl; 3) 1 mL of 6% H2O2; and 4) 1 mL of water (as control), respectively. Each sample was diluted to 5 mL with water, sealed in a volumetric flask and incubated at 25°C (water bath), and analyzed after 10 and 60 minutes’ exposure. Another two sets of samples were prepared and the study was repeated at 45°C (water bath). Mean residual concentration (μg/mL) and mean residual percentage of duplicate sets of samples were determined. Discoloration of the samples was also observed by the naked eye against a white background and recorded. The chromatograms were reviewed to ensure the analyte peak was pure.2
Linearity
Solution A (0.1% EDTA and 0.15% AA), as used by Ingram et al,12 was adapted for use in the preparation of standard solutions of apomorphine HCl (100 μg/mL). The 100 μg/mL solution was then used to prepare a set of standard solutions with concentrations of 5, 10, 20, 40, 50, 60, and 80 μg/mL. Each was analyzed in triplicate to obtain peak areas. Solution A was employed as the blank. Linearity of the standard plot was determined via least-squares regression analysis. The mean relative error of the observed versus calculated peak area and the mean value of relative standard deviations (RSDs) of the peak areas were calculated.2
Range
To assess the range of the method, the data from the standard curve were utilized to calculate the mean value of RSDs for each concentration involved in linearity assay. The accepted mean RSD was <3%.2
Precision – repeatability
Quality control (QC) standards of 50 μg/mL were freshly prepared daily and analyzed to evaluate intra-day and inter-day precision of the assay. The intra-day precision was determined by analyzing ten samples from freshly prepared QC solutions while the inter-day precision was obtained by analyzing QC solutions (in quadruplicate), which were freshly prepared daily for 10 days. The mean value of observed concentration, standard deviation, RSD, and the mean value of retention times for the intra-day and inter-day QC standards were calculated.2
Limit of detection and limit of quantitation
The limit of detection (LOD) was defined as the value of standard deviation of y-intercept of the regression line (σ)/slope (S) multiplied by 3.3 (ie, 3.3 σ/s) while the limit of quantitation (LOQ) was defined as the value of standard deviation of y-intercept of the regression line (σ)/Slope (S) multiplied by 10 (ie, 10 σ/s).2,13,14 The LOD and LOQ were calculated from the data obtained from standard curve.
Stability studies
Stability of apomorphine HCl in antioxidant solutions
The stability of 1) apomorphine HCl solution without antioxidant; 2) apomorphine HCl in 0.1% AA solution; 3) apomorphine HCl in 0.1% SMB solution; 4) apomorphine HCl in 0.1% EDTA solution; 5) apomorphine HCl in 0.1% AA plus 0.1% SMB solution; 6) apomorphine HCl in 0.1% AA plus 0.1% EDTA solution; and 7) apomorphine HCl in 0.1% SMB plus 0.1% EDTA solution were evaluated stored in sealed volumetric flasks at 25°C, 32°C, and 37°C, respectively in a water bath (±0.2°C) under typical laboratory conditions of light exposure. Solutions containing different antioxidants were adjusted to pH 4.0 using 0.01 M acetate buffer using a standardized pH meter (H8519N; Hanna Instruments, Woonsocket, RI, USA) before they were used in preparation of the sample solutions, which contained an initial concentration of 50 μg/mL apomorphine HCl. All of the samples were prepared in duplicate and analyzed immediately after preparation and subsequently sampled after 0.5, 1, 2, 4, 8, 24, 48, 72, 96, 168, and 336 hours, respectively. Each of the sample solutions was analyzed twice by HPLC, and the mean reading for the particular sample was determined.
The color of the samples was also observed every 24 hours while the pH of the samples was measured at the end of the kinetic runs. In order to be defined as a stable apomorphine HCl solution in this study (“Stability of apomorphine HCl in antioxidant solutions” section), the following criteria needed to be fulfilled: 1) the mean residual concentration (%) of both sets of samples, at the end of the study period, was within 95%–105% of the initial concentration of the samples and 2) no discoloration occurred in both sets of sample solutions that was visible to the naked eye, against a white background.
Stability of 10 mg/mL and 50 μg/mL (with and without acetate buffer) apomorphine HCl in 0.1% SMB solutions (purged with nitrogen)
To determine the effect of different initial concentrations of apomorphine HCl on the rate of degradation, two sets of 10 mg/mL apomorphine HCl solutions in 0.1% SMB were buffered to pH 4.00 using acetate buffer, while another two sets were prepared without buffer. Note that all of the samples described in this section were prepared using nitrogen-bubbled (60 minutes) water and the headspace was flushed with nitrogen in order to imitate apomorphine HCl injection (Apomine®) which is available commercially. Another two 50 μg/mL apomorphine HCl in 0.1% SMB solutions were prepared using the same method as above to imitate a diluted apomorphine HCl injection. All of the sample solutions were sealed in volumetric flasks and evaluated at 25°C in a water bath (±0.2°C) under typical laboratory conditions of light exposure. The stability of the sample solutions was analyzed immediately after preparation as well as after 24, 48, 72, and 168 hours. Each sample solution was analyzed twice by HPLC, and the mean reading for the particular sample was determined. The color and the pH of the sample solutions were observed and recorded after 168 hours. A stable apomorphine HCl solution occurred when the two criteria were fulfilled as defined in the “Stability of apomorphine HCl in antioxidant solutions” section.
Stability of apomorphine HCl solutions (10 mg/mL; without nitrogen purging) in 0.1% AA solution and 0.1% EDTA solutions
Two sets of 10 mg/mL apomorphine HCl solution, 0.1% AA solution (adjusted to pH 4.00 using acetate buffer) and two sets of 10 mg/mL in 0.1% EDTA solution (adjusted to pH 4.00 using acetate buffer) were prepared in sealed volumetric flasks and equilibrated at 25°C and 37°C, respectively, in a water bath (±0.2°C) under typical laboratory conditions of light exposure for 72 hours. To be defined as a stable apomorphine HCl solution in this study (in this section), the particular sample had to fulfill the criteria as defined in “Stability of apomorphine HCl in antioxidant solutions” section.
Results
LC–MS for apomorphine HCl and Compound 1 analysis
As indicated in Figure 1A and B, retention times for apomorphine HCl and Compound 1 were 1.400 minutes and 2.279 minutes, respectively, while m/z for apomorphine HCl and Compound 1 were found to be 268 and 264, respectively.
  | Figure 1 Chromatographs and mass spectra produced by liquid chromatography–mass spectrometry. |
Assay validation
Specificity
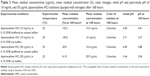
The data from specificity/stability indicating assay (Table 1) show that apomorphine HCl degraded rapidly in 0.02 M sodium hydroxide and produced a brown colored solution. Addition of hydrogen peroxide also significantly increased the degradation rate although the rate was slower compared to forced degradation in sodium hydroxide. Apomorphine HCl solutions showed similar stability in 0.02 M hydrochloric acid compared to the control solution (apomorphine HCl in water).
As shown in Figure 2A–D, the peak of apomorphine HCl and the peaks of its degradants could be distinguished clearly in the chromatograms produced from the forced degradation of apomorphine HCl. This indicated that the conditions employed in this study could separate apomorphine HCl from its degradants.
Linearity
For a concentration range of 5–80 μg/mL, the standard curve of apomorphine HCl calibration solutions in diluent A was linear (y =61.613x+11.672; R2=0.9998; n=7). The mean relative error of the observed versus calculated peak areas was 0.80%, while the mean RSD of the observed peak areas was 0.39%.
Range
The mean value of RSDs for each concentration of the samples involved in the linearity assay was 0.39%, which was less than 3%.
Precision – repeatability
The mean (±standard deviation) and RSD for observed intra-day concentration (n=10) of the QC standards were 50.3±0.5 μg/mL of apomorphine HCl with an RSD of 0.97%. The mean (±standard deviation) and RSD for observed inter-day concentration (n=10) of the QC standards were 51.0±0.9 μg/mL with an RSD of 1.79%.
The mean (±standard deviation) and RSD for observed intra-day and inter-day retention times (n=10) of the QC standards were 2.63±0.01 minutes with an RSD of 0.33% and 2.63±0.01 minutes with an RSD of 0.21%. The RSDs for intra-day and inter-day concentration and retention times of the QC standards were within the acceptable range of ≤2%.15
LOD and LOQ
The LOD and LOQ values were 1.05 μg/mL and 3.18 μg/mL, respectively.
Stability studies
Studies on the stability of apomorphine HCl in antioxidant solutions
The natural logarithm (ln) of mean residual concentrations (%) of apomorphine HCl solutions in different antioxidant solutions against time at 25°C, 32°C, and 37°C is illustrated in Figures 3–5.
Figures 3–5 indicate that the rates of oxidation of apomorphine HCl in the sample solutions increased with temperature. Table 2 shows that all sample solutions changed color at the end of the study, with the exception of those in 0.1% AA plus 0.1% SMB and sample solutions in 0.1% SMB plus 0.1% EDTA (at 25°C only) as well as sample solutions in 0.1% AA plus 0.1% EDTA (at 25°C only) which remained colorless. The results also indicated that the discoloration of sample solutions intensified over the study time and with increased temperature. The studies in 0.1% AA did not follow first-order kinetics over the 336 hours of testing. Overall it was only the apomorphine in 0.1% AA plus 0.1% SMB solution at pH 4.00 that retained >95% of the initial concentration and remained colorless over the duration of the study.
It was also found that the pH of most of the sample solutions after 336 hours did not markedly change from the initial pH of 4.00. The pH readings of all samples indicated the pH remained confined within a tight range (pH 4.00±0.20) with the exception of apomorphine HCl in 0.1% SMB sample solutions at 32°C and 37°C, as well as apomorphine HCl in 0.1% AA plus 0.1% SMB sample solutions (at 37°C).
Although apomorphine HCl in 0.1% EDTA (ie, 99.20% at 25°C) fulfilled the first criterion of stability, it did not fulfill the second criterion as it turned light blue at the end of the study. Therefore, only apomorphine HCl in 0.1% AA +0.1% SMB was considered stable when compared with other antioxidant systems in this study. None of the apomorphine HCl solutions were stable when stored at 37°C at the end of the study period except apomorphine HCl in 0.1% AA +0.1% SMB.
On the other hand, apomorphine HCl was found to be very unstable in only 0.1% SMB solutions where the apomorphine HCl in all experimental temperatures was essentially undetected at the end of the study, followed by apomorphine HCl in 0.1% AA solutions where the final concentration was approximately one-half of its initial concentration.
Studies on stability of 10 mg/mL and 50 μg/mL (with acetate buffer and without buffer) apomorphine HCl in 0.1% SMB solutions purged with nitrogen
The color of all sample solutions remained unchanged after 168 hours. Table 3 and Figure 6 illustrate that both 10 mg/mL apomorphine HCl in 0.1% SMB solutions purged with nitrogen (ie, imitated apomorphine HCl injection) with buffer and without buffer, retained approximately 100% of their initial concentration. On the other hand, 50 μg/mL apomorphine HCl in 0.1% SMB solution with buffer (ie, imitated diluted apomorphine HCl injection) lost approximately 80% of the initial concentration while the 50 μg/mL sample solution without buffer retained approximately 60% of the initial concentration. Thus, the 10 mg/mL apomorphine HCl solutions (both with buffer and without buffer and nitrogen purged) were considered stable as the remaining concentrations were within 95%–105% of the initial concentration, and there was no discoloration after 168 hours. In contrast to the 10 mg/mL sample solutions, both 50 μg/mL sample solutions (nitrogen purged) with buffer and without buffer were defined as unstable.
Stability of apomorphine HCl solutions (10 mg/mL) in 0.1% AA solution and 0.1% EDTA solutions (without nitrogen purging)
Table 4 shows that the mean residual concentration (%) of apomorphine HCl in 0.1% AA solution (10 mg/mL) and apomorphine HCl in 0.1% EDTA solution (10 mg/mL) after 72 hours were within 95%–105% of the initial concentration at 25°C and 37°C. None of the 10 mg/mL sample solutions changed in color at the end of the study period and therefore were considered stable as defined by the section “Stability of apomorphine HCl in antioxidant solutions”.
Discussion
This study has investigated the influence of selected antioxidant systems on the stability of apomorphine in solution in order to select the optimum antioxidant system. Two concentrations were selected, which were the commonly formulated one of 10 mg/mL and a diluted one which could be given as an infusion and represents a more difficult concentration to preserve. Of all antioxidant systems investigated in the section “Stability of apomorphine HCl in antioxidant solutions”, 0.1% AA +0.1% SMB solution provided the only environment where, over the temperature range of 25°C to 37°C, the concentration of apomorphine was >95% and the solution remained colorless for 50 μg/mL apomorphine HCl solution. On the other hand, apomorphine HCl appeared to be least stable in 0.1% SMB solution in this study, to the extent that the stability profile of apomorphine HCl solution (without any antioxidant) was more stable than when formulated in 0.1% SMB. This may indicate either SMB acts as a catalyst for the reaction when alone or specifically reacts with apomorphine under specific conditions. Abarca et al10 have proposed that the autoxidation of apomorphine (m/z 268) was initiated by the transfer of two electrons and two protons from apomorphine to an oxygen molecule with the formation of an intermediate and H2O2 before the intermediate product was further oxidized by H2O2 into apomorphine o-quinone (oxoapomorphine; MW 263), which caused the characteristic greenish apomorphine aqueous solution. Compound 1 (m/z 264) matched the description by Abarca et al10 and this indicated that Compound 1 was very likely to be oxoapomorphine. An earlier study by Wilcox et al9 showed that the degradation of apomorphine HCl could be delayed by increasing the concentration of either AA or SMB in the solution. Nevertheless, Wilcox et al9 concluded that neither AA nor SMB could significantly delay the degradation for more than 1–3 days when the solutions were stored at room temperature. This means that concentration increments of antioxidant for a single agent antioxidant in solution could not significantly stabilize apomorphine HCl in solution. Therefore, it is proposed that the combination of 0.1% AA and 0.1% SMB is a superior antioxidant system for apomorphine HCl solution, compared to other antioxidants evaluated in this study. The antioxidant combination will provide additional antioxidant activity to the system. The most easily oxidized antioxidant would be expected to react with the dissolved oxygen in the system, leaving more antioxidant to minimize any oxidation of apomorphine, especially in the 50 mg/mL solution.
Decker et al8 indicated that apomorphine HCl solution (in 0.1% SMB solutions) turned yellow in color after being subjected to autoclaving at 120°C. The authors also suggested that yellow compound(s) might arise from the decomposition of apomorphine or a reaction between apomorphine and SMB.8 The results from the section “Stability of apomorphine HCl in antioxidant solutions” supported this description where the yellow colored 50 μg/mL apomorphine HCl solution (in 0.1% SMB solution) was first observed on Day 11, Day 5, and Day 3 at 25°C, 32°C, and 37°C, respectively. Thus, we propose that the yellowish or brownish compound formation in 50 μg/mL apomorphine HCl in 0.1% AA solution or 0.1% AA plus 0.1% EDTA solution may be attributed to either the degradant(s) of AA16,17 or a product of reactions between AA and apomorphine HCl. It could also be the phenanthrene degradation product of apomorphine reported by Udvardy et al.1 As expected, the rate of degradation of apomorphine HCl (in all antioxidant solutions) increased with temperature, as shown by Wilcox et al9 previously. Notably, apomorphine HCl 50 μg/mL in 0.1% SMB solutions was stable for less than 5 hours at all experimental temperatures.
Moreover, from Figures 3–5, it is notable that the 50 μg/mL apomorphine HCl in 0.1% AA did not follow first-order kinetics, where the rate of degradation of apomorphine slowed after 96 hours. This could be contributed to by a small 0.1 pH unit reduction or diminished oxygen in the sample solutions. Burkman6 reported an exponential decay between the rate of degradation of apomorphine and the pH of apomorphine solutions. Similarly, a pH-dependent degradation pattern of the 50 μg/mL apomorphine HCl in 0.1% SMB was also observed in the “Stability of 10 mg/mL and 50 μg/mL (with and without acetate buffer) apomorphine HCl in 0.1% SMB solutions (purged with nitrogen)” section where apomorphine was more stable at a lower pH, where the 50 μg/mL apomorphine HCl in 0.1% SMB without buffer was stabilized by a 0.4 pH unit reduction compared to its counterpart with buffer, which only had a 0.12 pH unit reduction.
Interestingly, the results from sections “Stability of 10 mg/mL and 50 μg/mL (with and without acetate buffer) apomorphine HCl in 0.1% SMB solutions (purged with nitrogen)” and “Stability of apomorphine HCl solutions (10 mg/mL; without nitrogen purging) in 0.1% AA solution and 0.1% EDTA solutions” showed that apomorphine HCl in all 10 mg/mL samples remained stable, where the concentration of sample solutions were within 95%–105% with no discoloration during the study period, when subjected to either 0.1% SMB solutions (purged with nitrogen), 0.1% AA solution (without nitrogen purging) or 0.1% EDTA solution (without nitrogen purging). As discussed in the “Introduction” section, the similar concentration-dependent degradation pattern was also observed by Ng Ying Kin et al,7 where the authors described that 0.1 mg/mL apomorphine HCl in 0.125% SBM solution retained approximately 50% of its initial concentration after 6 weeks (with discoloration of solution after 3 weeks) when incubated at 4°C, while the counterpart 1 mg/mL apomorphine HCl in 0.125% SBM solution retained at least 95% of its initial concentration under the same incubation conditions. Similarly, Priston and Sewell18 also found that 10 mg/mL apomorphine HCl formulated in 0.1% SMB did not change in color and pH value with 98% remaining at 8°C after 14 days and with 96% remaining at 37°C after 7 days. The results from Ng Ying Kin et al7 and Priston Sewell18 indicated that apomorphine HCl solution with a higher concentration was more stable than its counterpart with a lower concentration. On the other hand, it is postulated that the 10 mg/mL apomorphine HCl in 0.1% AA solution and the 10 mg/mL apomorphine HCl in 0.1% EDTA solution in the “Stability of apomorphine HCl solutions (10 mg/mL; without nitrogen purging) in 0.1% AA solution and 0.1% EDTA solutions” section might have changed color if the study period was prolonged. This postulation was based on the observation by Priston and Sewell,18 where 10 mg/mL apomorphine HCl formulated in 0.1% AA plus 0.05% EDTA solution darkened in color, changed in odor, and lowered in pH values over the 7-day incubation period at 8°C and 37°C. However, Ng Ying Kin et al7 suggested that apomorphine has an unpredictable concentration-dependent discoloration pattern where the concentration of apomorphine HCl solution may affect its timing of discoloration. Therefore, the exact timing of discoloration of sample solutions in the section “Stability of apomorphine HCl solutions (10 mg/mL; without nitrogen purging) in 0.1% AA solution and 0.1% EDTA solutions” can only be determined by a study with a longer timeframe in the future.
From the results of the “Stability of 10 mg/mL and 50 μg/mL (with and without acetate buffer) apomorphine HCl in 0.1% SMB solutions (purged with nitrogen)” section, SMB, which is the antioxidant used in imitated apomorphine HCl injection could not effectively prevent the degradation of apomorphine HCl in the formulation (even though the sample was purged with nitrogen), especially when the product was diluted, with yellow colored compound(s) forming during the decomposition. However, if the 10 mg/mL imitated apomorphine HCl injection (purged with nitrogen) was not diluted, it had a relatively low oxygen to drug ratio compared to its 50 μg/mL counterpart to oxygen in the stability study. It should be noted that the water which was used to prepare samples in the section “Stability of 10 mg/mL and 50 μg/mL (with and without acetate buffer) apomorphine HCl in 0.1% SMB solutions (purged with nitrogen)” contained low residual dissolved oxygen even after purging with nitrogen.19,20 Furthermore, some limited reaeration of the sample solutions was possible when samples were withdrawn for analysis. Nevertheless, the study has found that the combination of 0.1% AA and 0.1% SMB solution provided the optimum antioxidant environment to limit the degradation, including visible color formation, for apomorphine HCl, even at a temperature as high as 37°C. Therefore, the authors recommend the addition of 0.1% AA to the current formulation of apomorphine HCl injection if it is to be used in other formulations.
One of the limitations of this study is that the duration of the study period is short (3–14 days), limiting the results to be applicable only to cases where apomorphine solution/infusion is prepared for immediate issue to patients. Thus, the long-term stability of apomorphine HCl in 0.1% AA and 0.1% SMB needs to be evaluated for long-term storage so that ready-to-use apomorphine infusion preparations with a lower concentration could be formulated for long-term infusions.
Conclusion
A combination of 0.1% AA +0.1% SMB solution provided the most stable pharmaceutical environment for apomorphine HCl solution at a concentration 50 μg/mL, where samples remained stable for at least 14 days at all experimental temperatures with respect to retention of concentration and absence of color formation. This enables the apomorphine injection to be further diluted and given as slow intravenous infusion for controlled administration. Apomorphine HCl 50 μg/mL in 0.1% SMB solutions was stable for less than 5 hours at all experimental temperatures. Therefore, the addition of 0.1% AA to the current formulation of apomorphine HCl injection, which contains SMB as an antioxidant, is recommended if it is to be used as an additive. Notably, 10 mg/mL apomorphine HCl solution remained stable for at least 72 hours (as defined by the experimental protocol) when 0.1% SMB, 0.1% AA or 0.1% EDTA were employed in the solution. This suggests that the degradation rate and discoloration of apomorphine in different antioxidant solutions was affected by the initial concentration of apomorphine HCl in solution. Future research should establish longer term stability data, which could be utilized in the formulation of ready-to-use apomorphine infusion preparations with lower concentrations.
Acknowledgments
We thank the School of Pharmacy, Curtin University, Western Australia, for funding and providing the facilities for this research. We also would like to thank Dr Madhu Page-Sharp for providing assistance and advice in LC–MS analysis as well as Mr Liew Weng Heng and Ms Cheah Kit Yee for their contribution in statistical data analysis.
Disclosure
The authors report no conflicts of interest in this work.
References
Udvardy A, Gyulai Z, Sipos A. Extensive study of the autooxidation products of apomorphine and its pharmacologically active derivatives. J Mol Struct. 2011;1002(1–3):37–44. | ||
Shabir GA. Step-by-step analytical methods validation and protocol in the quality system compliance industry. J Valid Technol. 2005;10: 314–325. | ||
Giesecke J. The absolute configuration of apomorphine. Acta Cryst. 1977;B33:302–303. | ||
Subramony JA. Apomorphine in dopaminergic therapy. Mol Pharm. 2006;3(4):380–385. | ||
Garrido JM, Delerue-Matos C, Borges F, Macedo TR, Oliveira-Brett AM. New insights into the oxidation pathways of apomorphine. J Chem Soc. 2002;(10):1713–1717. | ||
Burkman AM. Some kinetic and thermodynamic characteristics of apomorphine degradation. J Pharm Sci. 1965;54:325–326. | ||
Ng Ying Kin NM, Lal S, Thavundayil JX. Stability of apomorphine hydrochloride in aqueous sodium bisulphite solutions. Prog Neuropsychopharmacol Biol Psychiatry. 2001;25(7):1461–1468. | ||
Decker WJ, Corby DG, Combs HF. A stable parenteral solution of apomorphine. Clin Toxicol. 1981;18(7):763–772. | ||
Wilcox RE, Humphrey DW, Riffee WH, Smith RV. Stability of apomorphine in solutions containing ascorbic acid and bisulfite and effects of antioxidants on apomorphine-induced cage climbing and hypothermia in mice. J Pharm Sci. 1980;69(8):974–976. | ||
Abarca B, Ballesteros R, Bielsa P, et al. Opposite vascular activity of (R)-apomorphine and its oxidised derivatives. Endothelium-dependent vasoconstriction induced by the auto-oxidation metabolite. Eur J Med Chem. 2003;38(5):501–511. | ||
Priston MJ, Sewell GJ. Novel liquid chromatographic assay for the low-level determination of apomorphine in plasma. J Chromatogr B Biomed Appl. 1996;681(1):161–167. | ||
Ingram WM, Priston MJ, Sewell GJ. Improved assay for R(-)-apomorphine with application to clinical pharmacokinetic studies in Parkinson’s disease. J Chromatogr B Analyt Technol Biomed Life Sci. 2006;831(1–2):1–7. | ||
Snyder LR, Kirkland JJ, Dolan JW. Introduction to Modern Liquid Chromatography. New Jersey: John Wiley & Sons; 2011. | ||
Shrivastava S, Gupta V. Methods for the determination of limit of detection and limit of quantitation of the analytical methods. Chronicles of Young Scientists. 2011;2(1):21–25. | ||
Chatrabhuji P, Pandya C, Patel M. HPLC Method for Determination of APIs in Pharmaceutical Formulation. USA: Lulu Press, Inc; 2015. | ||
Bouwman Y, Le Brun P. Practical Pharmaceutics: An International Guideline for the Preparation, Care and Use of Medicinal Products. United Kingdom: Springer; 2015. | ||
Kurata T, Sakurai Y. Degradation of L-ascorbic acid and mechanism of nonenzymic browning reaction: Part II. Non-oxidative degradation of L-ascorbic acid including the formation of 3-deoxy-L-pentosone Part III. Oxidative degradation of L-ascorbic acid (degradation of dehydro-L-ascorbic acid). Agric Biol Chem. 1967;31(2):170–184. | ||
Priston M, Sewell G. The analysis of apomorphine formulations for ambulatory infusions. Pharm Pharmacol Commun. 1995;1(2):91–94. | ||
Butler IB, Schoonen MA, Rickard DT. Removal of dissolved oxygen from water: a comparison of four common techniques. Talanta. 1994;41(2):211–215. | ||
Degenhardt OS, Waters B, Rebelo-Cameirao A, Meyer A, Brunner H, Toltl NP. Comparison of the effectiveness of various deaeration techniques. Dissolut Technol. 2004;11(1):6–12. |
 © 2016 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2016 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.