Back to Archived Journals » Open Access Animal Physiology » Volume 7
Social isolation is associated with reduced neurogenesis, impaired spatial working memory performance, and altered anxiety levels in male rats
Authors Famitafreshi H, Karimian M , Fanaei H, Attari F, Fatima S
Received 10 March 2015
Accepted for publication 15 April 2015
Published 16 June 2015 Volume 2015:7 Pages 87—95
DOI https://doi.org/10.2147/OAAP.S84327
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 4
Editor who approved publication: Dr Peter Koulen
Hamidreza Famitafreshi,1 Morteza Karimian,2 Hamed Fanaei,3 Fatemeh Attari,4 Sulail Fatima1
1Department of Physiology, International Campus, 2Department of Physiology, Tehran University of Medical Sciences, Tehran, 3Department of Physiology, Zahedan University of Medical Sciences, Zahedan, 4Department of Neuroscience, School of Advanced Technologies in Medicine, Tehran University of Medical Sciences, Tehran, Iran
Background: Social isolation has some adverse behavioral effects. It has been shown that neurogenesis is essential for improvement of behavioral function. The aim of this study was to examine the effects of social isolation on neurogenesis, brain-derived neutrotrophic factor levels, learning abilities, and anxiety levels in rats.
Methods: Twenty male Sprague-Dawley rats were randomly divided into two groups, ie, an isolated group and a socialized group. After a 7-day adaption period, the animals received intraperitoneal bromodeoxyuridine (BrdU) 50 mg/kg for 14 days. Two types of memories were examined: spatial working memory using the Morris water maze and short-term memory using the Y-maze. Anxiety levels were examined using the elevated plus maze. Neurogenesis was assessed by immunostaining brain sections with anti-BrdU antibody.
Results: Neurogenesis was significantly reduced in the isolated group (10 cells/400×) as compared with the socialized group (232 cells/400×). Memory performance was markedly reduced in isolated animals than in socialized animals (working memory 50.87 seconds vs 31.71 seconds; reference memory 55.44 seconds vs 39.73 seconds; and in probe trials 24.72 seconds vs 18.11 seconds). Y-maze performance remained unchanged between the two groups (71.41 seconds vs 64.97 seconds). Anxiety levels were reduced in isolated animals, as indicated by more time spent in the open arms (73 seconds vs 11 seconds) and a higher number of entries into the open arms (4.1 vs 1.4) of the elevated maze. BDNF levels decreased significantly more in the isolated group than in the socialized group (467±37.69 vs 370.7±12.19, P=0.0311).
Conclusion: These results show that social isolation has adverse effects on the hippocampus and learning abilities, and may make one more susceptible to brain diseases like depression and Alzheimer’s disease.
Keywords: adaptation, learning, anxiety, susceptible, BDNF, memory, neurogenesis
Introduction
Social isolation refers to a complete absence of or insufficient contact with other members of society. It is not the same as loneliness, which is rooted in a temporary lack of contact with other humans. Social isolation has two components, ie, mental and physical.1 Evidence that social isolation might be related to fundamental aspects of cognition comes from animal studies showing that isolation may impair learning and alter anxiety levels,1 latent inhibition,2 social behavior,3 performance on the forced swim test,4 and reversal learning.5
Brain-derived neurotrophic factor (BDNF) is one of the major neurotrophic factors, primarily supporting the growth and survival of cholinergic, dopaminergic, and motor neurons. BDNF is synthesized by sensory neurons and glia and may have both autocrine and paracrine functions to mediate activity-dependent plasticity. It is highly expressed in brain areas that are known to regulate cognitive and emotional behaviors, such as the hippocampus and amygdala.6 The dentate gyrus of the hippocampus is essential for memory formation and learning. The amygdala is involved in regulation of anxiety, decision-making, and memory.
Neurogenesis occurs predominantly in two regions of brain, ie, the subventricular zone of the lateral ventricles and the subgranular zone of the dentate gyrus of hippocampal formation.7 However, there is some evidence for neurogenesis occurring in the prefrontal cortex and parts of the limbic system.8,9 To date, it remains unknown if neurogenesis is essential for normal brain functioning or it only sets in motion under diseased states. However, it is quite evident that neurogenesis can improve brain function after stroke10 and in diseases such as Alzheimer’s disease and depression.11,12 Different types of memory are attributed to different regions of brain. Nevertheless, it is quite unclear if these different forms of memory are interconnected functionally and how neurogenesis can affect them. Imayoshi et al demonstrated that continuous neurogenesis is essential for the structural and functional integrity of the forebrain cortex.12 Keeping these observations in view, we designed this study to examine the effect of social isolation on three different forms of memory (short-term memory, reference memory, and working memory), anxiety, and neurogenesis.
Materials and methods
Animals
Twenty male Sprague-Dawley rats weighting 200–250 g were randomly divided into two groups, ie, an isolated group and a socialized group. Animals in the isolated group were housed individually in cages covered with black plastic. In the socialized group, animals were randomly paired and housed in transparent cages. Housing took place under a standard 12-hour day/night cycle at room temperature (22°C), and the animals had free access to water and chow ad libitum. After a 7-day adaption period, the animals received intraperitoneal bromodeoxyuridine (BrdU, 50 mg/kg) for 14 days. This was followed by performance of behavioral tests like the Morris water maze and the Y-maze tasks. At the end of the study, the animals were anesthetized and euthanized. The brains were fixed with paraformaldehyde 4%, sliced (40 mm), and stained with anti-BrdU antibody. BrdU-positive cells were counted under a light microscope.
The experimental protocols followed in this study were conformed to the Guidelines for the Care and Use of Laboratory Animals published by National Institutes of Health (NIH Publication No. 85-23, revised 1996) and was further approved by the institutional ethical committee at Tehran University of Medical Sciences (Tehran, Iran).
Assessment of working memory
The Morris water maze is widely used to study spatial working memory and learning. This apparatus consisted of a large water-filled circular tank (183 cm in diameter) coated with non-toxic black tempera paint. For the hidden platform task, an escape platform (10 cm diameter) was placed in the south-east quadrant of the maze and submerged 0.5 cm below the water surface. For the visible platform task, the platform protruded 2 cm above the surface of the water. A large red geometric shape that provided extra-maze visual cues completely surrounded the maze. Since the animals were placed in opaque water and the platform appeared unnoticeable, they had to rely on extra-maze cues. As the animals become more familiar with the task, they are able to find the platform more quickly.
Animals’ performance during the task was assessed using a video camera positioned above the maze which was in turn connected to a computerized tracking system. The task was carried out in 2 days, with two trials per day. On day 3, the probe trial was performed. The first trial was considered for the visible test. In our experiments, we tested two types of memory, ie, reference memory and working memory. The first trial of each session was considered for evaluating reference memory; however, the other trials (second, third, and fourth) assessed working memory.13
Visible test
The visible platform version of the Morris water maze test was used for two reasons: first, to assess nonspatial learning; and second, to rule out the possibility that the spatial learning deficit detected might actually be a product of deficient escape motivation or impairment of vision and/or motor skills. For this, the location of the platform was made visible by using a brightly striped flag rising above the water, so as to provide animals with a spatial cue for escaping from water. This simple associative nonspatial task is believed to be independent of hippocampal function.
Spatial acquisition test
In these trials, the rats were trained to find a hidden platform using extra-maze cues. A transparent Lucite platform (10×10 cm) was submerged beneath the surface of the water (0.5 cm) in the south-east quadrant of the tank. Each rat participated in 16 spatial trials with invariable start positions. For each trial, a 60-second time period was provided to reach the platform. If the animal had failed to reach the platform within 60 seconds, the experimenter placed the animal on the platform. A 20-second rest period was observed between each trial. Swimming time (seconds), distance (cm), and swim speed (seconds per cm) were recorded. For swimming time and distance, lower values were indicative of better performance, and vice versa for swimming speed.
Probe trial for spatial memory
To examine how well the rats had learnt the location of the platform, one probe trial was conducted for 60 seconds. During this trial, the platform was removed and made unavailable for 60 seconds. For time spent in the fourth quadrant and swim distance traveled up to the fourth quadrant, higher numbers were indicative of better performance in contrast with the swimming speed. These parameters are used for assessing reference memory.14
Assessment of short-term memory
Short-term memory performance was assessed by recording spontaneous alternation behavior using a Y-maze composed of three equally spaced arms placed at a 120° angle from each other. Each arm was 40 cm long, 30 cm high, and 15 cm wide, converging on a triangular central area with 15 cm at its longest axis. This test was used to assess short-term memory involving many parts of brain, including the hippocampus, basal forebrain, septum, and prefrontal cortex. Each rat was placed at the start of the Y-maze and then allowed to move freely. The sequence of each arm entry recorded manually (ie, ABCBCAACACBABCB). Spontaneous alternation behavior, which is regarded as a measure of spatial memory, was defined as entry into all three arms on consecutive choices in overlapping triplet sets (ie, ABC, ABA, CAB, and CBC). The percent spontaneous alternation behavior was calculated as the ratio of actual to possible alternations. Percent alternation = actual alternation (ie, ABC, CBA =6)/maximal alternation (ie, ABCBCABCABCACBA =15–2 = 13)×100 = (6/13)×100 = 46.15%, where maximal alternation is the total number of arms entered minus 2. The test was done once for each animal.15
Assessment of anxiety levels
The elevated plus maze was used for assessing anxiety levels. It consisted of a plus-shaped apparatus with two open and two closed arms, each with an open roof. The arms were elevated 67 cm from the floor. Each animal was placed in the center of the apparatus and then allowed to move freely in the four arms. This model is based on rodents’ aversion to open places. The number of entries to open arms and time spent in open arms were recorded. Reduced anxiety levels were indicated by more time spent and increased number of entries into open arms.15
Measurement of BDNF levels
Hippocampus samples were obtained on the last day of the experiment and were homogenized. The samples were then centrifuged (4°C, 10,000× g for 10 minutes) and the supernatant was collected. BDNF was measured using a commercial enzyme-linked immunosorbent assay kit (Promega, Madison, WI, USA).
Immunohistochemistry
After completion of the adaptation period, BrdU 50 mg/kg was injected for 14 days intraperitoneally. BrdU is an analog of the thymine base incorporated into the DNA of newly proliferated neurons in the dentate gyrus of the hippocampus. At the end of day 14, the animals were anesthetized with ketamine 100 mg/kg and xylazine 10 mg/kg, and then sacrificed. It shall be noted that ketamine was used as an anesthetic drug and xylazine as a sedative drug to prevent stress in the animals. After thoracotomy, all animals were first perfused with normal saline, and then with paraformaldehyde 4% via intracranial infusion. After fixation, the brains were removed from the skull. For the first 2 days, the brains were kept in phosphate-buffered saline (PBS) + paraformaldehyde 4%, and then on day 3, in sucrose 10% + paraformaldehyde 4% + PBS. Throughout day 4, the brains were kept in sucrose 20% + paraformaldehyde 4% + PBS, and for the rest of the days they were kept in sucrose 30% + paraformaldehyde 4% + PBS. Cryosections (30 μm) were prepared from the dentate gyrus of the hippocampal region. Immunohistochemistry was performed for ten sections from each brain, five of which were stained for BrdU-positive neurons with an anti-BrdU antibody kit (5-bromo-2′-dU Labeling and Detection Kit II; Roche, Mannheim, Germany). BrdU-positive cells in the dentate gyrus were counted under a light microscope (400×).16 It should be taken in account that stained cells were counted homogenously in both groups. Therefore, if other proliferating cells were also stained, they would be in the count for both groups.
Statistical analysis
The data were analyzed using Statistical Package for the Social Sciences version 14 (SPSS Inc., Chicago, IL, USA) and GraphPad Prism version 5 (GraphPad Software, La Jolla, CA, USA). An independent two-tailed samples t-test was performed for all experiments. Data are shown as the mean ± standard error of the mean, and P<0.05 was considered to be statistically significant.
Results
Morris water maze
Reference memory and working memory
Our results showed that reference memory and working memory performance was markedly improved in socialized animals as compared with isolated animals (55.44±1.816 sec vs 39.73±3.275 sec, P<0.0001; and 50.87±1.451 sec vs 31.71±1.965 sec, P<0.0001, respectively, Figure 1A and B).
Speed and distance
Our analysis showed that distance traveled by rats in was significantly greater in the isolated group in comparison with the socialized group (1,473±44.91 cm vs 1,088 ±51.61 cm; P≤0.0001, Figure 2A). Swimming speed was also significantly faster in the isolated animals than in the paired ones (27.04±0.7067 cm/sec vs 23.64±0.5395 cm/sec, P=0.0004, Figure 2B).
Probe trials
The socialized animals spent more time in the fourth quadrant than the isolated animals (24.72±2.502 sec vs 18.11±1.991 sec, P=0.0480, Figure 3A). In addition, swimming speed in the fourth quadrant was significantly faster in isolated animals as compared with socialized animals (36.28±4.040 cm/sec vs 27.88 cm/sec, P=0.0268, Figure 3B).
Elevated plus maze for evaluation of anxiety and stress
Isolated animals spent more time in the open arms than socialized animals (73.50±36.44 sec vs 11.70±4.077 sec, P<0.0449; Figure 4A). The number of entries into open arms was significantly lower in paired animals as compared with isolated animals (4.167±1.662 times vs 1.400±0.3712 times, Figure 4B).
Short-term memory involving prefrontal Y-maze for evaluation of cortex
No significant differences were observed between the isolated group and the socialized group (71.4±9.009 sec vs 64.97±8.953 sec, P=0.618, Figure 5).
  | Figure 5 Number of right choices in Y-maze (n=10). |
BDNF levels
Our results showed that BDNF levels were significantly lower in the hippocampus of the isolated group as compared with the socialized group (467±37.69 pg/mg vs 370.7±12.19 pg/mg, P=0.0311, Figure 6).
  | Figure 6 BDNF levels in isolated and socialized group (n=8). |
Neurogenesis
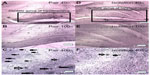
The number of BrdU-positive cells in the dentate gyrus of the hippocampus was significantly lower in the isolated group when compared with the socialized group (232.2±34.04 cells vs 10.00±2.517 cells, P≤ 0.0001, Figures 7 and 8).
Discussion
In the current study, we examined the effect of social isolation on memory, neurogenesis, and anxiety levels. We assessed reference memory and working memory using the Morris water maze.13 Both reference and working memory appeared to decline in isolated rats. In addition, working memory in the probe trial also declined in isolated rats. For short-term memory, we used the Y-maze and found that memory performance remained unaffected in the two groups. For assessment of anxiety levels, we used the elevated plus maze and found that anxiety levels declined in the isolated group. Furthermore, we found that neurogenesis was significantly reduced in the isolated group as compared with the socialized group.
Loneliness and social isolation are two related but distinct states. Loneliness is often described as a subjective feeling of isolation, not belonging, or lacking companionship.17,18 Steptoe et al considers loneliness as the psychological embodiment of social isolation.19 Nicholson defines social isolation as a state in which the individual lacks a sense of belonging socially, lacks a sense of engagement with others, has a minimal number of social contacts, and is deficient in fulfilling relationships.20 Other researchers define social isolation in a more structured manner, describing it as an objective and quantifiable reflection of a paucity of one’s social contacts.19 Previous studies have shown that social isolation impairs brain functions like learning and memory.1 Interestingly, it has been also associated with a higher incidence of Alzheimer’s disease.21 Keeping in view these findings, we designed a socialized and isolated model in rats for evaluating the effects of socialization and isolation on memory and learning.
Continuous neurogenesis is essential for the structural and functional integrity of the forebrain cortex.22 However, it remains unclear if neurogenesis occurs normally or is activated in the diseased state. It is quite evident that the hippocampus is involved in memory formation and neurogenesis is essential for the process. To illustrate the effects of social isolation on brain functions, we performed some brain function tests, such as for working memory, reference memory, and anxiety levels. The Morris water maze showed that isolation impairs memory storage and memory formation, thereby impairing normal brain functioning. Poor memory performance may be associated with dysfunction of inhibitory circuits in the dentate gyrus.23 It can be speculated that reduced neurogenesis may cause this dysfunction and thereby result in poor memory performance. Furthermore, social isolation can cause apoptosis of hippocampal cells, specifically hippocampal interneurons and decline in memory performance.24,25 Another anticipated cause of memory decline can be attributed to an increase in microglia cells.26 However, galanin, an inhibitory peptide, has been shown to lift up memory disturbances.27 In our study, reduced neurogenesis impaired both reference and working memory. In addition, socialization improved speed of swimming in spatial task acquisition trials, which is indicative of improved emotional reactivity and a better prognosis. It seems that emotional state can modulate learning involving the amygdala. Serotonergic receptors in the amygdala and prefrontal cortex change with emotional state.28
In this study, we performed the Y-maze test to assess short-term memory performance involving the prefrontal cortex. Our results show that isolation-induced reduction in neurogenesis may not affect the memory function of the prefrontal cortex, as indicated by intact short-term memory in the Y-maze task. Other disabilities, such as in motor, language, and visuospatial skills, may also accompany memory impairment. Personality changes, such as decreased energy, indifference, impulsivity, and irritability may be associated with memory impairment. Thus, decreased neurogenesis may lead to memory decline, which can also be accompanied by compromise of other brain functions. It has been shown that neurogenesis is not involved in all types of learning.29 Similarly, our results from the Y-maze showed no significant short-term memory decline in isolated rats.
In this study, we performed the elevated plus maze to assess if other brain functions are also affected by decreased neurogenesis. Our results show that isolation impairs the ability of the amygdala to regulate anxiety level. This was evident by an increased number of entries into the open arms and more time spent in the open arms. Likewise, a study in transgenic rats indicated increased anxiety-like behavior in rats with deficient neurogenesis.30 Yet, it remains unclear if neurogenesis occurs in the amygdala or hippocampal neurogenesis directs proper functioning of the amygdala. Chau et al demonstrated the role of the amygdala in facilitating neuroplasticity-mediated learning.31 It has been shown that the lowest anxiety levels are associated with intermediate levels of neurogenesis in rodents. Furthermore, it has demonstrated that exercise increases neurogenesis.32 The role of the amygdala in regulating neurogenesis is also quite evident.33 According to our results, isolated rats showed less prominent anxiety-like behavior on day 14 of the study. There may be two possible reasons for this: involvement of brain areas other than the amygdala and acclimation of the animals to the stressful environment.
Font et al has shown an association between angiogenesis, neurogenesis, and neuroplasticity.10 Further studies assessing neuroplasticity in the hippocampus during isolation and other brain regions such as the prefrontal cortex are recommended.
Studies have documented the molecular mechanisms responsible for the pathophysiological and neurochemical changes seen during social isolation. Haj-Mirzaian et al showed that N-methyl-D-aspartate (NMDA) receptors are involved in isolation-induced depression, and blockade of these receptors can reverse the depressant effects in mice.34 It has been demonstrated that the NR2B subunit of the NMDA receptor is responsible for hippocampal long-term potentiation that occur as chronic visceral pain. So if we consider anxiety and depression as learning antagonists of NMDA can decrease depression and anxiety.35 Furthermore, NMDA antagonist (MPEP) has been shown to improve anxiety and depression without having any adverse effects on the cortex, but antagonist of NMDA do not have such effects.36 Furthermore, blockade of GluN2B-containing NMDA receptors can show a more favorable side effect profile.37
In this study, BDNF levels were decreased in the isolated group. Decreased BNDF levels have been linked to reduced hippocampal volume38 and faster cognitive decline in Alzheimer’s disease.39 Change in corticosterone and insulin receptor density during memory impairment has been reported.40 Reduced neurogenesis can be the result of limited angiogenesis. Downregulation of angiogenic factors such as erythropoietin and vascular endothelial growth factor is the primary suspected cause of diminished angiogenesis.10 Moreover, increased calcium ions and astrocytes are also implicated as affecting neurogenesis.41 Recruitment and integration of newly generated neurons from neural stem cell niches is essential for neurogenesis and may be affected by vascular endothelial growth factor and other growth factors.42 In this study, neurogenesis was reduced in isolated animals. However, newly proliferated neurons may have migrated to regions like the amygdala. Hence, reduced neurogenesis alone is inadequate to cause impaired function of other brain regions, such as the prefrontal cortex. Further investigations for evidence of neurodegenerative diseases such as Alzheimer’s disease are recommended. Future studies can be based on ways to overcome social isolation and its consequences.
Conclusion
This study suggests that social isolation can affect working memory and reference memory, neurogenesis, and anxiety levels. Furthermore, isolation can affect cognitive processes and higher brain functions. Thus, preventing social isolation can inhibit memory deterioration and improve neurogenesis, thereby reducing cognitive deficits in patients with Alzheimer’s disease.
Acknowledgments
This research was funded by a doctoral grant from Tehran University of Medical Sciences. The authors thank Yaser Azizi for his help with preparation of the manuscript.
Disclosure
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
References
Huang HJ, Liang KC, Ke HC, Chang YY, Hsieh-Li HM. Long-term social isolation exacerbates the impairment of spatial working memory in APP/PS1 transgenic mice. Brain Res. 2011;1371:150–160. | |
Han X, Li N, Xue X, Shao F, Wang W. Early social isolation disrupts latent inhibition and increases dopamine D2 receptor expression in the medial prefrontal cortex and nucleus accumbens of adult rats. Brain Res. 2012;1447:38–43. | |
Shoji H, Mizoguchi K. Aging-related changes in the effects of social isolation on social behavior in rats. Physiol Behav. 2011;102:58–62. | |
Hong S, Flashner B, Chiu M, ver Hoeve E, Luz S, Bhatnagar S. Social isolation in adolescence alters behaviors in the forced swim and sucrose preference tests in female but not in male rats. Physiol Behav. 2011;105:269–275. | |
Han X, Wang W, Xue X, Shao F, Li N. Brief social isolation in early adolescence affects reversal learning and forebrain BDNF expression in adult rats. Brain Res Bull. 2011;86:173–178. | |
Ichisaka S, Katoh-Semba R, Hata Y, Ohshima M, Kameyama K, Tsumoto T. Activity-dependent change in the protein level of brain-derived neurotrophic factor but no change in other neurotrophins in the visual cortex of young and adult ferrets. Neuroscience. 2003;117:361–371. | |
Ming GL, Song H. Adult neurogenesis in the mammalian central nervous system. Annu Rev Neurosci. 2005;28:223–250. | |
Vessal M, Darian-Smith C. Adult neurogenesis occurs in primate sensorimotor cortex following cervical dorsal rhizotomy. J Neurosci. 2010;30:8613–8623. | |
Lieberwirth C, Liu Y, Jia X, Wang Z. Social isolation impairs adult neurogenesis in the limbic system and alters behaviors in female prairie voles. Horm Behav. 2012;62:357–366. | |
Font MA, Arboix A, Krupinski J. Angiogenesis, neurogenesis and neuroplasticity in ischemic stroke. Curr Cardiol Rev. 2010;6:238–244. | |
Rodriguez JJ, Verkhratsky A. Neurogenesis in Alzheimer’s disease. J Anat. 2011;219:78–89. | |
Sahay A, Hen R. Adult hippocampal neurogenesis in depression. Nat Neurosci. 2007;10:1110–1115. | |
Anisman H, McIntyre DC. Conceptual, spatial, and cue learning in the Morris water maze in fast or slow kindling rats: attention deficit comorbidity. J Neurosci. 2002;22:7809–7817. | |
Tanda K, Nishi A, Matsuo N, et al. Abnormal social behavior, hyperactivity, impaired remote spatial memory, and increased D1-mediated dopaminergic signaling in neuronal nitric oxide synthase knockout mice. Mol Brain. 2009;2:19. | |
Vila-Luna S, Cabrera-Isidoro S, Vila-Luna L, et al. Chronic caffeine consumption prevents cognitive decline from young to middle age in rats, and is associated with increased length, branching, and spine density of basal dendrites in CA1 hippocampal neurons. Neuroscience. 2012;202:384–395. | |
Spritzer MD, Ibler E, Inglis W, Curtis MG. Testosterone and social isolation influence adult neurogenesis in the dentate gyrus of male rats. Neuroscience. 2011;195:180–190. | |
Perissinotto CM, Stijacic Cenzer I, Covinsky KE. Loneliness in older persons: a predictor of functional decline and death. Arch Intern Med. 2012;172:1078–1083. | |
Luo Y, Hawkley LC, Waite LJ, Cacioppo JT. Loneliness, health, and mortality in old age: a national longitudinal study. Soc Sci Med. 2012;74:907–914. | |
Steptoe A, Shankar A, Demakakos P, Wardle J. Social isolation, loneliness, and all-cause mortality in older men and women. Proc Natl Acad Sci U S A. 2013;110:5797–5801. | |
Nicholson NR Jr. Social isolation in older adults: an evolutionary concept analysis. J Adv Nurs. 2009;65:1342–1352. | |
Wilson RS, Krueger KR, Arnold SE, et al. Loneliness and risk of Alzheimer disease. Arch Gen Psychiatry. 2007;64:234–240. | |
Imayoshi I, Sakamoto M, Ohtsuka T, et al. Roles of continuous neurogenesis in the structural and functional integrity of the adult forebrain. Nat Neurosci. 2008;11:1153–1161. | |
Hazra A, Gu F, Aulakh A, Berridge C, Eriksen JL, Ziburkus J. Inhibitory neuron and hippocampal circuit dysfunction in an aged mouse model of Alzheimer’s disease. PLoS One. 2013;8:e64318. | |
Filipovic D, Zlatkovic J, Inta D, Bjelobaba I, Stojiljkovic M, Gass P. Chronic isolation stress predisposes the frontal cortex but not the hippocampus to the potentially detrimental release of cytochrome c from mitochondria and the activation of caspase-3. J Neurosci Res. 2011;89(9):1461–1470. | |
Filipovic D, Zlatkovic J, Gass P, Inta D. The differential effects of acute vs chronic stress and their combination on hippocampal parvalbumin and inducible heat shock protein 70 expression. Neuroscience. 2013;236:47–54. | |
Wu LJ, Stevens B, Duan S, MacVicar BA. Microglia in neuronal circuits. Neural Plast. 2013;2013:586426. | |
Crawley JN. Galanin impairs cognitive abilities in rodents: relevance to Alzheimer’s disease. Cell Mol Life Sci. 2008;65:1836–1841. | |
van der Staay FJ, Schuurman T, van Reenen CG, Korte SM. Emotional reactivity and cognitive performance in aversively motivated tasks: a comparison between four rat strains. Behav Brain Funct. 2009;5:50. | |
Shors TJ, Townsend DA, Zhao M, Kozorovitskiy Y, Gould E. Neurogenesis may relate to some but not all types of hippocampal-dependent learning. Hippocampus. 2002;12:578–584. | |
Revest JM, Dupret D, Koehl M, et al. Adult hippocampal neurogenesis is involved in anxiety-related behaviors. Mol Psychiatry. 2009;14:959–967. | |
Chau LS, Galvez R. Amygdala’s involvement in facilitating associative learning-induced plasticity: a promiscuous role for the amygdala in memory acquisition. Front Integr Neurosci. 2012;6:92. | |
Fuss J, Ben Abdallah NM, Hensley FW, Weber KJ, Hellweg R, Gass P. Deletion of running-induced hippocampal neurogenesis by irradiation prevents development of an anxious phenotype in mice. PLoS One. 2010;5(9):pii e12769. | |
Kirby ED, Friedman AR, Covarrubias D, et al. Basolateral amygdala regulation of adult hippocampal neurogenesis and fear-related activation of newborn neurons. Mol Psychiatry. 2012;17:527–536. | |
Haj-Mirzaian A, Amiri S, Kordjazy N, et al. Blockade of NMDA receptors reverses the depressant, but not anxiogenic effect of adolescence social isolation in mice. Eur J Pharmacol. 2015;750C:160–166. | |
Chang CH, Hsiao YH, Chen YW, Yu YJ, Gean PW. Social isolation-induced increase in NMDA receptors in the hippocampus exacerbates emotional dysregulation in mice. Hippocampus. 2015;25:474–485. | |
Inta D, Filipovic D, Lima-Ojeda JM, et al. The mGlu5 receptor antagonist MPEP activates specific stress-related brain regions and lacks neurotoxic effects of the NMDA receptor antagonist MK-801: significance for the use as anxiolytic/antidepressant drug. Neuropharmacology. 2012;62:2034–2039. | |
Lima-Ojeda JM, Vogt MA, Pfeiffer N, et al. Pharmacological blockade of GluN2B-containing NMDA receptors induces antidepressant-like effects lacking psychotomimetic action and neurotoxicity in the perinatal and adult rodent brain. Prog Neuropsychopharmacol Biol Psychiatry. 2013;45:28–33. | |
Erickson KI, Prakash RS, Voss MW, et al. Brain-derived neurotrophic factor is associated with age-related decline in hippocampal volume. J Neurosci. 2010;30:5368–5375. | |
Laske C, Stellos K, Hoffmann N, et al. Higher BDNF serum levels predict slower cognitive decline in Alzheimer’s disease patients. Int J Neuropsychopharmacol. 2011;14:399–404. | |
Yau JL, Seckl JR. Local amplification of glucocorticoids in the aging brain and impaired spatial memory. Front Aging Neurosci. 2012;4:24. | |
Takano T, Tian GF, Peng W, et al. Astrocyte-mediated control of cerebral blood flow. Nat Neurosci. 2006;9:260–267. | |
Garzon-Muvdi T, Quinones-Hinojosa A. Neural stem cell niches and homing: recruitment and integration into functional tissues. ILAR J. 2009;51:3–23. |
 © 2015 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2015 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.