Back to Journals » Drug Design, Development and Therapy » Volume 12
Sinomenine inhibits the growth of melanoma by enhancement of autophagy via PI3K/AKT/mTOR inhibition
Authors Sun Z, Zheng L, Liu X, Xing W, Liu X
Received 1 November 2017
Accepted for publication 8 March 2018
Published 6 August 2018 Volume 2018:12 Pages 2413—2421
DOI https://doi.org/10.2147/DDDT.S155798
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 2
Editor who approved publication: Professor Manfred Ogris
Zheng Sun,1 Lingling Zheng,1 Xujun Liu,2 Wenlong Xing,3 Xinhai Liu4
1Department of Dermatology and Venereology, Beijing Luhe Hospital, Capital Medical University, Beijing, People’s Republic of China; 2Department of Biochemistry and Molecular Biology, School of Basic Medical Sciences, Peking University Health Science Center, Beijing, People’s Republic of China; 3Department of Cardiovasology, Beijing Chinese Medicine Hospital, Capital Medical University, Beijing, People’s Republic of China; 4Department of Plastic Surgery, Beijing Luhe Hospital, Capital Medical University, Beijing, People’s Republic of China
Background: Melanoma is a common skin tumor in adults with high metastasis and mortality rates. Thus, finding a better effective approach to treat melanoma has become very urgent. Sinomenine (SIN), the major active compound of Sinomenium acutum, has shown antitumorigenic activities in certain cancers. However, its role in melanoma remains unclear.
Purpose: This study aimed to explore the effects of SIN on melanoma in vitro and in vivo, in addition to exploring the underlying mechanism.
Methods: Mouse melanoma cell B16-F10 treated by SIN was analyzed by CCK8 assay and flow cytometry. Melanoma xenograft model was then established by subcutaneously injection with B16-F10 cells. Tumor growth was measured by immunohistochemistry. To further investigate the relative mechanism, the autophagy and PI3K/Akt/mTOR pathway were examined by immunofluorescence and Western blot.
Results: Our results revealed that SIN dose dependently inhibited the proliferation of B16-F10 cells in vitro and attenuated melanoma growth in vivo. In addition, SIN treatment promoted the apoptosis of B16-F10 cells in a dose-dependent manner, as demonstrated by the increase in apoptotic cells, Bax/Bcl-2 ratio, and caspase-3 activity. Moreover, preconditioning with SIN dramatically enhanced autophagy activity by increasing Beclin-1 and LC3II/LC3I expression, in addition to decreasing p62 expression and augmenting the number of LC3 puncta, in B16-F10 cells. More importantly, autophagy inhibitor chloroquine partly abolished SIN’s effects on cell growth and apoptosis. Furthermore, our results showed that SIN-triggered activation of autophagy was mediated by PI3K/Akt/mTOR signaling pathway.
Conclusion: Our study has identified a novel function of SIN and provided a molecular basis for potential applications of SIN in the treatment of melanoma and other cancers.
Keywords: sinomenine, melanoma, autophagy, PI3K/Akt/mTOR
Introduction
Melanoma is well known for its aggressive clinical behavior and therapeutic resistance.1,2 Although early stages of melanoma can be removed by surgery, with high patient survival rates, metastatic melanoma has poor prognosis. Despite recent progress in the treatment of late-stage melanoma, and in particular, the advances in the development of novel targeted therapy and immunotherapy approaches, the overall outcome of patients with metastatic melanoma remains poor.3 Therefore, it is necessary to discover new potential therapeutic agents for clinical studies.
Sinomenine (7,8-didehydro-4-hydroxy-3,7-dimethoxy-17-methylmorphinane-6-one; SIN) is an active compound of the Chinese medicine Sinomenium acutum, which has been used to treat rheumatoid arthritis in China for >2,000 years.4,5 SIN displays a variety of pharmacological activities, including anti-inflammatory, antirheumatic, antiangiogenic, and immunosuppressive effects.6–9 Furthermore, some researchers have demonstrated that SIN exhibits antitumor activities in many cancers, such as lung cancer, hepatocellular carcinoma, and breast cancer.10–12 However, the effects and potential mechanism of SIN on melanoma remain unclear.
Autophagy is a conserved intracellular degradation system that is well known to be activated downstream of PI3K/Akt/mTORC1.13,14 Downstream phosphorylation of Akt can activate tuberous sclerosis 1/2, which further promotes mTOR activation. mTOR functions by inhibiting the downstream molecular complex ULK1 to negatively regulate autophagy levels.15 Recent studies have shown that autophagic activity is elevated in different types of cancer and is hence considered a therapeutic target in several clinical trials.16,17 Nevertheless, the role of autophagy in tumor development and progression is controversial, since numerous studies have hinted at a role of autophagy in promoting apoptosis.18 These observations support the concept of context-dependent regulatory mechanisms of autophagy during cancer progression.19
Therefore, this study aimed to elucidate the biological function of SIN on melanoma in vitro and in vivo. In addition, the role of PI3K/Akt/mTOR-mediated autophagy on the protective effect of SIN was further examined.
Materials and methods
Reagents
SIN was purchased from AdooQ Bioscience (Nanjing, China). The primary antibody, including anti-Bax, anti-Bcl-2, anti-Beclin1, anti-p-p62/SQSTM1 (Thr269/Ser272), and anti-p62 were obtained from Abcam (Cambridge, UK). Anti-LC3, anti-pAkt (S473), anti-Akt, anti-pmTOR (S2448), and anti-mTOR antibodies were purchased from Sigma-Aldrich Co., (St Louis, MO, USA). LY294002 and CQ were obtained from Selleck Chemicals (Houston, TX, USA). IGF-1 was purchased from Abcam. Caspase-3 enzyme-linked immunosorbent assay (ELISA) kit was purchased from Sangon Biotech (Shanghai, China).
Cell culture
Mouse melanoma cell B16-F10 was purchased from American Type Culture Collection (ATCC; Manassas, VA, USA) and maintained in Dulbecco’s Modified Eagle’s Medium-H (DMEM-H; Thermo Fisher Scientific, Waltham, MA, USA) complemented with 10% FBS (Thermo Fisher Scientific). Cells were cultured at 37°C in a 5% CO2 incubator.
Cell viability assay
The cell viability was determined using the Cell Counting Kit-8 (CCK-8) assay. Melanoma cells were cultured in a 96-well plate and treated with a series of different doses of SIN for 24 hours. Then, CCK-8 solution was added to each well, and the absorbance at 450 nm was measured with a spectrophotometer (Bio-Rad Laboratories Inc, Hercules, CA, USA).
Flow cytometry
Apoptosis of B16-F10 cells was detected by flow cytometry using the annexin V and propidium iodide (PI) double-staining technique. Cells were treated with SIN for 24 hours, then collected, centrifuged, and incubated with binding buffer, which contained annexin V antibody and PI at room temperature for 15 minutes. Finally, cell apoptosis was detected by flow cytometry.
Western blot
Total protein samples were prepared from cells and tissues using standard protocols. The proteins (20 μg) in each sample were separated by 12% sodium dodecyl sulfate polyacrylamide gel electrophoresis and electrotransferred to polyvinylidene fluoride membranes for immunoblotting. After incubation with 5% nonfat milk, the membranes were incubated with primary and secondary antibodies. The blots were detected using a ChemiDoc XRS imaging system, and the optical density was measured using ImageJ analysis software.
Measurement of caspase-3 activity
Cells were treated with a series of different doses of SIN for 24 hours and collected using trypsin. The activity of caspase-3 was assessed using a caspase-3 ELISA kit (Sangon Biotech) according to the manufacturer’s recommendations. The optical density of the color reaction was spectrophotometrically measured at 405 nm.
Animals and experimental protocols
Six-week-old BALB/c mice were purchased from the Institute of Zoology, Chinese Academy of Medical Sciences. The experimental protocol was approved by the Institutional Animal Care Committee (IACUC) of Capital Medical University and was in accordance with the guidelines of IACUC of Capital Medical University. Mice were housed under standard conditions (25°C±2°C, 70% humidity, and 12-hour light–dark periods) in plastic cages with free access to food and water. All mice were randomly divided into three groups, including control group, model group, and SIN group. The mice in the model group were injected subcutaneously with B16-F10 cells, and the SIN group was injected subcutaneously with SIN (100 mg/kg/day) for continuous 35 days. Tumor volumes were measured every 5 days according to the following formula: (length [mm] × width [mm] × width [mm] × 0.52). Mice were killed on Day 35, and the tumor weight was measured.
Immunohistochemistry
Tumor sections were prepared essentially by the standard protocol.20 After the sequential processes of rehydration and antigen retrieval, the sections were incubated with antibody against PCNA overnight and subsequently incubated with secondary antibody for 30 minutes. Diaminobenzidine was used for performing color reactions. Sections were visualized with a fluorescence microscope.
Immunofluorescence
Immunofluorescence was performed essentially as previous described.21 B16-F10 cells were seeded on glass cover slips in six-well plates and were treated with SIN and/or CQ for 24 hours. After fixing in 4% paraformaldehyde for 10 minutes, cells were incubated with 0.2% Triton X-100 in PBS for 15 minutes and blocked at room temperature with 5% BSA for 1 hour. After washing twice with PBS, cells were incubated with anti-LC3 primary antibodies at 37°C overnight. Cells were then incubated with fluorescein isothiocyanate-labeled goat anti-rabbit IgG secondary antibody for 2 hours. Cells were visualized with a fluorescence microscope.
Statistical analysis
Data were analyzed with SPSS 19.0 software, and the results were expressed as mean ± SD. The statistical significance of the studies was analyzed using one-way analysis of variance. The difference was considered significant at P<0.05 or less.
Results
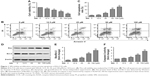
SIN inhibits proliferation and promotes apoptosis of B16-F10 cells
To gain insights into the antimelanoma function of SIN, B16-F10 melanoma cells were treated with SIN at a concentration range of 0–100 μM for 24 hours, and the cell viability was detected by the CCK-8 assay. As shown in Figure 1A, SIN dose dependently decreased the viability of B16-F10 cells. In addition, SIN promoted the apoptosis of B16-F10 cells in a dose-dependent manner (Figure 1B and C). Furthermore, SIN also increased the expression of Bax, decreased the expression of Bcl-2, and enhanced the activity of caspase-3, which play a crucial role in the progression of apoptosis (Figure 1D–F). Taken together, these findings indicate that SIN may inhibit proliferation and promote apoptosis of melanoma cells.
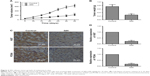
SIN relieves tumor growth of melanoma in vivo
To further investigate the inhibitory effect of SIN on tumor growth, a melanoma xenograft mouse model was studied. As shown in Figure 2A and B, the tumor volume and weight decreased remarkably with SIN treatment, compared with the results in the untreated group. Furthermore, we also tested the expression of proliferation-associated proteins in tumor tissues by immunohistochemistry. The data revealed that the expression levels of Ki67 and PCNA were significantly decreased by SIN treatment when compared with the control group (Figure 2C and D). These results suggest that SIN relieves tumor growth of melanoma in vivo.
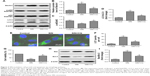
SIN modulates cell growth and apoptosis via autophagy pathway
Previous studies have shown that the regulation of autophagy can affect the progression of melanoma.22 SIN significantly increased the protein expression of Beclin1 and decreased the level of p-p62/SQSTM1 (Figure 3A–C). Moreover, SIN treatment markedly increased e LC3II/LC3I ratio (Figure 3A and D) and increased the amount of LC3-labeled puncta per cell (Figure 3E and F), suggesting that SIN enhanced autophagy in B16-F10 cells. Interestingly, blocking autophagy by CQ partly attenuated the SIN-induced reduction of cell viability and increase of cell apoptosis (Figure 3G–I). These results suggest that SIN modulates cell growth and apoptosis via the autophagy pathway.
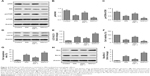
PI3K/Akt/mTOR pathway is involved in SIN-mediated proautophagic effects
The PI3K/Akt/mTOR signaling pathway is critical for the regulation of cell proliferation and apoptosis, as well as autophagy.23–25 Based on this, we further investigated whether SIN affects PI3K/Akt/mTOR activation in melanoma cells. As shown in Figure 4A–C, SIN treatment significantly decreased the phosphorylation of Akt and mTOR. IGF-1 is a well-known PI3K signaling activator that plays an important role in the regulation of cell proliferation and differentiation, and LY294002 is a common inhibitor of PI3K. Reactivating the PI3K/Akt/mTOR pathway by IGF-1 partly reversed the SIN-induced upregulation of autophagy and apoptosis, as well as the reduction of cell viability, in melanoma cells (Figure 4D–I). Interestingly, LY294002 further enhanced the effects of SIN on autophagy, apoptosis, and cell viability (Figure 4D–I). Taken together, these results indicate that SIN inhibits proliferation and promotes apoptosis of melanoma cells via a PI3K/Akt/mTOR-dependent autophagy pathway.
Discussion
SIN is originally derived from a medicinal herb and is used preferentially in the treatment of rheumatoid diseases in the Far East regions.4,5 It has been shown to be capable of alleviating inflammatory responses and regulating immunoreaction.6 In recent years, numerous studies have reported that SIN also has antitumor activities.10–12 The antitumor effects of SIN are mediated by different mechanisms. SIN induces the apoptosis of prostate cancer cells by blocking the activation of NF-κB.26 Sinomenine hydrochloride inhibits breast cancer metastasis by attenuating inflammation-related epithelial–mesenchymal transition and cancer stemness.27 In colon cancer, SIN displays an antitumor effect by regulating antibiotic resistance.28 In the present study, we first found that SIN also has an antitumor effect in melanoma brought about by promoting autophagy.
Abnormal cell proliferation is a key element of tumorigenesis, and thus inhibiting cell proliferation is considered an effective avenue for the development of novel antitumor therapeutics. We found, via cell viability assay and a mouse model, that SIN dose dependently inhibited melanoma cell proliferation in vitro and attenuated tumor growth in vivo. These results verified previously published findings indicating that SIN has an inhibitory effect on cancer cell proliferation.10,29,30 PCNA and Ki67 are widely used intrinsic markers of cell proliferation. Our results also showed that SIN may significantly inhibit the expressions of PCNA and Ki67 in melanoma tissues. Furthermore, our results showed that SIN dose dependently promoted apoptosis in B16-F10 cells, as demonstrated by the increases in apoptotic cells, Bax/Bcl-2 ratio, and caspase-3 activity.
Autophagy is a key mechanism in various physiological and physiopathological processes, including cell death and survival.31,32 Increasing evidence has indicated that autophagy plays a crucial role in the progression of cancer.16,17 In this research, a significant increase of Beclin1 and LC3-II, along with a remarkable decrease of p62, was observed in SIN-stimulated B16-F10 cells, suggesting that SIN conduces to induce autophagy in melanoma. Moreover, inhibition of autophagy by CQ partly abolished the SIN-induced decrease of cell viability and increase of cell apoptosis. These results indicated that SIN could suppress cell proliferation and promote cell apoptosis partly through enhancing autophagy in melanoma cells.
The PI3K/Akt/mTOR signaling pathway is an important intracellular mediator, which is critical for the regulation of cell survival and proliferation.33,34 Numerous studies have demonstrated that aberrant activation of PI3K/Akt/mTOR pathway is involved in the pathological process of melanoma, and its inhibition has become a useful therapy for melanoma.35,36 Moreover, it is reported that inhibition of the PI3K/Akt/mTOR signaling pathway can induce autophagy and apoptosis in prostate cancer stem cells.37 mTOR is composed of two complexes, including mTORC1 and mTORC2. mTORC1 functions as a transcriptional regulator of autophagy.38 Inhibition of mTOR induces the initiation of autophagy.15 Moreover, the Beclin1-induced formation of the preautophagy structure also needs the participation of PI3K.39 In this study, the expression levels of p-AKT and p-mTOR were significantly decreased after SIN treatment, suggesting that SIN could repress the PI3K/Akt/TOR signaling pathway in melanoma cells. In addition, reactivating PI3K/Akt/mTOR pathway by IGF-1 partly reversed the SIN-induced autophagy. Pretreatment with IGF-1 also attenuated the SIN-induced decrease of cell viability and increase of cell apoptosis. Furthermore, LY29002-mediated inhibition of PI3K/Akt/mTOR signaling further enhanced the effect of SIN on autophagy, apoptosis, and proliferation. These results strongly indicated that the PI3K/Akt/mTOR pathway is involved in the SIN-mediated proapoptotic and antiproliferative effects via autophagy.
Conclusion
Our investigation suggests that SIN regulation of autophagy via the PI3K/Akt/mTOR pathway is a critical component through which SIN protects against melanoma. Since melanoma shares its pathogenesis with many other cancers, our findings not only elaborate a novel mechanism of SIN’s antimelanoma function but also provide new insights into SIN’s potential applications in the treatment of other cancers and autophagy-related diseases.
Author contributions
Xinhai Liu designed the research. Zheng Sun and Lingling Zheng performed most of the experiments and the animal model studies. Xujun Liu and Wenlong Xing assisted in some experiments and data analysis. Xinhai Liu wrote the manuscript. All authors contributed toward data analysis, drafting and revising the paper and agree to be accountable for all aspects of the work.
Disclosure
The authors report no conflicts of interest in this work.
References
Syed DN, Lall RK, Majeed I, Liu F, Meyskens FL, Mukhtar H. Abstract 1179: unique metabolic profile of Vemurafenib-resistant melanoma cells: a quantitative proteomics approach. Cancer Res. 2015;75(15 suppl):1179. | ||
Winkler JK, Buder-Bakhaya K, Dimitrakopoulou-Strauss A, Enk A, Hassel JC. [Malignant melanoma: current status]. Radiologe. 2017;57(10):814–821. | ||
Boyle GM. Therapy for metastatic melanoma: an overview and update. Expert Rev Anticancer Ther. 2011;11(5):725–737. | ||
Lin X, Cai X, Ye J. The clinical observation on sinomenine for rheumatoid arthritis. J TCM Univ Hunan. 2009;29:52–54. | ||
Lao ZY. Sinomenine combined with methotrexate in treating rheumatoid arthritis. Chin J New Drugs Clin Rem. 2000;19:254–256. | ||
Wang Q, Li XK. Immunosuppressive and anti-inflammatory activities of sinomenine. Int Immunopharmacol. 2011;11(3):373–376. | ||
Yamasaki H. Pharmacology of sinomenine, an anti-rheumatic alkaloid from Sinomenium acutum. Acta Med Okayama. 1976;30(1):1–20. | ||
Kok TW, Yue PYK, Mak NK, Fan TPD, Liu L, Wong RNS. The anti-angiogenic effect of sinomenine. Angiogenesis. 2005;8(1):3–12. | ||
Yue C, Zhang J, Hou W, et al. Immunoregulatory effects of sinomenine on the T-bet/GATA-3 ratio and Th1/Th2 cytokine balance in the treatment of mesangial proliferative nephritis. Int Immunopharmacol. 2009;9(7–8):894–899. | ||
Jiang T, Zhou L, Zhang W, et al. Effects of sinomenine on proliferation and apoptosis in human lung cancer cell line NCI-H460 in vitro. Mol Med Rep. 2010;3(1):51–56. | ||
Lu XL, Zeng J, Chen YL, et al. Sinomenine hydrochloride inhibits human hepatocellular carcinoma cell growth in vitro and in vivo: involvement of cell cycle arrest and apoptosis induction. Int J Oncol. 2013;42(1):229–238. | ||
Song L, Liu D, Zhao Y, et al. Sinomenine inhibits breast cancer cell invasion and migration by suppressing NF-κB activation mediated by IL-4/miR-324-5p/CUEDC2 axis. Biochem Biophys Res Commun. 2015;464(3):705–710. | ||
Dibble CC, Manning BD. Signal integration by mTORC1 coordinates nutrient input with biosynthetic output. Nat Cell Biol. 2013;15(6):555–564. | ||
White E. Deconvoluting the context-dependent role for autophagy in cancer. Nat Rev Cancer. 2012;12(6):401–410. | ||
Jung CH, Ro SH, Cao J, Otto NM, Kim DH. mTOR regulation of autophagy. FEBS Lett. 2010;584(7):1287–1295. | ||
Rubinsztein DC, Codogno P, Levine B. Autophagy modulation as a potential therapeutic target for diverse diseases. Nat Rev Drug Discov. 2012;11(9):709–730. | ||
Amaravadi RK, Lippincott-Schwartz J, Yin XM, et al. Principles and current strategies for targeting autophagy for cancer treatment. Clin Cancer Res. 2011;17(4):654–666. | ||
Liu H, He Z, Von RT, Yousefi S, Hunger RE, Simon HU. Down-regulation of autophagy-related protein 5 (ATG5) contributes to the pathogenesis of early-stage cutaneous melanoma. Sci Transl Med. 2013;5(202):202ra123. | ||
White E, DiPaola RS. The double-edged sword of autophagy modulation in cancer. Clin Cancer Res. 2009;15(17):5308–5316. | ||
He Q, Sha S, Sun L, Zhang J, Dong M. GLP-1 analogue improves hepatic lipid accumulation by inducing autophagy via AMPK/mTOR pathway. Biochem Biophys Res Commun. 2016;476(4):196–203. | ||
Wu J, Kong F, Pan Q, et al. Autophagy protects against cholesterol-induced apoptosis in pancreatic beta-cells. Biochem Biophys Res Commun. 2017;482(4):678–685. | ||
Tomic T, Botton T, Cerezo M, et al. Metformin inhibits melanoma development through autophagy and apoptosis mechanisms. Cell Death Dis. 2011;2(9):e199. | ||
Mitra A, Raychaudhuri SK, Raychaudhuri SP. IL-22 induced cell proliferation is regulated by PI3K/Akt/mTOR signaling cascade. Cytokine. 2012;60(1):38–42. | ||
Park KR, Nam D, Yun HM, et al. β-Caryophyllene oxide inhibits growth and induces apoptosis through the suppression of PI3K/AKT/mTOR/S6K1 pathways and ROS-mediated MAPKs activation. Cancer Lett. 2011;312(2):178–188. | ||
Singh BN, Kumar D, Shankar S, Srivastava RK. Rottlerin induces autophagy which leads to apoptotic cell death through inhibition of PI3K/Akt/mTOR pathway in human pancreatic cancer stem cells. Biochem Pharmacol. 2012;84(9):1154–1163. | ||
Fan J, Wang JC, Chen Y, et al. Sinomenine induces apoptosis of prostate cancer cells by blocking activation of NF-kappa B. Afr J Biotechnol. 2011;10(17):3480–3487. | ||
Li X, Li P, Liu C, et al. Sinomenine hydrochloride inhibits breast cancer metastasis by attenuating inflammation-related epithelial-mesenchymal transition and cancer stemness. Oncotarget. 2011;8(8):13560–13574. | ||
Liu Z, Duan ZJ, Chang JY, et al. Sinomenine sensitizes multidrug-resistant colon cancer cells (Caco-2) to doxorubicin by downregulation of MDR-1 expression. PLoS One. 2014;9(6):e98560. | ||
Song L, Liu D, Zhao Y, et al. Sinomenine reduces growth and metastasis of breast cancer cells and improves the survival of tumor-bearing mice through suppressing the SHh pathway. Biomed Pharmacother. 2018;98:687–693. | ||
Deng F, Ma YX, Liang L, Zhang P, Feng J. The pro-apoptosis effect of sinomenine in renal carcinoma via inducing autophagy through inactivating PI3K/AKT/mTOR pathway. Biomed Pharmacother. 2018;97:1269–1274. | ||
Yu L, Strandberg L, Lenardo MJ. The selectivity of autophagy and its role in cell death and survival. Autophagy. 2008;4(5):567–573. | ||
Mizumura K, Choi AM, Ryter SW. Emerging role of selective autophagy in human diseases. Front Pharmacol. 2014;5:244. | ||
Malemud CJ. The PI3K/Akt/PTEN/mTOR pathway: a fruitful target for inducing cell death in rheumatoid arthritis? Future Med Chem. 2015;7(9):1137–1147. | ||
Wang Y, Chen B, Wang Z, et al. Marsdenia tenacissimae extraction (MTE) inhibits the proliferation and induces the apoptosis of human acute T cell leukemia cells through inactivating PI3K/AKT/mTOR signaling pathway via PTEN enhancement. Oncotarget. 2016;7(50):82851–82863. | ||
Sinnberg T, Lasithiotakis K, Niessner H, et al. Inhibition of PI3K-AKT-mTOR signaling sensitizes melanoma cells to cisplatin and temozolomide. J Invest Dermatol. 2009;129(6):1500–1515. | ||
Cheng H, Zhang X, Su JJ, Li QL. [Study of gambogenic acid-induced apoptosis of melanoma B16 cells through PI3K/Akt/mTOR signaling pathways]. Zhongguo Zhong Yao Za Zhi. 2014;39(9):1666–1669. | ||
Kumar D, Shankar S, Srivastava RK. Rottlerin induces autophagy and apoptosis in prostate cancer stem cells via PI3K/Akt/mTOR signaling pathway. Cancer Lett. 2014;343(2):179–189. | ||
Martina JA, Chen Y, Gucek M, Puertollano R. MTORC1 functions as a transcriptional regulator of autophagy by preventing nuclear transport of TFEB. Autophagy. 2012;8(6):903–914. | ||
He C, Levine B. The Beclin 1 interactome. Curr Opin Cell Biol. 2010;22(2):140–149. |
 © 2018 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2018 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.