Back to Journals » Drug Design, Development and Therapy » Volume 14
Sinapultide-Loaded Microbubbles Combined with Ultrasound to Attenuate Lipopolysaccharide-Induced Acute Lung Injury in Mice
Authors Liu D, Chen Y, Li F, Chen C, Wei P, Xiao D, Han B
Received 17 September 2020
Accepted for publication 19 November 2020
Published 22 December 2020 Volume 2020:14 Pages 5611—5622
DOI https://doi.org/10.2147/DDDT.S282227
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 2
Editor who approved publication: Professor Anastasios Lymperopoulos
Dong Liu,1– 3 Yanjun Chen,1,2 Fang Li,1,2 Cunwu Chen,1,2 Peipei Wei,1,2 Deli Xiao,4,5 Bangxin Han1,2
1School of Biological and Pharmaceutical Engineering, West Anhui University, Lu’an 237012, People’s Republic of China; 2Anhui Engineering Laboratory for Conservation and Sustainable Utilization of Traditional Chinese Medicine Resources, Lu’an 237012, People’s Republic of China; 3School of Biomedical Sciences and Medical Engineering, Southeast University, Nanjing 210009, People’s Republic of China; 4Department of Analytical Chemistry, China Pharmaceutical University, Nanjing 210009, People’s Republic of China; 5Key Laboratory of Biomedical Functional Materials, China Pharmaceutical University, Nanjing 210009, People’s Republic of China
Correspondence: Deli Xiao
Department of Analytical Chemistry, China Pharmaceutical University, Nanjing 210009, People’s Republic of China
Tel +86 25 86185160
Email [email protected]
Bangxin Han
School of Biological and Pharmaceutical Engineering, West Anhui University, Lu’an 237012, People’s Republic of China
Tel +86 564 3305073
Email [email protected]
Purpose: Pulmonary surfactants (eg, sinapultide) are widely used for the treatment of lung injury diseases; however, they generally induce poor therapeutic efficacy in clinics. In this study, sinapultide-loaded microbubbles (MBs) were prepared and combined with ultrasound (US) treatment as a new strategy for improved treatment of lung injury diseases.
Methods: The combination treatment strategy of MBs combined with ultrasound was tested in a lipopolysaccharide (LPS)-induced mouse model of alveolar epithelial cells (AT II) and acute lung injury. Firstly, cytotoxicity, cytokines, and protein levels in LPS-mediated AT II cells were assessed. Secondly, the pathological morphology of lung tissue, the wet/dry (W/D) weight ratio, cytokines, and protein levels in LPS-mediated acute lung injury mice after treatment with the MBs were evaluated. Moreover, histology examination of the heart, liver, spleen, lung and kidney of mice treated with the MBs was performed to initially evaluate the safety of the sinapultide-loaded MBs.
Results: Sinapultide-loaded MBs in combination with ultrasound treatment significantly reduced the secretion of inflammatory cytokines and increased the expression of surfactant protein A (SP-A) in AT II cells. Furthermore, the pathological morphology of lung tissue, the wet/dry (W/D) weight ratio, interleukin-6 (IL-6), tumor necrosis factor-α (TNF-α) and SP-A expression level of mice treated with MBs and ultrasound were significantly improved compared to those of non-treated mice. In addition, the histology of the examined organs showed that the MBs had a good safety profile.
Conclusion: Sinapultide-loaded MBs combined with ultrasonic treatment may be a new therapeutic option for lung injury diseases in the clinic.
Keywords: sinapultide, microbubbles, lipopolysaccharide, lung injury, safety
Introduction
Acute lung injury (ALI) is a kind of systemic inflammation of the respiratory system with pulmonary infiltration by immune cells, hypoxemia and edema. ALI can progress to a more severe stage, ie, the acute respiratory distress syndrome (ARDS).1 ALI is caused by various factors, and the main pathological features are injury of alveolar epithelial cells, atelectasis, severe pulmonary edema, and microcirculation disturbance lead by microthrombosis, etc.2 Despite the significant progress in ALI treatment, effective therapy for ALI is still lacking.3,4
Pulmonary surfactants (PS) are mixtures of lipids and proteins secreted by type II alveolar epithelial cells. The main function of PS lies in maintaining normal respiratory mechanics by reducing alveolar surface tension to prevent alveolar collapse. Besides, it also plays important roles in providing uniform lung inflation, improving efficiency of airway clearance, and alleviating lung inflammatory reaction.5,6 Recently, several studies have demonstrated that the decrease of pulmonary surfactant secretion and related component changes in alveolus is one of the typical pathological features of ALI/ARDS, and exogenous surfactants of animal origin were widely used for the treatment of lung injury diseases.7,8 Nevertheless, safety issues like the spread risk of animal-related diseases are potential risks. Besides, the ratio of surface-active proteins to lipid is uncontrollable because animal-derived agents vary between batches. Therefore, the synthetic PS can be a choice to avoid such problems. Sinapultide (KL4), a novel 21-amino-acid peptide, was used as surfactant protein analog in the formulation to mimic the function of the critical human pulmonary surfactant protein (SP-B). Surfaxin®, the only peptide-based synthetic pulmonary surfactant (sinapultide) in clinical application, was approved by the Food and Drug Administration (FDA) in 2012 for the treatment of neonatal respiratory distress syndrome (NRDS).9,10 Compared with animal-derived pulmonary surfactants, such synthetic surfactants have a higher bio-safety and batch homogeneity. As a conventional suspension formulation, the dosage form and treatment method of Surfaxin® does not allow for high dose in a simple treatment, which considerably compromises the patient compliance of the medication. Therefore, we constructed novel sinapultide-loaded lipid microbubbles for pulmonary delivery based on the formulation of Surfaxin®. The film dispersion and sonication method was used to prepare sinapultide-loaded lipid microbubbles. Briefly, the optimized sinapultide-loaded microbubbles formulation was composed of 0.1 mg of sinapultide, 23.22 mg of DPPC (Dipalmitoyl phosphatidylcholine), 7.68 mg of POPG. Na (Phosphatidyl glycerol monosodium salt), 4.21 mg of PA (Palmitic acid). The prescribed ingredients were synchronously dissolved in chloroform with ultrasound. Then, the organic solvent was evaporated under reduced pressure in a rotary evaporator. After all the organic solvent was removed from the mixture, the film was obtained after they were dried at 30°C in a vacuum oven for 12 hours. Then, 1 mL of phosphate buffered saline (PBS) (pH 7.4) was added to the sinapultide-loaded lipid film in a radius flask, after that, a water ultrasonic bath was carried through ultrasonic. Then, 1 mL aliquots of the precursor milk-white emulsion were placed in a 3 mL glass vial, and the vial was capped and sealed immediately. Since SF6 was required as the filling gas, the air headspace of the vial was replaced using a self-made gas exchange apparatus. Microbubbles were produced by the homogenizer, and the more detailed preparation process was previously reported.11 In this study, the therapeutic effect of sinapultide MBs integrated with ultrasound to inflammatory alveolar epithelial cells as well as lung injury animal models were investigated. Though PS was widely used for lung injury diseases, to the best of our knowledge the use of sinapultide MBs combined with ultrasound effect for treatment of ALI has not yet been reported.
As lipopolysaccharide (LPS) has been confirmed as an important mediator of ALI, which directly induces toxicities in lung endothelial cells, and activates neutrophils and macrophages to release pro-inflammatory cytokines, therefore, in the current study it was used to establish the model of alveolar epithelial cells (AT II) and acute lung injury in mice.12,13 We demonstrated that treatment of MBs combined with ultrasound could significantly improve pulmonary edema associated with LPS-induced ALI in mice, and the secretion levels of inflammatory factors and SP-A were significantly improved compared to the control group. In addition, the sinapultide-loaded MBs exhibited a good safety profile in mice. In conclusion, sinapultide-loaded MBs combined with ultrasound have the potential to be used for the treatment of lung injury diseases in the future.
Materials and Methods
Materials
Sinapultide suspension and MBs were prepared by using a previously described method.11 LPS was purchased from the Sigma Chemical Co. (St. Louis, MO, USA). TNF-α, IL-6 and SP-A ELISA kits were provided by Thermo Fisher Scientific Inc. (Shanghai, People's Republic of China). Fetal bovine serum (FBS), trypsin ethylene diamine tetra-acetic acid (EDTA), RPMI (Roswell Park Memorial Institute)-1640, and a cell apoptosis detection kit was purchased from the Nanjing Kaiji Biotechnology Co., Ltd. (Nanjing, Jiangsu Province, People's Republic of China). Other chemicals used were of reagent grade.
Animals
BALB/c mice (male, 6–8 weeks old) were purchased from the Experimental Animal Center of Southeast University (Jiangsu, People's Republic of China). The mice were kept with food and water ad libitum in a temperature-controlled room (21–23°C). The animals used for the experiment were treated according to the protocols evaluated and approved by the ethical committee of Southeast University (Nanjing, People's Republic of China).
In vitro Cell Experiments
Cell culture: Primary AT II cells were purchased from Shanghai Baili Biotechnology Co., Ltd. (Shanghai, People's Republic of China) and grown at 37°C in a humidified incubator with 5% (v/v) CO2 in RPMI 1640 supplied with 5% (v/v) fetal bovine serum, 1% penicillin/streptomycin and 1% (w/v) glutamine. To maintain self-renew and multiple differentiation potential, cells were cultured at appropriate confluence (70–80%) and harvested by 0.25% (w/v) trypsin-ethylene diamine tetra-acetic acid (EDTA) solution.14 After the cell culture bottle was incubated in the cell incubator for 2 minutes, the culture bottle was placed under the microscope to observe the cell morphology. When the cell body became round and appeared to float in a mass, an appropriate amount of medium was added to terminate digestion, and the cell suspension was sucked out into a centrifuge tube, then the residual cells on the wall and bottom of the culture bottle were gently blown with a dropper, and observed under a microscope to determine whether the cells were completely blown. All cell suspensions collected after blowing were centrifuged at a speed of 1000 rpm for 5 minutes, and then the cultured cells of three generations were placed in a cryopreservation tube and stored in a refrigerator at −80°C. All AT II cells were collected after fourth passages for the following experiments.
Determination of intervention dose: To determine the LPS dose and incubation time, cells were collected and incubated with different concentrations of LPS solution at 37°C in a humidified incubator with 5% (v/v) CO2 for 24 hours and 48 hours respectively, and the levels of TNF-α, IL-6 and SP-A were detected at the time point by ELISA kits. Based on the preliminary experimental results, AT II cells were incubated with various concentrations of sinapultide MBs preparations in 24-well culture plates with 20 μg/mL LPS solution, and cytokines as well as protein levels were detected by ELISA kits after 24 hours. All of the experiments were prepared in parallel.
Cell viability assay: MTT (Methyl-thiazolyl-tetrazolium) assay was used to measure cell viability. Cells were seeded in a 96-well plate (3 × 103 cells per well), and a sinalpultide suspension group, sinalpultide MBs group, group of suspension combined with ultrasonic, group of sinalpultide MBs combined ultrasonic as well as a control group were set up respectively. The concentration of sinalpultide in suspension and microbubble was 0.1 mg/mL, and the ultrasound parameters were set as follows: frequency 0.5 MHz; intervention time 40 seconds; pressure 24.6 kPa.15 After 24 hours incubation, cells were washed twice with PBS, MTT reagent was added to each well and co-incubated with cells for 4 hours at 37°C. The cell activity was determined by measuring the changes in absorbance at 490 nm using a microplate reader.
Cytokines, and protein levels in LPS-mediated AT II cells: Cells were seeded in a 48-well plate (1 × 104 cells per well), and then added with sinalpultide preparations as a cell viability assay experiment. After ultrasonication and incubation for 24 hours, cell supernatant was collected and detected by ELISA method.
Experimental Design for in vivo Experiments
A LPS-induced ALI model was established by intratracheal instilling of LPS (500 μg/mL) to mice (10 mg/kg), and then 90 mice were divided into five groups: a control group, a sinalpultide suspension group, a sinalpultide MBs group, a group of suspension combined with ultrasonic, and a group of sinalpultide MBs combined with ultrasonic.16 The LPS-induced ALI mice were administered through the nasal cavity with sinalpultide preparations (0.1mg/mL) with a drug dose of 5.8 mL/kg, and normal saline was given to the control group.17,18 After drug administration of 6, 12, and 24 hours, the BALF as well as lung tissues were collected for six samples one time. All animal experiments were performed in accordance with the Care and Use of Laboratory Animals established by Southeast University.
Lung W/D Weight Ratio
Lung tissue edema was estimated by determining the lung W/D weight ratios. The fresh upper part of the right lung tissue was blotted dry before weighed, and dried in an oven at 80°C for 48 hours, then weighed again to calculate the lung W/D weight ratio.
Cytokines, and Protein Levels in BALF
Mice were anaesthetized, and bronchoalveolar lavage fluid (BALF) samples were obtained by flushing the lung (1.0 mL ×3 times). BAL fluid samples were centrifuged at 1500 rpm for 5 minutes at 4°C, then refrigerated and stored at -80°C before taking measurements. The levels of inflammatory cytokines IL-6, TNF-α and surfactant protein level (SP-A) in BAL fluid were measured using ELISA kits under the manufacturer’s instructions.
Histology Examination
Right lung lobes were removed and washed with normal saline, then fixed with 10% buffered formalin at room temperature. The lung tissue was embedded in paraffin, cut into 3 mm sections, and stained with haematoxylin and eosin (H&E) using standard methods.19
In vivo Safety Evaluation
To investigate the preliminary safety of sinapultide-MBs, 18 mice were divided into three groups (n = 6). The mice were administered with normal saline, sinapultide suspension, and sinapultide-MBs, respectively, by daily intranasal doses for 14 days with a drug dose of 2.32 mg/kg per day. The body weight was recorded during the 14 days. On day 14 after initial administration, the mice were euthanized and their hearts, livers, spleens, lungs and kidneys were taken. The organs were fixed in 10% formalin, embedded in paraffin and sectioned and stained with H&E. In addition, the organ coefficient was calculated to evaluate the safety of preparations.
Statistical Analysis
The data obtained were expressed as mean ± SD (standard deviation). Statistical analysis was performed using Student’s t-test for two groups and one-way analysis of variance for multiple groups. A difference with p < 0.05 was regarded as statistically significant. All experiments were conducted at least in triplicate.
Results
Effects of Sinapultide MBs on LPS-Mediated Model of AT II Cells
To verify the establishment of the model by LPS, levels of TNF-α, IL-6 and SP-A were evaluated in mice treated with different doses of LPS (0, 5, 10, 15, 20 μg/mL) (Figure S1). The levels of TNF-α and IL-6 increased gradually with the rise of LPS dose, and the TNF-α level was 4.38 and 6.11 times higher than the control group after LPS (20 μg/mL group) treatment for 24 hours and 48 hours, respectively. Similarly, the level of IL-6 secretion was significantly higher than that of the control group under LPS intervention (20 μg/mL), which was 3.96 and 5.79 times higher at 24 hours and 48 hours, respectively. Besides, the SP-A level was reduced as the increase of LPS dose, and the SP-A secretion level at 48 hours post treatment was lower than that at 24 hours after treatment. Based on the results, the LPS dosage of 20 μg/mL and an incubation time of 24 hours were selected to establish the model. In the screening experiment of drug intervention dose, sinapultide-loaded MBs with a concentration of 0.1 mg/mL was selected, and results showed that the levels of TNF-α and IL-6 were significantly different when the microbubble dosage of sinapultide was 2.0 μg/mL and 5.0 μg/mL. However, SP-A level showed a significant difference with the control group only when the microbubble dosage was 5.0 μg/mL (Figure S2), therefore, the intervention dosage of sinapultide preparations was set as 5.0 μg/mL.
The cytotoxicity of each group of preparation to LPS-mediated AT II cells was examined with MTT assay using a model group without drug intervention as control (Figure 1). Compared with the control group, the viability of AT II cells was much higher in the intervention group of sinapultide preparations (p < 0.01). Furthermore, the number of apoptotic cells in the group of microbubbles of sinapultide combined with ultrasound was reduced compared with other drug intervened groups. Therefore, the treatment strategy based on microbubbles combined with ultrasound induced the most significant improvement of cell function.
Cytokines of TNF-α and IL-6 as well as pulmonary surfactant (SP-A) secretion level were used as indicators to estimate the effect of microbubble preparation combined with ultrasound on LPS-mediated AT II cells (Figure 2). Results show that the expression of TNF-α and IL-6 in the microbubble preparation intervened group was significantly lower than that in the non-intervention group, while the expression of SP-A level was increased significantly (p < 0.01). Compared with the microbubble preparation intervened group, the expression level of inflammatory factors was further reduced and the secretion of SP-A was further improved in the sinapultide microbubble combined with ultrasonic group (p < 0.01). The above results further confirmed that the microbubble preparation combined with ultrasound could promote the function recovery of injured AT II cells effectively.
Effects of Sinapultide MBs on Lung Histopathologic Changes in LPS-Induced ALI Mice
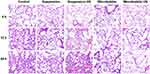
Histopathological analyses were performed to investigate the effects of sinalpultide preparations on LPS-induced histological changes in mice. Firstly, the method of instilling intratracheal was used to prepare LPS-induced ALI model, and the lung tissues of the LPS group exhibited diffused pathological changes compared with the control group after 24 hours (Figure S3), which demonstrated the establishment of the model of acute lung injury. The H&E staining sections of lung tissues from sinalpultide preparation group of mice are shown in Figure 3. LPS-induced mice with non-intervention were set as controls, and the lung tissues exhibited characteristics of histological changes during the study, including interstitial edema, obvious inflammatory cells infiltration, hemorrhage and perivascular exudates. However, these histopathologic changes were markedly improved after administration of sinalpultide preparations, which suggests that the supplement of pulmonary surfactant can improve pulmonary edema in lung injury diseases. Though the pulmonary edema of sinapultide suspension group was improved compared with the control group. Moreover, lung tissue lesions of the suspension combined with ultrasound group showed no further improvement, which showed that the single ultrasound intervention had a limited impact on the acute lung injury. Nevertheless, compared with the group without ultrasound intervention, the pathological structure of lung tissues in the sinalpultide MBs combined with ultrasound group was clearer, and the infiltration degree as well as pulmonary interstitial edema was further improved, which points to the combination of microbubbles and ultrasound having a positive treatment effect on the lung injury diseases.
Effects of Sinapultide MBs on Lung W/D Weight Ratio in LPS-Induced ALI Mice
Lung tissue of mice in each group was collected at 3, 6, 12 and 24 hours after LPS administration to obtain the ratio of lung wet/dry weight (Figure S4 and Table S1), and results showed that the lung wet/dry weight ratio of the treatment group at different time periods was increased gradually compared with the control group. Furthermore, the ratio of wet/dry lung weight was significantly different from that of the control group after 12 hours, and the data measured at 24 hours was also significantly different from that at 12 hours, which further proved the successful establishment of an acute lung injury model by intranasal administration of LPS.
The lung W/D weight ratio was measured at 12 hours and 24 hours after drug administration to estimate pulmonary edema. As shown in Figure 4 and Table 1, the lung W/D weight ratio of of sinalpultide microbubble preparation group was obviously higher than that of the control group at 12 hours (p < 0.05) and 24 hours (p < 0.01). Additionally, compared with the group without ultrasound intervention, the pulmonary tissue edema was further improved after collaborative ultrasound at 24 hours (p < 0.05), and the proportional coefficient to the lung W/D weight was decreased from 4.382 ± 0.274 to 3.966 ± 0.191 at 12 hours.
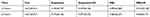 |
Table 1 Determination of W/D Weight Ratio in Lung Tissues of Different Preparation Groups (n = 6) |
Cytokine and Protein Levels Analyzed by BALF
Cytokines of TNF-α and IL-6 as well as pulmonary surfactant (SP-A) secretion level were used to estimate the effect of microbubble preparation combined with ultrasound on lung tissue function of ALI mice. Results showed that the release level of TNF-α and IL-6 in the microbubble group and the microbubble combined ultrasound group were significantly reduced, and the content of SP-A was increased notably compared with the control group (p < 0.01; Figure 5). From the experimental results, ultrasound intervention can play a synergistic role with microbubbles, and the level of inflammatory factors secretion as well as the expression of pulmonary surfactant was further improved.
Safety Evaluation
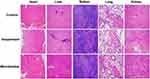
We evaluated the safety of the preparation for 14 days after treatment with the formulations. Compared with the control group of normal saline, no inflammation, degeneration or necrosis was found in the pathological tissue sections of the organs in the sinalpultide suspension and microbubble preparation group (Figure 6), showing the lower toxicity and higher safety of microbubble preparation.20 Through the measurement of body weight during continuous administration, the weight growth curve was drawn. As shown in Figure 7, compared with the normal saline group, there is no significant difference in body weight compared to the suspension group and the microbubble group. After 14 days of continuous administration, the main organs of the mice were obtained and the organ coefficients were calculated (Table 2). Results showed that there was no significant difference in organ coefficients between the sinalpultide suspension group and the normal saline group. In summary, it could be concluded that the sinalpultide microbubble preparations did not cause further damage of the liver, spleen, heart, lung and kidney compared to the control group.
 |
Table 2 Organ Coefficient of Main Organs in Mice After Administration for 14 Days at a Dose of 2.32 mg/kg per Day (n = 6) |
Discussion
Recently, with the development of ultrasound diagnosis and treatment, ultrasound combined with microbubbles has been widely used as a targeted therapeutic strategy in the field of gene therapy and drug delivery.21,22 Additionally, there are a few reports of the application of microbubbles in vivo through the regulation of ultrasound energy. Surfaxin® is the only total synthetic pulmonary surfactant medicine on the market to date. Though it has been widely used in the treatment of respiratory distress syndrome, there are still many shortcomings for suspension dosage form, such as a large single dose, a single treatment method, and further improvement of bioavailability.23,24 Accordingly, the method of sinapultide-loaded microbubbles combined with ultrasound provide a new option for the treatment of such disease. Firstly, the efficacy of microbubbles was better than that of suspension at the same dose; secondly, by using the effect of ultrasonic force, results showed that the appropriate ultrasonic energy can promote the recovery of cell physiological function. In this study, we studied the effects of sinapultide-loaded microbubbles combined with ultrasound in AT II cells and LPS-induced ALI in mice. Sinapultide MBs pretreatment reduced the inflammatory cytokines levels, improved the expression of SP-A, and decreased the lung W/D weight ratio. Histological analysis further verified that sinapultide MBs-ultrasound combination method significantly attenuated lung tissue injury. Besides, sinapultide MBs showed a good security for future clinical application.
LPS has been reported as a main cause of ALI, and the replication of ALI cells and animal models by LPS is one of the important means for scientific research.25 In this study, the mice model of acute lung injury was established by LPS, and the mice showed obvious lung injury symptoms, such as thickening of alveolar wall, pulmonary congestion, pulmonary interstitial edema, etc.26 The function of alveolar epithelial cells affects the occurrence and development of ALI, for AT II will involve in the interaction between inflammatory cells by secreting cytokines and inflammatory mediators. As the initial mediator of inflammatory cascade reaction, TNF- α is an important cytokine in pulmonary inflammation. In addition, the secretion of cytokine IL-6 can also promote inflammatory response and tissue damage, and various influencing factors interact with each other within the ALI process.27,28 In the experiment, the concentration of TNF-α and IL-6 in cell supernatant and BALF were increased after being stimulated by LPS, indicating that the inflammatory cytokines activated and released by LPS is one of the mechanisms of lung injury. Further, from the experiment, we were able to discover that the therapeutic method of sinapultide MBs combined ultrasound can reduce the secretion of pro-inflammatory cytokines effectively, which confirms that this combination method is effective in the treatment of acute lung injury.
The protein of SP-A is synthesized and secreted by the type of epithelial cells in the alveoli, which holds the largest proportion in AT II cells. The main function of SP-A is to regulate local immune and inflammatory reactions, reduce the surface tension of air-liquid on the alveoli, and prevent excessive collapse of alveoli during exhalation etc.29 The results of the present study showed that the expression of SP-A in lung tissue decreased significantly after LPS was administered. One potential mechanism is that the degeneration and necrosis of AT II cells leads to hyposecretion of PS during the ALI process. Additionally, the increase of pulmonary capillary permeability results in the bidirectional leakage of macromolecules as well as SP-A in the blood vessels. Besides, the release of large amounts of elastase and inflammatory cell metabolites will degrade SP-A and further decrease the content of SP-A level.30 The abnormality of PS quality and quantity promotes the occurrence and development of lung injury, and the aggravation of lung injury can further lead to PS dysfunction. In the experiment, after being administered with sinalpultide MBs, the expression of SP-A was increased and the pathological changes of lung tissue were alleviated significantly in ALI mice. It is suggested that sinalpultide microbubble preparation has a certain therapeutic effect on lung injury, and promoting PS expression may be one of its working mechanisms.
Pulmonary edema and severe hypoxemia are typical manifestations of acute lung injury, and the lung W/D weight was used to evaluate the degree of pulmonary edema. The improvement of pulmonary edema not only increased the diffusion distance of gas exchange in lung and then improved the diffusion dysfunction of the lung tissue, but also improved lung compliance and strengthened the ventilation of blood flow balance. One study has shown that hypoxemia is directly related to the ratio of W/D weight of lung tissue, meaning that the decrease of W/D weight ratio to lung tissue indicates the improvement of hypoxemia and acute lung injury.31 The integrity of alveolar epithelial cells will be damaged and the function will be affected while LPS interfere with lung tissue, and the alveolar fluid clearance ability of alveolar epithelial cells will be affected leading to the occurrence of pulmonary edema. Therefore, the innovative treatment of microbubble preparation combined with ultrasound for the improvement of pulmonary tissue edema indirectly shows that it improves the function of alveolar epithelial cells and restores the pulmonary water clearance ability. Though appropriate ultrasound energy can promote the recovery of physiological function at cell level for the mechanical effect of ultrasound, the mechanism of ultrasound combination method to promote the function of alveolar epithelial cells needs to be further studied. It’s speculated that the mechanism may be related to the mechanical effect and cavitation effect of ultrasound. On the one hand, appropriate intensity of ultrasound stimulation can promote cell growth and functional recovery, on the other hand, the effect of ultrasonic force makes it easy to fuse between microbubbles and cells.32–35
Conclusions
In conclusion, sinalpultide-loaded microbubbles combined with ultrasonic treatment significantly decreased the production of IL-6 and TNF-α in the ALI mouse model. Importantly, the level of SP-A was increased and the pulmonary edema in LPS-induced ALI was significantly attenuated by the combination treatment. Furthermore, sinalpultide-loaded microbubbles showed satisfying initial safety in mice after repeated administration. Collectively, these results suggest that sinalpultide-loaded MBs combined with -ultrasound may be a potential novel strategy for the management of lung injury diseases in clinics.
Acknowledgments
This investigation was financially supported by the National Natural Science Foundation of China (31901383), High level talent project of West Anhui University (WGKQ202001012), Key projects of excellent young talents support program of Anhui universities (gxyqZD2020040) and the Natural Science Foundation of Anhui Province (1808085QH233). We thank Professor Fang Yang and Professor Ning Gu for technical support and for careful help in preparing sinapultide microbubbles as well as in vivo experiments.
Author Contributions
All authors made a significant contribution to the work reported, whether that was in the conception, study design, execution, acquisition of data, analysis and interpretation, or in all these areas; took part in drafting, revising or critically reviewing the article; gave final approval of the version to be published; have agreed on the journal to which the article has been submitted; and agree to be accountable for all aspects of the work.
Disclosure
The authors report no conflicts of interest in this work.
References
1. Wheeler AP, Bernard GR. Acute lung injury and the acute respiratory distress syndrome: a clinical review. Lancet. 2007;369(9572):1553–1564. doi:10.1016/S0140-6736(07)60604-7
2. Levy BD. Acute lung injury and the acute respiratory distress syndrome. Respir Tract Pediatr Crit Ill Inj. 2012;25–36.
3. Xu Y, Xiang J, Zhao H, et al. Human amniotic fluid stem cells labeled with up-conversion nanoparticles for imaging-monitored repairing of acute lung injury. Biomaterials. 2016;100:91–100. doi:10.1016/j.biomaterials.2016.05.034
4. Zhou F, Zhang Y, Chen J, et al. Liraglutide attenuates lipopolysaccharide-induced acute lung injury in mice. Eur J Pharmacol. 2016;791:735–740. doi:10.1016/j.ejphar.2016.10.016
5. Ware LB, Matthay MA. Resolution of alveolar edema: mechanisms and relationship to clinical acute lung injury. Acute Respir Distress Syndr. 2016;233:255–279.
6. Zhang L-N, Sun J-P, Xue X-Y, et al. Exogenous pulmonary surfactant for acute respiratory distress syndrome in adults: a systematic review and meta-analysis. Exp Ther Med. 2013;5(1):237–242. doi:10.3892/etm.2012.746
7. Steingrub M, Tidswell M. Pulmonary-artery versus central venous catheter to guide treatment of acute lung injury. N Engl J Med. 2006;354:2213–2224.
8. Standiford TJ, Ward PA. Therapeutic targeting of acute lung injury and acute respiratory distress syndrome. Transl Res. 2016;167(1):183–191. doi:10.1016/j.trsl.2015.04.015
9. Braide-Moncoeur O, Tran NT, Long JR. Peptide-based synthetic pulmonary surfactant for the treatment of respiratory distress disorders. Curr Opin Chem Biol. 2016;32:22–28. doi:10.1016/j.cbpa.2016.02.012
10. Guagliardo R, Pérez-Gil J, De Smedt S, et al. Pulmonary surfactant and drug delivery: focusing on the role of surfactant proteins. J Control Release. 2018;291:116–126. doi:10.1016/j.jconrel.2018.10.012
11. Liu D, Zhang Z, Qin Z, et al. Sinapultide-loaded lipid microbubbles and the stabilization effect of sinapultide on the shells of lipid microbubbles. J Mater Chem B. 2018;6(9):1335–1341. doi:10.1039/C7TB02799K
12. Zeng M, Sang W, Chen S, et al. 4-PBA inhibits LPS-induced inflammation through regulating ER stress and autophagy in acute lung injury models. Toxicol Lett. 2017;271:26–37. doi:10.1016/j.toxlet.2017.02.023
13. Lei J, Wei Y, Song P, et al. Cordycepin inhibits LPS-induced acute lung injury by inhibiting inflammation and oxidative stress. Eur J Pharmacol. 2018;818:110–114. doi:10.1016/j.ejphar.2017.10.029
14. Wang Q, Chen B, Cao M, et al. Response of MAPK pathway to iron oxide nanoparticles in vitro treatment promotes osteogenic differentiation of hBMSCs. Biomaterials. 2016;86:11–20. doi:10.1016/j.biomaterials.2016.02.004
15. Yang F, Li M, Cui H, et al. Altering the response of intracellular reactive oxygen to magnetic nanoparticles using ultrasound and microbubbles. Sci China Mater. 2015;58(6):467–480. doi:10.1007/s40843-015-0059-9
16. Bai GZ, Yu HT, Ni YF, et al. Shikonin attenuates lipopolysaccharide-induced acute lung injury in mice. J Surg Res. 2012;182:303–311. doi:10.1016/j.jss.2012.10.039
17. Lal MK, Sinha SK. Lucinactant (Surfaxin™) for prevention and treatment of respiratory distress syndrome in newborns. Pediatr Health. 2009;3:427–434.
18. Yu PJ, Wan LM, Wan SH, et al. Standardized myrtol attenuates lipopolysaccharide induced acute lung injury in mice. Pharm Biol. 2016;54:3211–3216. doi:10.1080/13880209.2016.1216132
19. Liu D, Xing J, Xiong F, et al. Preparation and in vivo safety evaluations of antileukemic homoharringtonine-loaded PEGylated liposomes. Drug Dev Ind Pharm. 2017;43(4):652–660. doi:10.1080/03639045.2016.1275670
20. Shi Y, Lammers T. Combining nanomedicine and immunotherapy. Acc Chem Res. 2019;52(6):1543–1554. doi:10.1021/acs.accounts.9b00148
21. Yang F, Li M, Liu Y, et al. Glucose and magnetic-responsive approach toward in situ nitric oxide bubbles controlled generation for hyperglycemia theranostics. J Control Release. 2016;228:87–95. doi:10.1016/j.jconrel.2016.03.002
22. Duan L, Yang L, Jin J, et al. Micro/nano-bubble-assisted ultrasound to enhance the EPR effect and potential theranostic applications. Theranostics. 2020;10(2):462–487. doi:10.7150/thno.37593
23. Chen S, Ding J-H, Bekeredjian R, et al. Efficient gene delivery to pancreatic islets with ultrasonic microbubble destruction technology. Proc Natl Acad Sci U S A. 2006;103(22):8469–8474. doi:10.1073/pnas.0602921103
24. Silveyra P, Floros J. Genetic variant associations of human SP-A and SP-D with acute and chronic lung injury. Front Biosci. 2012;17:407–416. doi:10.2741/3935
25. Bosma KJ, Taneja R, Lewis JF. Pharmacotherapy for prevention and treatment of acute respiratory distress syndrome. Drugs. 2010;70(10):1255–1282. doi:10.2165/10898570-000000000-00000
26. Middleton EA, Rondina MT, Schwertz H, et al. Amicus or adversary revisited: platelets in acute lung injury and acute respiratory distress syndrome. Am J Respir Cell Mol Biol. 2018;59:18–35. doi:10.1165/rcmb.2017-0420TR
27. Ren J. Relationship between serum TNF-α and IL-6 levels and respiratory function in patients with chronic obstructive pulmonary disease. Int J Lab Med. 2017;38:1781–1783.
28. Joshi L, Chelluri LK, Valluri V, et al. Association of TNF-α, IL-10 and IL-6 promoter polymorphisms in pulmonary tuberculosis patients and their household contacts of younger age group. Comp Immunol Microbiol Infect Dis. 2018;56:20–26. doi:10.1016/j.cimid.2017.12.001
29. Kishore U, Greenhough TJ, Waters P, et al. Surfactant proteins SP-A and SP-D: structure, function and receptors. Mol Immunol. 2006;43(9):1293–1315. doi:10.1016/j.molimm.2005.08.004
30. Haque R, Umstead TM, Ponnuru P, et al. Role of surfactant protein-A (SP-A) in lung injury in response to acute ozone exposure of SP-A deficient mice. Toxicol Appl Pharmacol. 2007;220(1):72–82. doi:10.1016/j.taap.2006.12.017
31. Chen H, Bai C, Wang X, Kishore U, Greenhough TJ, Waters P. The value of the lipopolysaccharide-induced acute lung injury model in respiratory medicine. Expert Rev Respir Med. 2018;56(6):773–783. doi:10.1586/ers.10.71
32. Matsuura K, Hirotsune M, Nunokawa Y, et al. Acceleration of cell growth and ester formation by ultrasonic wave irradiation. J Ferment Bioeng. 1994;77(1):36–40. doi:10.1016/0922-338X(94)90205-4
33. McMillan JR, Watson IA, Ali M, Jaafar W. Evaluation and comparison of algal cell disruption methods: microwave, waterbath, blender, ultrasonic and laser treatment. Appl Energy. 2013;103:128–134. doi:10.1016/j.apenergy.2012.09.020
34. Wang H, Ding YF, Wei K, et al. Oxymatrine liposomes for intervertebral disc treatment: formulation, in vitro and vivo assessments. Drug Des Devel Ther. 2020;14:921–931. doi:10.2147/DDDT.S242493
35. Yang F, Gu N, Chen D, et al. Experimental study on cell self-sealing during sonoporation. J Control Release. 2008;131(3):205–210. doi:10.1016/j.jconrel.2008.07.038
 © 2020 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2020 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.