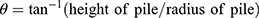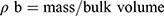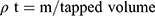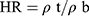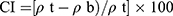Back to Journals » Drug Design, Development and Therapy » Volume 14
Simvastatin-Nicotinamide Co-Crystals: Formation, Pharmaceutical Characterization and in vivo Profile
Authors Khan FM, Ahmad M , Idrees HA
Received 4 July 2020
Accepted for publication 25 September 2020
Published 19 October 2020 Volume 2020:14 Pages 4303—4313
DOI https://doi.org/10.2147/DDDT.S270742
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 2
Editor who approved publication: Professor Anastasios Lymperopoulos
Fahad Mehmood Khan,1 Mahmood Ahmad,2 Hafiz Arfat Idrees1
1Faculty of Pharmacy and Alternative Medicine, The Islamia University of Bahawalpur, Punjab 63100, Pakistan; 2Faculty of Pharmacy, University of Central Punjab, Punjab 54000, Pakistan
Correspondence: Mahmood Ahmad Tel +92 300 9682258
Email [email protected]
Purpose: To enhance the solubility and dissolution profile of simvastatin (SIM) through co-crystallization with varying ratios of nicotinamide (NIC) using various co-methods.
Materials and Methods: Twelve SIM:NIC co-crystal formulations (F01–F12) were prepared using dry grinding, slurry, liquid-assisted grinding, and solvent-evaporation methods, and their properties compared. Optimized formulations were selected on the basis of dissolution profiles and solubility for in vivo studies. The angle of repose, Carr Index and Hausner ratio were calculated to evaluate flow properties. Differential light scattering (DLS) was used to estimate particle-size distribution. Scanning electron microscopy (SEM) was employed to evaluate surface morphology. Thermal analyses and Fourier-transform infrared (FTIR) spectroscopy were used to determine the ranges of thermal stability and physical interaction of formulated co-crystals. X-ray powder diffraction (XPD) spectroscopy was used to determine the crystalline nature. Solubility and dissolution studies were undertaken to determine in vitro drug-release behaviors.
Results: Micromeritic analyses revealed the good flow properties of formulated co-crystals. DLS showed the particle size of co-crystals to be in the nanometer range. SEM revealed that the co-crystals were regular cubes. Thermal studies showed the stability of co-crystals at > 300°C. FTIR spectroscopy revealed minor shifts of various peaks. XPD spectroscopy demonstrated co-crystal formation. The formulations exhibited an improved dissolution profile with marked improvements in solubility. In vivo studies showed a 2.4-fold increase in Cmax whereas total AUC(0–∞) was increased 4.75-fold as compared with that of SIM tablets.
Conclusion: Co-crystallization with NIC improved the solubility and dissolution profile and, hence, the bioavailability of the poorly water-soluble drug SIM.
Keywords: pharmacokinetics, solubility enhancement, simvastatin, nicotinamide, co-crystal
Introduction
“Co-crystals” are crystalline solids in which interrelating components in stoichiometric ratios, with different structures, are formed as a single crystalline phase. Pharmaceutical co-crystals belong to a subclass of co-crystals wherein one of the components is a drug molecule (or an active pharmaceutical ingredient (API)) and the second is a benign food or drug grade additive (generally regarded as safe). Pharmaceutical co-crystals have become important for solid-state screening because they demonstrate the physicochemical properties of APIs. Co-crystals have been investigated with regard to increasing bioperformance, and their effect on bioavailability in preclinical studies has been very encouraging.1–3 Indeed, co-crystallization is now a promising concept to improve solubility and, thus, the bioavailability of drug products.
Simvastatin (SIM) is a lipid-lowering drug and belongs to class of drugs called “statins”. It is a prodrug that lowers the cholesterol level by inhibiting releases of 3-hydroxy-3-methyl-glutaryl-coenzyme A reductase. The hydroxy acid form of SIM is pharmacologically active, thanks to the chemical and enzymatic actions on the prodrug.4 SIM reduces high glucose levels and mitigates vascular events in people with type-2 diabetes mellitus.5
Use of drugs that have poor solubility in water leads to problems with respect to dissolution and bioavailability. SIM has low solubility in water (30 µg/mL). Hence, SIM is placed in class II of the Biopharmaceutical Classification System, and its bioavailability is only 5%.6–9 Thus, improvement in its release in vitro may lead to bioavailability enhancement. Attempts have been made to enhance SIM solubility using different methods and different dosage formulations.10,11
We wished to enhance the solubility and dissolution profile of SIM through co-crystallization with varying ratios of NIC using various methods. Increases in solubility and dissolution were studied with respect to increases in the molar ratio of co-crystals. The formulated co-crystals were characterized using micromeritics, drug release (in vitro and in vivo), solubility studies, determination of molecular size by differential light scattering (DLS), Fourier transform infrared (FTIR) spectroscopy, thermal analyses (thermogravimetric analysis (TGA) and differential scanning calorimetry (DSC)), X-ray powder diffraction (XPD) spectroscopy, and scanning electron microscopy (SEM).
Materials and Methods
SIM (C25H38O5) (Purity 98.78% and batch number 201–002.180) was provided by Bio Fine Pharmaceuticals Lab (Pakistan). Nicotinamide (NIC; C6H6N2O) and sodium hydroxide were purchased from Sigma–Aldrich (Saint Louis, MO, USA). Methanol, potassium hydroxide, hydrochloric acid and potassium dihydrogen phosphate were bought from Merck (Kenilworth, NJ, USA).
Co-Crystallization of Simvastatin and Nicotinamide
The formulations were synthesized using SIM and NIC in equimolar ratios via the methods indicated in Table 1. In the co-grinding method, SIM and NIC were milled together using a pestle and mortar at the indicated stoichiometric ratios for 45 min without solvent addition. In the LAG method, the same ingredients were milled for 30 min using a pestle and mortar but with methanol addition. For the slurry method, SIM and NIC were slurried overnight at 300 rpm using a magnetic stirrer, and the solution was left to evaporate slowly for 12–16 h at room temperature. In the solvent-evaporation method, SIM and NIC were solubilized in methanol (as the common solvent) and then the solution was left for 20–24 h at room temperature to evaporate slowly.
 |
Table 1 Compositions of SIM-NIC Co-Crystals in Molar Ratios |
Characterization of Formulated Co-Crystals
Micromeritics Properties of Simvastatin and Its Co-Crystals
The fixed-funnel method was used to evaluate the angle of repose (Ɵ). The latter characterizes the frictional force within a powder, and a value of 20–30° indicates good flow properties.12 Ɵ was determined using Equation (1):
Bulk density (ρƅ) and tapped density (ρt) were calculated with a known mass of SIM and powdered formulations. Equation (2) and Equation (3) were used to calculate ρƅ and ρt, respectively.12
The Hausner ratio (HR) predicts the flow properties related to inter-particle friction. HR <1.25 indicates good flowability.12 The HR was calculated employing the pre-determined ρb and ρt using Equation (4):
The Carr Index (CI) is an index of flow rate, particle size, and cohesiveness. A CI of 5–18% denotes the suitability of tablets. The CI was calculated using Equation (5).12
In vitro Dissolution Studies of Formulated Co-Crystals
Drug release in vitro was evaluated in acidic (pH 1.2) and basic (pH 7.4) media using 50 mg-equivalent of pure drug and co-crystal formulations. A USP type-II apparatus was operated at 37± 0.5°C with 900 mL of media in each dissolution vessel, and sink conditions were maintained at 75 rpm. Aliquots of media were taken in labeled glass vials at various times (0, 10, 15, 30, 45, 60, and 90 min) using adjustable pipettes. Samples were filtered, diluted, and analyzed at 238 nm in a UV–visible spectrophotometer (UV-1601; Shimadzu, Tokyo, Japan). All samples were analyzed in triplicate.
Solubility Studies of Formulated Co-Crystals
Solubility studies were undertaken by adding an excess amount of sample (drug or co-crystal) to 10 mL of each medium (ie, aqueous, acidic (pH 1.2) or basic (pH 7.4) media). The mixture of sample and medium was stirred at 400 rpm up to 48 h at room temperature. Then, samples were withdrawn in triplicate, passed through filter paper (number 42; Whatman, Maidstone, UK) and diluted before spectrophotometry. Samples were collected in triplicate.13
Determination of Particle Size
DLS was used with the help of the Nano-series Zetasizer (ZEN3600; Malvern Instruments, Malvern, UK) installed with Malvern Zetasizer v7.01.
FTIR Spectroscopy
FTIR spectroscopy for pure SIM and moisture-free, powdered co-crystals was undertaken using a Tensor 27 FTIR spectrophotometer (Bruker, Billerica, MA, USA) with OPUS software. The resolution was 2 cm−1 in the frequency range 4000 cm−1 to 400 cm−1.14
Thermal Analyses
TGA and DSC were carried out using the thermal analysis system Q600 SDT (TA Instruments, New Castle, DW, USA) over the temperature range 50–400°C at a heating rate of 10°C/min using 0.5–3.0 mg of sample.15
XRD Spectroscopy
XPD spectroscopy for pure drug and co-crystals was undertaken using a D8 Advance X-ray Diffractometer (Bruker). Data were collected using 2–5 mg of sample in the temperature range 5–70°C under-defined conditions (tube voltage=40 kV, current=40 mA, and 2ø=0.001°) using a wide angle.15
SEM
A scanning electron microscope (EVO® LS 10; Carl Zeiss, Jena, Germany) was used for microscopic evaluation of the pure drug and formulated co-crystals. The coating was made with gold-sputtered atoms.16
In vivo Experimental Design
In vivo analyses were conducted using high-performance liquid chromatography (on a 1100 series (Agilent Technologies, Santa Clara, CA, USA)) equipped with a pump (LC-10), degasser, injector (20-µL loop) and UV detector. The column was a stainless-steel ODS Hypersil™ C18 (5-µm particle size and 4.6 mm×250 mm) with flow rate of 1 mL/min and run time of 15 min. The mobile phase consisted of acetonitrile:monobasic sodium phosphate (Buffer pH of 4.5 adjusted with 5N sodium hydroxide) in a 65:35 ratio. For in vivo studies, a crossover single-dose study was conducted on rabbits (n=36)17–19 as per protocol and approval from “Pharmacy Research Ethics Committee” of Faculty of Pharmacy and Alternative medicine, the Islamia University of Bahawalpur (Ref No. 11–2017/PREC). Each blood sample was centrifuged and the plasma separated and stored at −70°C in an ultra-refrigerator using Eppendorf™ tubes. For analyses, each frozen sample was removed from the ultra-refrigerator and maintained at room temperature. Plasma and a precipitating agent were vortex-mixed and then centrifuged to obtain a clear supernatant. Each sample (10 µL), before injection into the HPLC system, was passed through a syringe filter.
Pharmacokinetics
A non-compartmental model was applied and pharmacokinetic parameters (Cmax (µg/mL), Tmax (h), T1/2 (h), AUC(0–∞) (μg/mL.min), AUMC(0–∞) (μg/mL.min2), MRT (h), Cl (L/min) and Vz (L)) were evaluated using PKSolver 2.0.20
Statistical Analyses
Results are presented as the mean±SD. Data obtained were analyzed using analysis of variance through Excel™ 2016 (Microsoft, Redmond, WA, USA).
Results
Micromeritics Evaluation of Co-Crystals
The flow properties of co-crystals were studied by calculating the Ɵ, HR and CI (Table 2).
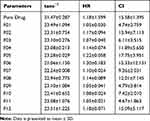 |
Table 2 Micromeritic Properties of Formulated SIM-NIC Co-Crystals |
Drug Release in vitro
In vitro release of the pure drug was in the range 1.69–12.16% at pH 1.2, and 1.71–15.12% at pH 7.4. Spectrophotometry showed that maximum drug release was obtained from the co-crystal F11 in both media.
The in vitro drug-release patterns at pH 7.4 and pH 1.2 are shown in Figure 1. The order of drug release from all formulated co-crystals and pure drug in both media was: F02 > F03 > F04 > F01 > SIM for SIM:NIC at a 1:1 ratio; F07 > F06 > F08 > F05 > SIM for SIM:NIC at a 1:2 ratio; F11 > F10 > F12 > F09 > SIM for SIM:NIC at a 1:4 ratio. Drug release in basic medium was higher than that in acidic medium. For the formulated SIM:NIC (1:1) co-crystals, the release was 7.67–68.96%, 8.08–82.87%, 8.00–75.90% and 7.89–72.78%, whereas for SIM:NIC (1:2) co-crystals it was 9.57–71.96%,10.11–79.70%, 9.08–88.77% and 9.89–75.78%. Drug release increased with an increase in the molar ratio of conformer. For SIM:NIC (1:4), the release in basic medium was 9.57–71.96%,10.55–85.70%,10.99–97.73% and 10.88–82.78%. Maximum drug release was recorded for F11 at pH 1.2 and pH 7.4, respectively.
 |
Figure 1 Comparative dissolution profile of SIM and all co-crystal formulations in acidic and basic media. |
Solubility Studies
Solubility studies were conducted on optimized formulations. For this purpose, F02, F07, and F11 were selected, one from each pair of ratios, having the highest dissolution profile. In the formulated co-crystals, solubility was improved significantly when compared with that of the pure drug. Moreover, F02 showed increases in percentage solubility of 172.89%, 297.34%, and 334.17% in water, acidic medium, and basic medium, respectively. Similarly, F07 had enhanced solubility, with 424.89% in water, 425.29% in acidic medium, and 485.99% in basic medium. The percentage increase in the solubility of F11 in water, acidic medium, and basic medium was 504.44%, 505.06% and 641.52%, respectively.
Particle Size
The average particle size (Z-Average) of SIM:NIC (1:1), SIM:NIC (1:2) and SIM:NIC (1:4) was 298.7±62.5, 242.7±11.7, and 231.3±10.6 nm, respectively, as shown in Figure 2. The Polydispersity Index (PDI) of these co-crystals was 0.142, 0.151, and 0.182, respectively.
 |
Figure 2 Zeta sizer analysis of F02; SIM:NIC (1:1), F07; SIM:NIC (1:2) and F11; SIM:NIC (1:4). |
FTIR Spectroscopy
FTIR spectra for pure SIM and formed co-crystals were obtained to ascertain any intermolecular interactions between SIM and NIC (Figure 3). Pure SIM exhibited characteristic peaks at 3558 cm−1 (hydroxyl stretching); 3011, 2956, and 2872 cm−1 (methine stretching), and 1706 cm−1 (ester-lactone carbonyl stretching).21 In contrast, NIC showed characteristic –NH2 asymmetrical and symmetrical stretching vibrations at 3348 and 3148 cm−1, respectively. A –C=O absorption band showed a characteristic peak at 1677 cm−1, –NH bending vibrations were observed at 1615 cm−1 and –CN stretching vibrations were observed at 1394 cm−1.22
 |
Figure 3 FTIR of SIM and co-crystals formulations F02; SIM:NIC (1:1), F07; SIM:NIC (1:2) and F11; SIM:NIC (1:4). |
XRD Spectroscopy
XRD spectroscopy revealed the crystallinity of the formulated co-crystals. XRD spectroscopy of pure SIM showed characteristic peaks at diffraction angles of 9.46°, 10.08°, 10.72°, 15.02°, and 17.64°.23 However, an increase in crystallinity was observed24 with more intense and sharper peaks of the formulated co-crystals at diffraction angles of: 9.39°, 15.25°, 17.8°, 22.18°, 25.42°, 27.52° for F02; 99.41°, 10.12°, 10.48°, 10.52°, 10.6°,10.74°, 10.76°, 10.82°, 10.84°, 20.22°, and 20.83° for F07; 9.42°, 10.12°, 10.41°, 10.50°, 10.62°,10.76°, 10.77°, 10.82°, 10.84°, and 20.86° for F11 (Figure 4).
 |
Figure 4 PXRD spectra of SIM and co-crystals formulations F02; SIM:NIC (1:1), F07; SIM:NIC (1:2) and F11; SIM:NIC (1:4). |
Thermal Analyses
Pure SIM exhibited a sharp endothermic peak in the DSC curve at 138.8°C, which denoted its melting point.4,14 The DSC thermogram of the optimized formulation SIM:NIC (1:1) showed a preliminary endothermic peak at 103.12°C and an additional peak at 218.5°C (Figure 5A). In the DSC curve of the optimized formulations SIM:NIC (1:2) and SIM:NIC (1:4), the initial endothermic peak appeared at 99.12°C and 103.57°C, followed by an additional endothermic peak at 208.83°C and 200.97°C, respectively. The TGA thermogram of pure SIM exhibited a maximum mass loss (58.37%) at >300°C after 14.36 min, whereas a slight mass loss (5.89%) was observed at >400°C after 16.54 min. The TGA thermograms of optimized formulation SIM:NIC (1:1) showed a preliminary mass loss (9.22%) at >90°C, with a maximum mass loss (19.25%) at >200°C. A further mass loss of 7.78% was observed at >300°C. For optimized formulations SIM:NIC (1:2) and SIM:NIC (1:4), a smaller mass loss (8.72% and 9.16%, respectively) was observed at 91–92°C, whereas a maximum mass loss (23.55% and 20.57%, respectively) occurred at 190–200°C, with some mass loss (7.54% and 7.48%, respectively) at 310–320°C (Figure 5B).
 |
Figure 5 DSC analysis (A) and TGA (B) of (a) SIM and co-crystals formulations (b) F02; SIM:NIC (1:1), (c) F07; SIM:NIC (1:2) and (d) F11; SIM:NIC (1:4). |
SEM
Microscopic evaluation of the pure drug and formulated SIM:NIC co-crystals was done by SEM and the results are shown in Figure 6.
 |
Figure 6 SEM analysis; SIM at 100x (A), SIM:NIC (1:4) co-crystals at 100x (B) and at 1000x (C). |
Pharmacokinetics
Pharmacokinetic parameters were calculated with the help of pK Solver based on a non-compartmental system. The concentration–time profile in plasma of the drug and formulated co-crystals is shown in Figure 7. All comparative values of pharmacokinetic parameters are stated in Table 3 comprehending enhanced Cmax, with improved total AUC from co-crystals as compared to simvastatin tablets.
 |
Table 3 Pharmacokinetic Parameters Following Oral Administration of SIM and SIM-GLU (F11) co-crystals to Healthy Rabbits (n=12) |
 |
Figure 7 Mean ± SD Plasma concentration time profile of SIM and SIM: NIC co-crystals (n=12). |
Discussion
The formulated co-crystals had a CI value in the range of 5–18% and HR <1.25. Hence, all formulations showed good flowability and compressibility when compared with the micrometric properties of the pure drug.
The dissolution and release profiles of various low-solubility drugs in different media can be improved upon co-crystallization.25,26 In the present study, for all formulations, dissolution and solubility were improved in the aqueous phase, acidic medium, and basic medium. Moreover, the drug-release profile for all SIM:NIC formulations (1:1, 1:2 and 1:4) and pure drug were higher at pH 7.4 than the corresponding drug-release profile at pH 1.2. In basic medium, salt formation for SIM is not feasible.27 Thus, the increase in drug release is most likely due to co-crystallization. The formulation F11 exhibited the maximum release and solubility of the drug. This phenomenon was likely due to formation of more hydrogen bonds because the solvent-evaporation method has been reported to produce co-crystals with new hydrogen bonds.28
The PDI value denotes size uniformity within a formulation. PDI >1 indicates low uniformity, and vice versa.4 The formulated co-crystals had low PDI (0.142–0.182), which suggested the uniformity of particle size. The FTIR spectra of the formulated co-crystals showed new peaks. The peak shifts indicate formation of new bonds. Co-crystallization may cause interactions among various single-component crystalline-phase molecules. It is most likely that these interactions were responsible for the new molecular conformations seen in the FTIR spectra of the formulated co-crystals.29
The DSC and TGA curves for the formulated co-crystals differed from those of the original components. Formation of new phases was confirmed further because the co-crystals showed distinct thermal behaviors, and transition was observed relative to that of the individual components. The peak in the DSC curve of the pure drug was retained in all formulations, indicating the compatibility of the drug and NIC.30 Multiple endothermic peaks with broad endotherms may have been due to different crystal structures with different degrees of crystallinity.9 It has been reported that co-crystals obtained using different preparation methods show different endothermic peak temperatures, to some extent.31 TGA revealed the thermal stability of formulated co-crystals because maximum mass losses were observed at very high temperatures when compared with those of the original components.
The co-crystals showed distinct XRD patterns, which may reflect differences in the processes used in their preparation. XRD spectra further supported the notion of formation of new and different crystalline structures.29 Further screening using different methods may be helpful in obtaining clearer understanding of the structures of the formulated co-crystals.
SIM appeared as rod-shaped crystals.23 In contrast, optimized formulated co-crystals showed asymmetrical-to-spherical crystals with irregular surfaces. These analyses provide further evidence of crystal formation, and also support the results from XRD and DSC. SEM images suggested that particle size was reduced, a feature which may contribute to increased solubility.
There was a significant difference in the pharmacokinetic parameters of tablets and formulated co-crystals. Pharmaceutical co-crystals can increase Cmax and total AUC by many fold. This increase with no change in Tmax could be because various co-crystal characteristics influence the pharmacokinetics of an API in different ways.32
Conclusion
Co-crystals of SIM with NIC prepared by solvent evaporation showed significantly higher in vitro drug release, solubility, as well as a corresponding in vivo pharmacokinetic profile when compared with those of pure SIM. We demonstrated the potential usefulness of co-crystallization in solubility enhancement of SMI and the possibility to improve its bioavailability.
Acknowledgments
The authors acknowledge the Pharmacy Department, Islamia University Bahawalpur, for providing essential equipment and resources to undertake this work.
Disclosure
The authors report no conflicts of interest in this work.
References
1. Almarsson Ö, Peterson ML, Zaworotko M. The A to Z of pharmaceutical cocrystals: a decade of fast-moving new science and patents. Pharm Patent Anal. 2012;1(3):313–327. doi:10.4155/ppa.12.29
2. Korotkova EI, Kratochvíl B. Pharmaceutical cocrystals. Procedia Chem. 2014;10:473–476. doi:10.1016/j.proche.2014.10.079
3. Babu NJ, Nangia A. Solubility advantage of amorphous drugs and pharmaceutical cocrystals. Cryst Growth Des. 2011;11(7):2662–2679. doi:10.1021/cg200492w
4. Gu F, Ning J, Fan H, Wu C, Wang Y. Preparation and characterization of simvastatin/DMβCD complex and its pharmacokinetics in rats. Acta Pharmaceutica. 2018;68(2):145–157. doi:10.2478/acph-2018-0016
5. Banes-Berceli AK, Shaw S, Ma G, et al. Effect of simvastatin on high glucose-and angiotensin II-induced activation of the JAK/STAT pathway in mesangial cells. Am J Physiol Renal Physiol. 2017;291(1):F116–F121. doi:10.1152/ajprenal.00502.2005
6. Dahan A, Miller JM, Amidon GL. Prediction of solubility and permeability class membership: provisional BCS classification of the world’s top oral drugs. AAPS J. 2009;11(4):740–746. doi:10.1208/s12248-009-9144-x
7. Murtaza G. Solubility enhancement of simvastatin: a review. Acta Pol Pharm. 2012;69(4):581–590.
8. Sopyan I, Fudholi A, Muchtaridi M, Sari IP. Simvastatin-nicotinamide co-crystal: design, preparation and preliminary characterization. Trop J Pharm Res. 2017;16(2):297–303. doi:10.4314/tjpr.v16i2.6
9. Rao M, Mandage Y, Thanki K, Bhise S. Dissolution improvement of simvastatin by surface solid dispersion technology. Dissolution Technol. 2010;17(2):27–34. doi:10.14227/DT170210P27
10. Savjani KT, Gajjar AK, Savjani JK. Drug solubility: importance and enhancement techniques. ISRN Pharm. 2012;2012:10. doi:10.5402/2012/195727
11. Qiao N, Li M, Schlindwein W, Malek N, Davies A, Trappitt G. Pharmaceutical cocrystals: an overview. Int J Pharm. 2011;419(1):1–11. doi:10.1016/j.ijpharm.2011.07.037
12. Rajurkar V, Sunil N, Ghawate V. Tablet formulation and enhancement of aqueous solubility of efavirenz by solvent evaporation Co-Crystal technique. Med Chem S. 2015;2:2161–2444.
13. Zhou Z, Li W, Sun W-J, et al. Resveratrol cocrystals with enhanced solubility and tabletability. Int J Pharm. 2016;509(1–2):391–399. doi:10.1016/j.ijpharm.2016.06.006
14. Jun SW, Kim M-S, Kim J-S, et al. Preparation and characterization of simvastatin/hydroxypropyl-β-cyclodextrin inclusion complex using supercritical antisolvent (SAS) process. Eur J Pharmaceutics Biopharm. 2007;66(3):413–421. doi:10.1016/j.ejpb.2006.11.013
15. Sopyan I, Fudholi A, Muchtaridi M, Puspitasari IA. Novel of cocrystallization to improve solubility and dissolution rate of simvastatin. Int J PharmTech Res. 2016;9(6):483–491.
16. Deng JH, Lu TB, Sun CC, Chen JM. Dapagliflozin-citric acid cocrystal showing better solid state properties than dapagliflozin. Eur J Pharmaceutical Sci. 2017;104:255–261. doi:10.1016/j.ejps.2017.04.008
17. Sohail M, Ahmad M, Minhas MU, Ali L, Khalid I, Rashid H. Controlled delivery of valsartan by cross-linked polymeric matrices: synthesis, in vitro and in vivo evaluation. Int J Pharm. 2015;487(1):110–119. doi:10.1016/j.ijpharm.2015.04.013
18. Ullah M, Hussain I, Sun CC. The development of carbamazepine-succinic acid cocrystal tablet formulations with improved in vitro and in vivo performance. Drug Dev Ind Pharm. 2016;42(6):969–976. doi:10.3109/03639045.2015.1096281
19. Bugnon D, Potel G, Caillon J, et al. In vivo simulation of human pharmacokinetics in the rabbit. Bull Math Biol. 1998;60(3):545–567. doi:10.1006/bulm.1997.0013
20. Balakumar K, Raghavan CV, Abdu S. Self nanoemulsifying drug delivery system (SNEDDS) of rosuvastatin calcium: design, formulation, bioavailability and pharmacokinetic evaluation. Colloids Surf B Biointerfaces. 2013;112:337–343. doi:10.1016/j.colsurfb.2013.08.025
21. Patel R, Patel M. Preparation, characterization, and dissolution behavior of a solid dispersion of simvastatin with polyethylene glycol 4000 and polyvinylpyrrolidone K30. J Dispers Sci Technol. 2008;29(2):193–204. doi:10.1080/01932690701706946
22. Lin H-L, Zhang G-C, Huang Y-T, Lin S-Y. An investigation of indomethacin–nicotinamide cocrystal formation induced by thermal stress in the solid or liquid state. J Pharm Sci. 2014;103(8):2386–2395. doi:10.1002/jps.24056
23. Trask AV, Motherwell WS, Jones W. Physical stability enhancement of theophylline via cocrystallization. Int J Pharm. 2006;320(1–2):114–123. doi:10.1016/j.ijpharm.2006.04.018
24. Sonar P, Behera A, Banerjee S, Gaikwad D, Harer S. Preparation and characterization of Simvastatin solid dispersion using skimmed milk. Drug Dev Ind Pharm. 2015;41(1):22–27. doi:10.3109/03639045.2013.845836
25. Krishnam Raju K, Sudhakar B, Murthy KVR. Factorial design studies and biopharmaceutical evaluation of simvastatin loaded solid lipid nanoparticles for improving the oral bioavailability. Isrn Nanotechnol. 2014;2014:1–8. doi:10.1155/2014/951016
26. Shiraki K, Takata N, Takano R, Hayashi Y, Terada K. Dissolution improvement and the mechanism of the improvement from cocrystallization of poorly water-soluble compounds. Pharm Res. 2008;25(11):2581–2592. doi:10.1007/s11095-008-9676-2
27. Chow SF, Chen M, Shi L, Chow AH, Sun CC. Simultaneously improving the mechanical properties, dissolution performance, and hygroscopicity of ibuprofen and flurbiprofen by cocrystallization with nicotinamide. Pharm Res. 2012;29(7):1854–1865. doi:10.1007/s11095-012-0709-5
28. Setyawan D, Sari R, Yusuf H, Primaharinastiti R. Preparation and characterization of artesunate-nicotinamide cocrystal by solvent evaporation and slurry method. Asian J Pharm Clin Res. 2014;7(1):62–65.
29. Abdelbary G, Amin M, Salah S. Self nano-emulsifying simvastatin based tablets: design and in vitro/in vivo evaluation. Pharm Dev Technol. 2013;18(6):1294–1304. doi:10.3109/10837450.2012.672989
30. Shimono K, Kadota K, Tozuka Y, Shimosaka A, Shirakawa Y, Hidaka J. Kinetics of co-crystal formation with caffeine and citric acid via liquid-assisted grinding analyzed using the distinct element method. Eur J Pharm Sci. 2015;76:217–224. doi:10.1016/j.ejps.2015.05.017
31. Hosny KM, Khames A, Elhady SSA. Preparation and evaluation of orodispersible tablets containing hydroxylbutyl-β-cyclodextrin-simvastatin solid dispersion. Trop J Pharm Res. 2013;12(4):469–476.
32. Shan N, Perry ML, Weyna DR, Zaworotko MJ. Impact of pharmaceutical cocrystals: the effects on drug pharmacokinetics. Expert Opin Drug Metab Toxicol. 2014;10(9):1255–1271. doi:10.1517/17425255.2014.942281
 © 2020 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2020 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.