Back to Journals » Drug Design, Development and Therapy » Volume 12
Short- versus long-term dual antiplatelet therapy after second-generation drug-eluting stent implantation: a systematic review and meta-analysis of randomized controlled trials
Authors Li H, Guo W, Dai W , Li L
Received 13 February 2018
Accepted for publication 17 April 2018
Published 22 June 2018 Volume 2018:12 Pages 1815—1825
DOI https://doi.org/10.2147/DDDT.S165435
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 3
Editor who approved publication: Professor Anastasios Lymperopoulos
Hongqing Li, Wenqin Guo, Weiran Dai, Lang Li
Department of Cardiology, The First Affiliated Hospital of Guangxi Medical University, Guangxi Cardiovascular Institute, Nanning, Guangxi, China
Background: The optimal dual antiplatelet therapy (DAPT) duration after second-generation drug-eluting stent (DES) implantation remains unclear. We aim to evaluate the efficacy and safety of short-term (≤6 months) and long-term (≥12 months) DAPT after second-generation DES implantation.
Methods: Randomized controlled trials (RCTs) were searched in PubMed, the Cochrane Library, the Embase and ClinicalTrials.gov in the English language. The endpoints included all-cause mortality, cardiac death, non-cardiac death, myocardial infarction (MI), stent thrombosis (ST), stroke, all bleeding, and major bleeding. The effect estimate was expressed by using the hazard ratio (HR) with 95% CI and random effect models.
Results: Seven RCTs with 13,571 patients were included in this study. In terms of survival endpoints, there was no significant difference in all-cause mortality (HR: 0.91; 95% CI: 0.71–1.17), cardiac death (HR: 0.93; 95% CI: 0.67–1.29), and non-cardiac death (HR: 0.89; 95% CI: 0.62–1.28) in the 2 groups. Moreover, there was no significant difference in ischemic outcomes, including MI (HR: 1.15; 95% CI: 0.91–1.45), ST (HR: 1.11; 95% CI: 0.75–1.66), and stroke (HR: 0.85; 95% CI: 0.53–1.35) in the 2 groups. In terms of bleeding endpoints, there was no significant difference in all bleeding (HR: 0.81; 95% CI: 0.64–1.04) and major bleeding (HR: 0.82; 95% CI: 0.49–1.36) in the 2 groups. The subgroup analysis showed that the proportion of patients with acute coronary syndrome was not associated with the benefit of long-term versus short-term DAPT.
Conclusion: Short-term DAPT is not inferior to long-term DAPT in patients implanted with second-generation DES.
Keywords: dual antiplatelet therapy, second-generation drug-eluting stent implantation, meta-analysis
Introduction
Dual antiplatelet therapy (DAPT) is the standard therapy for patients with coronary artery disease after percutaneous coronary intervention (PCI). Second-generation drug-eluting stent (DES) is widely used in clinical practice. However, the optimal DAPT duration for second-generation DES remains unclear.
The 2016 American College of Cardiology/American Heart Association (ACC/AHA) guidelines recommend at least 6 months of DAPT after DES implantation in patients with stable ischemic heart disease (SIHD).1 For patients with acute coronary syndrome (ACS), the guidelines recommend at least 12 months of DAPT after DES implantation. Moreover, the guidelines further suggest that the DAPT duration should be made individually according to the risk of ischemia and bleeding. However, a DAPT duration after second-generation DES implantation was not recommended in the guidelines. The guidelines of the 2017 European Society of Cardiology (ESC) update on DAPT in coronary artery disease (CAD) recommends that DAPT should be considered for 6 months after coronary stent implantation in patients with stable CAD,2 regardless of the stent type. For patients with ACS and without contraindications after coronary stent implantation, the guidelines recommend a DAPT duration of 12 months. If patients have a high bleeding risk, the guidelines recommend a DAPT duration of 3 months for patients with stable CAD and 6 months for patients with ACS.
Studies have confirmed that second-generation DES is more beneficial than first-generation DES3–5 as it reduced the late ST risk. Therefore, it is reasonable to shorten the DAPT duration after second-generation DES implantation. Currently, some studies have evaluated the efficacy and safety of short-term DAPT duration after second-generation DES implantation.6–12 Because of the limitations of sample size and low event rates, the results were without statistical power. Accordingly, we performed a meta-analysis to evaluate the efficacy and safety of short- (≤6 months) and long-term (≥12 months) DAPT durations after second-generation DES implantation.
Methods
Search strategy
The study search was performed by 2 investigators (H-QL and W-QG). We searched for studies in PubMed, the Cochrane Library, Embase and ClinicalTrials.gov in English. The retrieval time was limited from January 1, 2000 to July 31, 2017. With the keywords of second-generation drug-eluting stent, dual antiplatelet therapy (DAPT) and randomized controlled trials, two investigators searched for RCTs independently. After reading the studies, if disagreement existed, a third investigator (W-RD) discussed the disagreement with H-QL and W-QG to make the final decision. Because these analyses were based on previously published studies, there was no requirement for ethical approval and patient consent.
Inclusion and exclusion criteria
The inclusion criteria included the following: 1) object of study: patients received DAPT after second-generation DES implantation; 2) intervention: short-term (≤6 months) DAPT; 3) comparison: long-term (≥12 months) DAPT; 4) outcomes: all-cause mortality, cardiac death, non-cardiac death, MI, ST, stroke, all bleeding and major bleeding; 5) study type: RCTs.
The exclusion criteria included the following: 1) the object of study included non-second-generation DES implantation; 2) the studies did not report outcomes as per the inclusion criteria; 3) the intervention or comparison did not include short-term (≤6 months) DAPT or long-term (≥12 months) DAPT; 4) any observational study.
Data extraction and endpoint
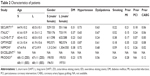
The investigators extracted the characteristics of the patients, including age and gender, the presence of diabetes mellitus, hypertension, prior MI, prior coronary artery bypass grafting (CABG), and prior PCI, and the proportion of ACS in patients. The endpoints of our study included all-cause mortality, cardiac death, non-cardiac death, MI, ST, stroke, all bleeding and major bleeding. The definitions of these endpoints were given according to the original definition in the studies.
Quality assessment
Assessment of the risk of bias was performed by using the Cochrane Handbook risk of bias instrument. The content of the bias included selection bias, performance bias, detection bias, attrition bias, reporting bias and other biases. The degree of assessment included low risk, unclear risk and high risk.
Statistical analyses
The statistical analyses were performed by using STATA, version 14.0 (StataCorp LLC, College Station, TX, USA). We expressed the estimated effect by using the hazard ratio (HR) with 95% CI and random effect models. If the studies reported an HR with 95% CI, we extracted them directly. If an HR with 95% CI was not reported in the studies, we estimated by using the following formula: log-HR =2× (events group1 − events group2)/(events group1 + events group2), variance log-HR =4/(events group1 + events group2).13,14 P-values <0.05 indicated statistical significance. We used the I2 statistic to check heterogeneity, and I2<25% was considered low, 25≤I2≤50% was considered moderate, and >50% was considered high heterogeneity. A sensitivity analysis was performed by excluding studies one by one, and then the analysis was repeated. If the direction of the overall effect was consistent, the results were considered stable. We plotted the funnel plot and performed an Egg’s test to assess publication bias.
Results
According to the search strategy, 651 relevant articles were identified. After finding duplicates and scanning the titles and abstracts, we read the full text of the 14 articles. Four RCTs did not include an intervention of short-term (≤6 months) DAPT,15–18 and 2 RCTs did not report specific results about second-generation DES.19,20 We excluded the IVUS-XPL trial because the patients were implanted with long DES.21 As a result, there was a total of 7 RCTs,6–12 including 13,571 patients, in our study. In total, 6,766 patients were in the short-term DAPT group, and 6,805 patients were in the long-term DAPT group. The details of article searching and the reasons for exclusion can be seen in Figure 1. The characteristics of the studies and patients are shown in Tables 1 and 2. All the included studies were RCTs with allocation concealment without selective reporting and open label trials. The quality assessment of the studies is shown in Figure 2. The definition of major bleeding was slightly different in the included studies. Other endpoints had the same definition.
  | Figure 1 Details of the searched articles and the reasons for exclusion. |
  | Figure 2 Quality assessment of the studies. |
Among the included studies, there was 1 RCT that compared results at 3 and 12 months, 1 RCT that compared at 6 and 18 months, 2 RCTs that compared at 6 and 24 months, and 3 RCTs that compared at 6 and 12 months. The second-generation DES was used in 100% of patients in 5 RCTs (SECURITY https://ClinicalTrials.gov/ct2/show/NCT00944333, ITALIC https://ClinicalTrials.gov/ct2/show/NCT01476020, I-LOVE-IT 2 https://ClinicalTrials.gov/ct2/show/NCT01681381, OPTIMIZE https://ClinicalTrials.gov/ct2/show/NCT01113372, and NIPPON https://ClinicalTrials.gov/ct2/show/NCT01514227). Some patients in the EXCELLENT https://ClinicalTrials.gov/ct2/show/NCT00698607 and PRODIGY https://ClinicalTrials.gov/ct2/show/NCT00611286 trials were implanted with other stents, eg, 25% in the EXCELLENT and 50% in the PRODIGY trial; however, there were subgroups utilized to analyze patients with second-generation DES. The endpoints of all-cause mortality, cardiac death, non-cardiac death, MI, ST and stroke were reported in all the included studies. All bleeding was not reported in NIPPON and PRODIGY, and major bleeding was not reported in PRODIGY.
For all-cause mortality, there were 126 patients in the short-term DAPT group and 138 patients in the long-term DAPT group. For cardiac death, there were 69 patients in the short-term DAPT group and 74 patients in the long-term DAPT group. For non-cardiac death, there were 57 patients in the short-term DAPT group and 64 patients in the long-term DAPT group. There were no significant differences in all-cause mortality (HR: 0.91; 95% CI: 0.71–1.17, P=0.48, I2=5.1%), cardiac death (HR: 0.93; 95% CI: 0.67–1.29, P=0.68, I2=0%), and non-cardiac death (HR: 0.89; 95% CI: 0.62–1.28, P=0.53, I2=2.4%) (Figure 3).
  | Figure 3 Survival endpoints of the studies. |
In terms of MI, there were 149 patients in the short-term DAPT group and 138 patients in the long-term DAPT group. For ST, there were 52 patients in the short-term DAPT group and 46 patients in the long-term DAPT group. For stroke, there were 42 patients in the short-term DAPT group and 51 patients in the long-term DAPT group. There were no significant differences in ischemic outcomes, including MI (HR: 1.15; 95% CI: 0.91–1.45, P=0.26, I2=0%), ST (HR: 1.11; 95% CI: 0.75–1.66), P=0.59, I2=0%), and with stroke risk (HR: 0.85; 95% CI: 0.53–1.35, P=0.50, I2=14.8%) (Figure 4).
  | Figure 4 Ischemic endpoints of the studies. |
For all bleeding, there were 115 patients in the short-term DAPT group and 142 patients in the long-term DAPT group. For major bleeding, there were 41 patients in the short-term DAPT group and 48 patients in the long-term DAPT group. The risks of all bleeding (HR: 0.81; 95% CI: 0.64–1.04, P=0.1, I2=0%) and major bleeding (HR: 0.82; 95% CI: 0.49–1.36, P=0.44, I2=27%) were similar in the 2 groups (Figure 5).
  | Figure 5 Bleeding endpoints of the studies. |
The results of the subgroup analysis are shown in Table 3. The proportion of patients with ACS was not associated with the overall benefit of long- versus short-term DAPT duration. The results of the sensitivity analysis are shown in Table 4, and funnel plots for the publication bias are shown in Figure 6.
  | Table 4 Outcome of the sensitivity analysis |
  | Figure 6 Funnel plots of the publication bias. |
Discussion
Comparing short-term (≤6 months) with long-term (≥12 months) DAPT duration after second-generation DES implantation, our study found that there were no significant differences in survival, ischemia and bleeding outcomes.
Short- versus long-term DAPT duration after second-generation DES
Second-generation DES was implemented with new frame material, new anti-proliferative drugs, such as everolimus, zotarolimus and biolimus, and new biodegradable polymeric coating. These factors reduce the risk of in-stent restenosis and late or very late stent thrombosis compared with first-generation DES. It is reasonable to shorten the DAPT duration after second-generation DES implantation. The SECURITY trial, including 1,399 patients following second-generation DES implantation, showed that a 6-month DAPT duration was not inferior to a 12-month DAPT duration regarding the risk of MACE (including cardiac death, ST, MI, stroke and major bleeding).10 The result was similar to EXCELLENT, which showed that the primary outcomes (death, ischemia and bleeding endpoints) had no significant differences between a 6- and 12-month DAPT duration.12 ITALIC showed that patients receiving a 6-month DAPT duration with second-generation DES had similar outcomes compared with 24 months of DAPT duration.11 Moreover, OPTIMIZE compared a DAPT duration of 3 with 12 months and showed that the risk of ST tended to increase in patients with SCAD, and there was a low risk of ACS with a DAPT duration of 3 months; however, a 3-month DAPT duration was not inferior in the risk of all-cause death, MI, stroke and major bleeding compared with a 12-month DAPT duration.7 The results of NIPPON, including 3,307 patients, also showed that a 6-month DAPT duration was not inferior to an 18-month DAPT duration after second-generation DES implantation.6 However, the above studies are not without limitations. For example, the patients were recruited slowly over time, which results in selection bias. Additionally, these studies are without statistical power.
Comparison with other studies
Palmerini et al22 compared DAPT durations of 3, 6 and 12 months after DES using a network meta-analysis. They found that patients receiving a 3-month DAPT duration had a tendency for an increased risk of ischemic complications, although this risk was not found in patients with SCAD. Moreover, they found that the risk of bleeding tended to increase when the DAPT duration was increased. However, this study included patients with first-generation DES and did not further analyze patients with second-generation DES. Huang et al23 compared shorter DAPT durations with longer DAPT durations after second-generation DES implantation using a meta-analysis; with 5 RCTs and 8,407 patients, they showed that longer (≥12 months) DAPT durations had no significant effect compared to shorter DAPT durations.23 Thus, the latest study was not included in this study. Our study focused on DAPT duration for patients with second-generation DES and included all the available and relevant evidence.
Complications of this study
The 2016 ACC/AHA guidelines for DAPT recommend that patients with ACS should have at least a 12-month DAPT duration after DES. The guidelines of the 2017 ESC recommend that if there are no contraindications, patients with ACS after coronary stent implantation should be considered for a DAPT duration of 12 months. If patients can tolerate DAPT without bleeding complications, a DAPT duration longer than 12 months should be considered. The results of I-LOVE-IT 2 showed that a 6-month DAPT duration was not inferior to a 12-month DAPT duration after the biodegradable polymer DES implantation. Compared with other trials, 82% of patients in this study were patients with ACS. This suggested that not all patients with ACS need to receive 12 months of DAPT duration after the biodegradable polymer DES implantation. It is reasonable to shorten the DAPT duration to 6 months for patients with a high risk of bleeding or for those that cannot tolerate DAPT. Nevertheless, the results of this study require more trials to make a definitive conclusion.
The results of our study suggested that a short-term DAPT duration is not inferior to a long-term DAPT duration for patients with second-generation DES. The subgroup analysis of our study showed that the proportion of patients with ACS was not associated with the overall benefit of long- versus short-term DAPT durations. However, patients with ACS are at high risk for myocardial infarction and stent thrombosis after PCI. The results of the latest SMART-DATE trial showed that 6-month DAPT increased the risk of myocardial infarction compared with 12-month DAPT in patients with ACS undergoing PCI with current-generation DES.24 DAPT can be considered for patients with ACS without excessive risk of bleeding. Due to the high risk of patients with ACS and the limitation of our subgroup analysis, the efficacy and safety of short-term DAPT for patients with ACS require more clinical trials to be evaluated. Most patients included in our study were at a low risk; thus, our conclusions may not be applicable to patients at high risk, such as those with complex PCI, lower-extremities artery disease and prior stent thrombosis. These patients were at increased risk of ischemic complications; thus, long DAPT durations may be considered in these patients. Giustino et al25 evaluated the efficacy and safety of different DAPT durations for patients with complex PCI. Complex PCI was defined as one of the following features: 3 vessels treated, ≥3 stents implanted, ≥3 lesions treated, bifurcation with 2 stents implanted, total stent length >60 mm, or chronic total occlusion. The results showed that patients with complex PCI had a higher risk of ischemic events. Compared with short-term DAPT (3 or 6 months), long-term DAPT (≥12 months) significantly reduced the risk of cardiac ischemic events.25 For patients with prior stent thrombosis, a study from Armstrong et al26 showed that patients with an initial stent thrombosis are at high risk for recurrent stent thrombosis; thus, a longer DAPT duration for these patients may be considered.
Limitations
There are several limitations in our study. First, due to the lack of individual data of patients with ACS, our study was unable to solely evaluate the efficacy and safety in patients with ACS. We conducted a subgroup analysis according to the proportion of patients with ACS in the studies. Second, the definition of major bleeding is different in the included studies, which may lead to biases in the conclusions of a safe endpoint. Third, because the definition of major adverse cardiovascular events (MACE) is different in the included studies, we did not use it as an endpoint in our study. Regarding the included MACE, we evaluated the efficacy and safety by indexes of death, ischemia and bleeding. Fourth, most of the P2Y12 inhibitor drugs in this study were clopidogrel; thus, our conclusion may only apply to DAPT with clopidogrel. Finally, most patients included in this study were at a low risk; therefore, our conclusions may not applicable to patients at high risk.
Conclusion
Short-term (≤6 months) DAPT duration is not inferior to long-term (≥12 months) DAPT duration for patients with second-generation drug-eluting stents. Due to the limitations of this study, our conclusions need to be confirmed by more clinical trials.
Acknowledgment
This study was supported by the First Affiliated Hospital of Guangxi Medical University.
Disclosure
The authors report no conflicts of interest in this work.
References
Levine GN, Bates ER, Bittl JA, et al. 2016 ACC/AHA Guideline focused update on duration of dual antiplatelet therapy in patients with coronary artery disease: a report of the American College of Cardiology/American Heart Association Task Force on Clinical Practice Guidelines. J Am Coll Cardiol. 2016;68(10):1082–1115. | ||
Valgimigli M, Bueno H, Byrne RA, et al. 2017 ESC focused update on dual antiplatelet therapy in coronary artery disease developed in collaboration with EACTS: the task force for dual antiplatelet therapy in coronary artery disease of the European Society of Cardiology (ESC) and of the European Association for Cardio-Thoracic Surgery (EACTS). Eur Heart J. 2018;39(3):213–260. | ||
Wu G, Sun G, Zhao R, Sun M. Clinical outcomes of second- versus first-generation drug-eluting stents in patients with acute myocardial infarction: a meta-analysis of randomized controlled trials. Arch Med Sci. 2014;10(4):643–650. | ||
Kedhi E, Joesoef KS, McFadden E, et al. Second-generation everolimus-eluting and paclitaxel-eluting stents in real-life practice (COMPARE): a randomised trial. Lancet. 2010;375(9719):201–209. | ||
Lange RA, Hillis LD. Second-generation drug-eluting coronary stents. N Engl J Med. 2010;362(18):1728–1730. | ||
Nakamura M, Iijima R, Ako J, et al. Dual antiplatelet therapy for 6 versus 18 months after biodegradable polymer drug-eluting stent implantation. JACC Cardiovasc Interv. 2017;10(12):1189–1198. | ||
Feres F, Costa RA, Abizaid A, et al. Three vs twelve months of dual antiplatelet therapy after zotarolimus-eluting stents: the OPTIMIZE randomized trial. JAMA. 2013;310(23):2510–2522. | ||
Han Y, Xu B, Xu K, et al. Six versus 12 months of dual antiplatelet therapy after implantation of biodegradable polymer sirolimus-eluting stent: randomized substudy of the I-LOVE-IT 2 trial. Circ Cardiovasc Interv. 2016;9(2):e003145. | ||
Valgimigli M, Borghesi M, Tebaldi M, et al. Should duration of dual antiplatelet therapy depend on the type and/or potency of implanted stent? A pre-specified analysis from the PROlonging Dual antiplatelet treatment after Grading stent-induced Intimal hyperplasia studY (PRODIGY). Eur Heart J. 2013;34(12):909–919. | ||
Colombo A, Chieffo A, Frasheri A, et al. Second-generation drug-eluting stent implantation followed by 6- versus 12-month dual antiplatelet therapy: the SECURITY randomized clinical trial. J Am Coll Cardiol. 2014;64(20):2086–2097. | ||
Didier R, Morice MC, Barragan P, et al. 6- versus 24-month dual antiplatelet therapy after implantation of drug-eluting stents in patients nonresistant to aspirin: final results of the ITALIC trial (is there a life for DES after discontinuation of clopidogrel). JACC Cardiovasc Interv. 2017;10(12):1202–1210. | ||
Gwon HC, Hahn JY, Park KW, et al. Six-month versus 12-month dual antiplatelet therapy after implantation of drug-eluting stents: the Efficacy of Xience/Promus Versus Cypher to Reduce Late Loss After Stenting (EXCELLENT) randomized, multicenter study. Circulation. 2012;125(3):505–513. | ||
Elmariah S, Mauri L, Doros G, et al. Extended duration dual antiplatelet therapy and mortality: a systematic review and meta-analysis. Lancet. 2015;385(9970):792–798. | ||
Parmar MK, Torri V, Stewart L. Extracting summary statistics to perform meta-analyses of the published literature for survival endpoints. Stat Med. 1998;17(24):2815–2834. | ||
Lee CW, Ahn JM, Park DW, et al. Optimal duration of dual antiplatelet therapy after drug-eluting stent implantation: a randomized, controlled trial. Circulation. 2014;129(3):304–312. | ||
Collet JP, Silvain J, Barthelemy O, et al. Dual-antiplatelet treatment beyond 1 year after drug-eluting stent implantation (ARCTIC-Interruption): a randomised trial. Lancet. 2014;384(9954):1577–1585. | ||
Helft G, Steg PG, Le Feuvre C, et al. Stopping or continuing clopidogrel 12 months after drug-eluting stent placement: the OPTIDUAL randomized trial. Eur Heart J. 2016;37(4):365–374. | ||
Mauri L, Kereiakes DJ, Yeh RW, et al. Twelve or 30 months of dual antiplatelet therapy after drug-eluting stents. N Engl J Med. 2014;371(23):2155–2166. | ||
Kim BK, Hong MK, Shin DH, et al. A new strategy for discontinuation of dual antiplatelet therapy: the RESET Trial (REal Safety and Efficacy of 3-month dual antiplatelet Therapy following Endeavor zotarolimus-eluting stent implantation). J Am Coll Cardiol. 2012;60(15):1340–1348. | ||
Schulz-Schupke S, Byrne RA, Ten Berg JM, et al. ISAR-SAFE: a randomized, double-blind, placebo-controlled trial of 6 vs. 12 months of clopidogrel therapy after drug-eluting stenting. Eur Heart J. 2015;36(20):1252–1263. | ||
Hong SJ, Shin DH, Kim JS, et al. 6-month versus 12-month dual-antiplatelet therapy following long everolimus-eluting stent implantation: the IVUS-XPL randomized clinical trial. JACC Cardiovasc Interv. 2016;9(14):1438–1446. | ||
Palmerini T, Della Riva D, Benedetto U, et al. Three, six, or twelve months of dual antiplatelet therapy after DES implantation in patients with or without acute coronary syndromes: an individual patient data pairwise and network meta-analysis of six randomized trials and 11,473 patients. Eur Heart J. 2017;38(14):1034–1043. | ||
Huang H, Li Y, Sun M. Shorter (≤6 months) vs longer (≥12 months) dual antiplatelet therapy after second-generation drug-eluting stents implantation: a meta-analysis of randomized controlled trials. Eur Heart J Suppl. 2016;18(Suppl A):A54–A62. | ||
Hahn JY, Song YB, Oh JH, et al. 6-month versus 12-month or longer dual antiplatelet therapy after percutaneous coronary intervention in patients with acute coronary syndrome (SMART-DATE): a randomised, open-label, non-inferiority trial. Lancet. 2018;391(10127):1274–1284. | ||
Giustino G, Chieffo A, Palmerini T, et al. Efficacy and safety of dual antiplatelet therapy after complex PCI. J Am Coll Cardiol. 2016;68(17):1851–1864. | ||
Armstrong EJ, Sab S, Singh GD, et al. Predictors and outcomes of recurrent stent thrombosis: results from a multicenter registry. JACC Cardiovasc Interv. 2014;7:1105–1113. |
 © 2018 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2018 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.