Back to Journals » Drug Design, Development and Therapy » Volume 12
Senkyunolide A protects neural cells against corticosterone-induced apoptosis by modulating protein phosphatase 2A and α-synuclein signaling
Authors Gong S, Zhang J, Guo Z, Fu W
Received 6 January 2018
Accepted for publication 12 April 2018
Published 25 June 2018 Volume 2018:12 Pages 1865—1879
DOI https://doi.org/10.2147/DDDT.S161748
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 2
Editor who approved publication: Dr Sukesh Voruganti
This paper has been retracted.
Shenglan Gong,1 Jin Zhang,2 Zhouke Guo,3 Wenjun Fu1
1South China Research Center for Acupuncture and Moxibustion, School of Basic Medical Science, Guangzhou University of Chinese Medicine, Guangzhou, Guangdong 510006, People’s Republic of China; 2Department of Anatomy, Guangzhou University of Chinese Medicine, Guangzhou, Guangdong 510006, People’s Republic of China; 3Department of Neurology and Psychology, Shenzhen Affiliated Hospital of Guangzhou University of Chinese Medicine, Shenzhen, Guangdong 518033, People’s Republic of China
Background: Depression is characterized by a pathological injury to the hippocampal neurons. Senkyunolide A (SenA) is one of the major active components of Dan-zhi-xiao-yao-san, which is widely used in the treatment of depression-related disorders.
Materials and methods: In the present study, it was hypothesized that the antidepressant effect of Dan-zhi-xiao-yao-san depended on the function of SenA and the authors attempted to reveal the molecular mechanism associated with the treatment. An in vitro depression model was induced using corticosterone (Cort), and the effect of SenA on the cell viability, apoptosis, and protein phosphatase 2A/α-synuclein (PP2A/α-syn) signaling was detected. To validate the mechanism driving the therapeutic effect of SenA, activity of PP2A and α-syn was modulated and the effect on neural cells was evaluated.
Results: The results showed that SenA protects Cort-induced cell apoptosis in PC12 cells. In addition, SenA increased Cort-induced reduction of PP2A activity, while it decreased the expression of p-PP2A, α-syn, and p-α-syn (Ser129). Further, modulation of PP2A activity with specific inhibitor okadaic acid (OA) increased Cort-induced cell apoptosis, while PP2A activator D-erythro-sphingosine (SPH) exhibited an opposite effect. The neuroprotective effects of SenA on neural cells also depended on inhibition of α-syn function, the regulation of which would influence the activity of PP2A in a negative loop.
Conclusion: Collectively, the results suggested that the neuroprotective effects of SenA were exerted by modulating activities of PP2A activities and α-syn. The findings partially explained the mechanism associated with the neuroprotective effect of SenA.
Keywords: α-synuclein, corticosterone, depression, neuroprotection, protein phosphatase 2A, senkyunolide A
Introduction
As one of the most common life-threatening psychiatric disorders, depression has a high prevalence worldwide.1,2 Complicated pathogenesis of depression is now being characterized, which makes the disorder a tough issue to be handled in clinical settings. Currently, it is well conceived that hyperactivation of the hypothalamic–pituitary–adrenal (HPA) axis is implicated in the onset and progression of depression.2–4 Hyperactivation of HPA is characterized by higher levels of glucocorticoids in the circulating blood,5,6 representing a typical pathogenic symptom in depression patients.2,4,5 In laboratory research, high glucocorticoid levels cause depression-like behavior in animals, and induce pathological injury to the hippocampal neurons.2,6 For example, rat pheochromocytoma cell-line PC12 is one of the widely used neuronal cell-lines for neuroscience-related studies. The cell-line expresses relatively high levels of glucocorticoid receptors and has typical neuron features, as a result of which it is used as an effective candidate for an in vitro depression model induced by glucocorticoids.7–9 Administration of PC12 with classic antidepressants has been found to have cytoprotective effects in glucocorticoids-induced in vitro models,10,11 suggesting that antidepressants may exert their effect on neural cells by antagonizing glucocorticoid-induced neurotoxicity.
Traditional antidepressants, such as monoamine oxidase inhibitor, tricyclic antidepressants, selective serotonin reuptake inhibitor, etc., have achieved considerable treatment outcomes during clinical applications.12–14 However, these therapies are accompanied by various side effects.15,16 Thus, the development of better-tolerated, safer, and powerful antidepressants is now imperative.
Dan-zhi-xiao-yao-san is a Traditional Chinese Medicine (TCM) formula used for treating multiple diseases in China and other Asian countries.17 The formula comprises several herbs, including Atractylodis Macrocephale rhizoma, Bupleuri radix, Angelica sinensis, Poria, Glycyrrhizae radix, tree peony bark, Gardenia jasminoides, Paeonia lactiflora pall, mint, and roasted ginger, active components of which are identified to be genipside, paeonol, ferulic acid, paeoniflorin, and senkyunolide A (SenA).18–20 TCM has been used for thousands of years for treating depression-related conditions in China, and Dan-zhi-xiao-yao-san has been proved to be one of the most effective formulae. Dong et al21 demonstrated that the treatment of rats with generalized anxiety disease using Dan-zhi-xiao-yao-san relieved the animals of depression symptoms by protecting neural cells in Papez’s circuit against apoptosis. Similar results were reported by Li et al22 and Xu et al.23 However, Dan-zhi-xiao-yao-san consists of several components, which restrains the universal application of the therapy for patients with different symptoms. To promote the effective management of depression patients, exploration of the key active components of Dan-zhi-xiao-yao-san contributing to the antidepression effect demands prompt solution.
Therefore, in the present study, we first tested the neural cell protecting effect of several major compounds in Dan-zhi-xiao-yao-san in a corticosterone (Cort)-induced depression cell model. Based on the results, SenA was found to protect neural cells from Cort-induced apoptosis. Subsequently, the possible mechanism driving the neural protective effect of SenA was investigated by focusing on protein phosphatase 2A (PP2A)/α-synuclein (α-syn) pathways, the latter one of which was proved to be the key contributor to the pathogenesis of depression.24,25 Findings outlined in the current study demonstrated that the antidepression effect of Dan-zhi-xiao-yao-san depended on the neuroprotective effects of SenA, which was exerted by modulating activities of PP2A activities and α-syn.
Materials and methods
Chemicals
Genipside, paeonol, ferulic acid, paeoniflorin, and SenA were purchased from PureOne Biotechnology (Shanghai). Cort (200 μM for treatment of PC12 cells),8 Okadaic acid (OA) (PP2A inhibitor, 2 μM for treatment of PC12 cells),26 and D-erythro-sphingosine (SPH) (PP2A agonist, 10 nM for treatment with PC12 cells)27 were purchased from Sigma-Aldrich Co (St. Louis, MO, USA).
Cell culture
Rat pheochromocytoma cell-line PC12 cells were obtained from the American Type Cell Culture Collection (Manassas, VA, USA) and were maintained in Dulbecco’s Modified Eagle’s Medium (DMEM) supplemented with 10% horse serum and 5% fetal bovine serum (FBS), and 1% penicillin/streptomycin at 37°C in a humidified 5% CO2 atmosphere.
Cell viability assay
The cell viability was evaluated by CCK-8 assay (Dojindo Molecular Technologies, Inc., Rockville, MD, USA). Briefly, PC12 cells were plated on a 96-well plate; after 24 h, the cells were treated with different drug for another 24 h and then washed with D-Hanks buffer solution. Finally, 200 μL CCK-8 solution was added to each well and incubated for 3 h at 37°C. The optical density (OD) of each well was recorded at 450 nm on a Microplate Reader (Varioskan Flash, Thermo Scientific, Waltham, MA, USA).
Lactate dehydrogenase (LDH) leakage assay
The release of LDH is a marker for cellular toxicity. LDH activity was measured using a LDH diagnostic kit (88953; Thermo Fisher Scientific, Waltham, MA, USA) according to the manufacturer’s instructions. Briefly, PC12 cells were seeded in 24-well culture plates at a density of 1×105 cells/well. After treatment with SenA in the presence or absence of Cort, the medium was collected for analysis of LDH activities.
Flow cytometric analysis of cell apoptosis
At the end of drug treatment, the cells were freshly harvested and suspended in a 1:1 (v/v) mixture of PBS and 0.2 M Na2HPO4-0.1 M citric acid (pH 7.5). Following the fixation with ice-cold ethanol at 4°C for 1 h, the cells were resuspended in binding buffer and then incubated in a buffer containing 200 ng/mL Annexin V-FITC conjugates (Sigma, MO, USA) at room temperature for 15 min. Subsequently, the cells were stained with PI (Sigma, MO, USA) (300 ng/mL) for 10 min. The stained cells were analyzed on a FACSCalibur flow cytometer (BD Biosciences, USA).
Hoechst staining
The deformation of cell nuclei due to apoptosis was detected using a Hoechst staining kit according to the manufacturers’ instruction (Beyotime, Shanghai, People’s Republic of China). The results were observed under a fluorescence microscope at 460 nm.
Immunofluorescence staining
At the end of drug treatment, the cells were fixed with 4% paraformaldehyde in PBS, permeabilized with 0.5% Triton X-100 and then blocked with 5% normal goat serum. The cells were incubated with anti-α-syn (Abcam, Cambridge, UK) and anti-PP2A (Abcam), antibodies in 1% BSA at 4°C overnight. After washing with phosphate-buffered saline (PBS) three times, the cells were incubated with Anti-Rabbit IgG H&L (HRP) (Abcam, UK) for 50 min. Cell nuclei were indicated by staining with 4′-6-diamidino-2-phenylindole (DAPI) for 15 min. The immunofluorescence images were obtained using FV10i confocal microscopy (Olympus Corporation, Tokyo, Japan).
Western blotting analysis
The cells were harvested by RIPA Lysis and Extraction Buffer (89900, Thermo Fisher Scientific). Denatured protein samples were resolved on SDS-PAGE and transferred to PVDF membrane (Millipore, Billerica, MA, USA). After blocking with non-fat milk, the membrane was incubated overnight at 4°C with α-syn (Abcam), p-α-syn (Abcam), PP2A (Abcam), p-PP2A (Abcam), and GAPDH (Abcam) antibodies, followed by incubation with the anti-rabbit HRP-conjugated secondary antibodies (Abcam). Chemiluminescence detection was performed using enhanced chemiluminescence (ECL) advance Western blotting detection reagents (GE Healthcare, Little Chalfont, Buckinghamshire, UK).
Real-time PCR analysis
PC12 cells were used to analyze the expression of α-syn and internal control β-actin by quantitative real-time polymerase chain reaction (PCR). Briefly, total RNA was extracted using TRIzol reagent (Invitrogen, CA, USA) and used for cDNA synthesis with 2 μg RNA and a High-Capacity cDNA Reverse Transcription kit. The expression of these three genes was conducted by real-time PCR using a kit from DBI (Shanghai, People’s Republic of China). The expression level of genes was calculated with normalization to housekeeping gene β-actin. Three independent experiments were carried out, and each experiment was performed in three analytical replicates. The forward and reverse primers for α-syn are shown in Table 1.
  | Table 1 α-synuclein, PP2A, and β-actin primers |
Determination of PP2A activities by ELISA assay
PP2A activities were detected by ELISA.28 Briefly, the cell lysis buffer with Triton X-100 and protease inhibitors (10 μg/mL Aprotinin, 10 μg/mL Leupeptin, 100 μM PMSF, and 10 μg/mL Pepstatin A) were used for cell homogenization. Homogenized cell samples were centrifuged at 10,000× g for 10 min at 4°C. Supernatant was collected for the protein phosphatase assay. The pNPP (p-Nitrophenyl phosphate) is a colorimetric substrate used for measuring the activity of serine/threonine phosphatases. Assay buffer for PP2A was as following: 40 mM Tris-HCl, pH 8.4, 34 mM MgCl2, 4 mM EDTA, and 4 mM DTT. Upon dephosphorylation by phosphatases, pNPP turns yellow and is read at absorbance 405 nm.
Plasmid construction and cell transfection
Overexpression of α-syn were performed by ligating ORF of α-syn into pcDNA3.0 vector. Specific α-syn siRNA (sense: 5′-GGCUUAUGAAAUGCCUUCAUU-3′; antisense: 5′-UUCCGAAUACUUUACGGAAGU-3′) was obtained from Sango Biotech. (Shanghai, People’s Republic of China). The wide type vector of α-syn and mutant type vector for mutation of Ser129 to Ala129 were obtained from Sango Biotech. (Shanghai, People’s Republic of China). Transfection was performed with Lipofectamine® P3000 Reagent (Thermo Fisher Scientific). After 72 h of transfection, the cells were collected and used for subsequent experiments.
Statistical analysis
Data was expressed as mean±standard deviation (SD). Differences between the groups were identified by one-way ANOVA followed by post hoc test with Fisher’s least significant difference method. Statistical significance was accepted when two-tailed P-value was smaller than 0.05. The statistical analyses were performed using Graphpad Prism version 6.0 (GraphPad Software, Inc., San Diego, CA, USA).
Results
Identification of potential active components involved in the neuroprotective effect of Dan-zhi-xiao-yao-san
To determine the compound that could contribute to the antidepression effects of Dan-zhi-xiao-yao-san, the neuroprotective effect of major compounds in Dan-zhi-xiao-yao-san formula on Cort-induced injury in PC12 cells was detected with CCK-8 assay. The cells were pretreated with geniposide, paeonol, ferulic acid, paeoniflorin, and SenA at a concentration ranging from 0 to 2 mg/L for 24, 48, and 72 h. It was observed that geniposide, paeonol, ferulic acid, and paeoniflorin had no influence on the viability of PC12 cells (Figure 1A–D); however, SenA at the concentration range of 0.125–0.5 mg/L significantly increased cell viability impaired by Cort in a time- and dose-dependent manner (Figure 1E). However, for SenA at 2 mg/L, evident cytotoxicity on PC12 cells was observed, which indicated that application of SenA in practice should be carefully handled. Given that SenA at 0.5 mg/L concentration was the most effective concentration to protect neural cells against cytotoxicity of Cort, the concentration was chosen for subsequent studies.
Administration of SenA on PC12 attenuated Cort-induced (200 μm) cytotoxicity and inhibited PP2A phosphorylation and α-syn signaling
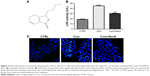
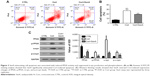
The chemical structure of SenA is shown in Figure 2A. To further confirm the neuroprotective effect of SenA, the production of LDH release was also measured. LDH is a soluble cytosolic enzyme, which is widely presented in eukaryotic cells29 and represents cell injury due to apoptosis and necrosis.29 As shown in Figure 2B, the level of released LDH was significantly increased by Cort as compared with the control group (P<0.01). In contrast, pretreatment of cells with 0.5 mg/L of SenA effectively decreased Cort-induced LDH release. In addition, Hoechst staining showed that SenA could protect Cort-induced cell apoptosis: less Hoechst-positive (bright blue) cells were detected in the Cort+SenA group (Figure 2C). The effect of SenA on Cort-induced apoptosis was further validated using flow cytometric analysis, supporting the notion that SenA had a neuroprotective effect against Cort-induced cell apoptosis (Figure 3A and B).
In addition, Cort upregulated the expression of α-syn and p-α-syn (Ser129) which is the most frequent modifier of α-syn (Figure 3C).30 PP2A is an upstream regulator for α-syn phosphorylation.28 The results verified the close relation between PP2A/α-syn and depression in that Cort also induced the phosphorylation of PP2A. In contrast, SenA attenuated the effect of Cort on these factors (Figure 3C), suggesting that PP2A and α-syn were involved in the neuroprotective effect of SenA.
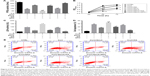
PP2A contributed to the neuroprotective effect of SenA
To further examine the role of PP2A in the neuroprotective effect of SenA, PC12 cells were treated with PP2A inhibitor OA (15 min before Cort administration) or PP2A activator SPH (15 min before Cort administration) alone or with SenA. OA significantly inhibited PP2A activities as reflected by ELISA assay, whereas PP2A activator SPH enhanced PP2A activities (Figure 4A). Based on the results of the CCK-8 assay, it was demonstrated that OA further decreased cell viability, whereas SPH exerted an opposite effect (Figure 4B). Similar change patterns were also detected for LDH production and cell apoptosis (Figure 4C–E). Concatenated treatment of PC12 cells with SenA and SPH have synergistic effects on cell viability and LDH release caused by Cort (Figure 4B–D). Moreover, the results of immunofluorescence staining further confirmed the effects of different treatments on the expression and distribution of PP2A, with OA decreasing and SPH increasing the level of PP2A (Figure 5). Taken together, the results evidently inferred the role of PP2A in mediating the effect of SenA on PC12 cells: suppression of PP2A activities and promotion of Cort-induced cell injury, while activating PP2A attenuated Cort-induced cell injury. Remarkably, SenA and PP2A agonist SPH could synergistically protect Cort-induced neural cell damages.
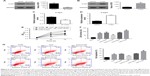
Alpha-syn was involved in the Cort-induced neural cell injury, and α-syn inhibition contributed to neuroprotective effects of SenA
As α-syn has been implicated in progression of depression,24 it was wondered whether the impairments of Cort on PC12 cells were related to the function of α-syn. Therefore, the expression of α-syn was bilaterally modulated in Cort-treated PC12 cells (Figure 6A–D). As shown in Figure 6E–G, knockdown of α-syn in Cort-treated PC12 cells decreased release of LDH and cell apoptosis and increased cell viability. On the contrary, induced expression of α-syn in Cort-treated PC12 cells further promoted the negative effect of Cort on PC12 cells by increasing production of LDH and cell apoptosis and decreasing cell viability (Figure 6E–G). Taken together, the results affirmed the involvement of α-syn in the Cort-induced neural cell injury.
Moreover, the neuroprotective effect of SenA was also dependent on the inhibition of α-syn, which has been conceived to be the downstream effector of PP2A.28 CCK-8 assay showed that knockdown of the expression of α-syn increased cell viability, whereas overexpression of α-syn decreased cell viability (Figure 7A). The results of LDH assay and Annexin V-FITC/PI assay further confirmed the key role of α-syn inhibition in the neuroprotective effect of SenA: knockdown of α-syn strengthened the effect of SenA on neural cell injury by decreasing the Cort-induced LDH production and cell apoptosis and overexpressing α-syn counteracted the effect of SenA on Cort-induced impairments (Figure 7B–D). However, it was interesting to find that modulation of α-syn also influenced the activity of PP2A, which was reported to be the upstream regulator of α-syn: knockdown of α-syn increased the activity of PP2A, while overexpression of α-syn decreased the activity of PP2A (Figure 7E). The immunofluorescence staining results further confirmed that knockdown of the expression of α-syn decreased; whereas overexpression of α-syn increased Cort-induced expression of α-syn levels (Figure 8).
Function of α-syn in the neural cell injury depended on the phosphorylation of Ser129
The function of α-syn in the Cort-induced cell injury and SenA treatment was investigated to confirm whether α-syn and p-ser-129 phosho-α-syn contribute to the neuroprotective effects of SenA in a Cort-induced depression cell model. The Ser129 was mutated to Ala129 to prevent the phosphorylation of Ser129 in α-syn product (Figure 9A and B). The mutation of Ser129 to Ala129 significantly attenuated the Cort-induced suppression of cell viability, as reflected by CCK-8 assay (Figure 9C). Moreover, LDH assay and Annexin V/FITC-PI assay further confirmed the role of phosphorylation state of α-syn in Cort-induced cell injury (Figure 9D–F), the inhibition of which had a synergetic effect with SenA. Additionally, similar to the results of bilateral modulation of α-syn expression: WT α-syn had an inhibiting effect on PP2A activity, while mutation of Ser129 to Ala129 had an inducing effect (Figure 9G). These results suggested that the effect of α-syn during neural cell injury critically depended on the normal phosphorylation of Ser129.
Discussion
Dan-zhi-xiao-yao-san, a TCM formula, is used in the treatment of diseases such as menopausal syndrome, anemia, functional uterine bleeding, hepatitis, as well as emotional diseases such as depression.17,26 In previous studies, researchers have shown that application of Dan-zhi-xiao-yao-san is effective in controlling progression of depression by maintaining the viability of neural cells.21–23 However, as a formula, Dan-zhi-xiao-yao-san consists of different herbs with various active compounds that may contribute to the antidepression effect. Exploring the major compound involved in the antidepression function of the formula and to explain the underlying mechanisms associated with the treatment will expand the application of Dan-zhi-xiao-yao-san. Therefore, in the current study, we assessed the anti-apoptosis effect of several major compounds isolated from Dan-zhi-xiao-yao-san, and the results showed that SenA was the active compound that exerted a protective effect on PC12 cells against Cort-induced apoptosis. Further, by focusing on the PP2A/α-syn pathway, our study showed that the neuroprotective effects of Sen A were exerted by inhibiting the activity of α-syn through mediation of PP2A.
SenA is one of the major ingredients of Rhizoma Chuanxiong, and used in the treatment of cardio and cerebrovascular diseases.31–33 Further, SenA has also been employed for the treatment of migraine in a mice model.34 However, its protecting role against depression was barely reported. Our results showed that SenA at a dose range of 0.125–0.5 mg/L effectively suppressed Cort-induced apoptosis in PC12, evidently demonstrating that SenA was the key active compound involved in the neural protection function of Dan-zhi-xiao-yao-san. However, our results also showed that overdose of SenA (2 mg/L) was clearly cytotoxic to PC12 cells, which reminded that application of SenA against depression or other neural disorders in practice should be carefully monitored.
To explain the possible mechanism underlying the neural protecting effect of SenA, the activity of PP2A and α-syn was detected. It was found that Cort administration suppressed the activity of PP2A, while it induced the expression and phosphorylation of α-syn, thereby representing the initiation of pro-depression signaling as previously reported.24,25 Reduced activity of PP2A generally results in overexpression and phosphorylation of α-syn,35 which will in turn increase the phosphorylation of PP2A and inhibit the activity of the factor.36 In the current study, pretreatment of SenA counteracted the effect of Cort on PP2A/α-syn, indicating the possibility that the neural protecting effect of the agent depended on the activity of PP2A/α-syn. Therefore, the functions of both factors were modulated in PC12 cells in the presence of Cort and/or SenA.
By using PP2A inhibitor OA and activator SPH, our results showed that function of PP2A was essential for the neural protecting effect of SenA. Co-incubation of PP2A activator promoted the effect of SenA, which is consistent with a previous report demonstrating that the PP2A ligand exerts a neuroprotective effect.37 Subsequently, the expression of α-syn was bilaterally modulated, and it was found that, without the function of α-syn, administration of Cort failed to induce cell apoptosis to some extent. When the expression of α-syn was modulated in SenA-treated PC12 cells, the results showed that overexpression of the factor blocked the protection of SenA on PC12 cells, while suppression of the factor promoted the effect in a similar way to that of PP2A inhibitor. Taken together, the data showed that Cort induced neural cell damage by suppressing the activity of PP2A, and induced the phosphorylation of the indicator, which further activated the function of α-syn, and treatment of SenA was dependent on the inhibition of α-syn by inducing PP2A activity.
In addition, phosphorylation of α-syn at Ser129 is the most frequent modifier of α-syn and plays an important role for α-syn-induced cell death.38,39 Whether the phosphorylation was crucial to the function of α-syn in neural cell apoptosis and treatment of SenA or not needs further investigation. Therefore, the Ser129 of α-syn mutated to Ala129. Our results showed that mutation decreased Cort-induced apoptosis, while strengthening the neuroprotective effects of SenA, thus suggesting that phosphorylation at Ser-129 of α-syn was also essential for Cort-induced cell apoptosis. Together, our data suggested that α-syn levels as well as phosphorylation of Ser-129 were prerequisites for Cort-induced neural cell damages, suppression of which would be a promising therapeutic strategy for the treatment of depression and other neural disorders. Our study also reported the regulating function of α-syn on the activity of PP2A: not only the changes in the expression level of α-syn but also the alternation in the phosphorylation status that influenced the PP2A activity. The results provided further explanation to our results: interaction between PP2A and α-syn played a key role in the maintenance of normal function of neural cells, and the neuroprotective effect of SenA depended both on the activation of PP2A and suppression of α-syn. Although PP2A has been previously reported to act as an upstream regulator of α-syn, during the disorders, the two factors could influence the activity of each other in a negative loop.
Conclusion
Our study showed that SenA plays an important role in the antidepression effect of Dan-zhi-xiao-yao-san, which exerted its function by protecting neural cells against cell apoptosis. With a series of molecular detection, it was observed that the function of SenA depended on the induced activity of PP2A, which would block the expression and phosphorylation of α-syn and p-α-syn (Ser-129). However, the current study only provided a preliminary explanation on the pathways driving the neural protecting effect of SenA. Except for the overall protective effect on neural cells, our data also inferred the cytotoxicity of high-dose SenA on PC12 cells. Thus, the detailed mechanism involved in the treating of SenA against neural disorders needs further investigation for the successful application of the agent.
Acknowledgment
This work is supported by National Natural Science foundation of China (No 81774182), Shenzhen Science and Technology Project (No. JCYJ20160428174825490), Elite Youth Education Program of Guangzhou University of Chinese Medicine (No QNYC20140104), GZUCM Science Fund for Creative Research Groups (No 2016KYTD10).
Disclosure
The authors report no conflicts of interest in this work.
References
Heim C, Binder EB. Current research trends in early life stress and depression: review of human studies on sensitive periods, gene–environment interactions, and epigenetics. Exp Neurol. 2012;233(1):102–111. | ||
Kalia M. Neurobiological basis of depression: an update. Metabolism. 2005;54(5 Suppl 1):24–27. | ||
Schatzberg AF. Anna-Monika Award Lecture, DGPPN Kongress, 2013: the role of the hypothalamic–pituitary–adrenal (HPA) axis in the pathogenesis of psychotic major depression. World J Biol Psychiatry. 2015;16(1):2–11. | ||
Stetler C, Miller GE. Depression and hypothalamic-pituitary-adrenal activation: a quantitative summary of four decades of research. Psychosom Med. 2011;73(2):114–126. | ||
Tsigos C, Chrousos GP. Hypothalamic–pituitary–adrenal axis, neuroendocrine factors and stress. J Psychosom Res. 2002;53(4):865–871. | ||
Nestler EJ, Barrot M, DiLeone RJ, Eisch AJ, Gold SJ, Monteggia LM. Neurobiology of depression. Neuron. 2002;34(1):13–25. | ||
Zheng M, Liu C, Pan F, et al. Protective effects of flavonoid extract from Apocynum venetum leaves against corticosterone-induced neurotoxicity in PC12 cells. Cell Mol Neurobiol. 2011;31(3):421–428. | ||
Mao QQ, Xian YF, Ip SP, Tsai SH, Che CT. Protective effects of peony glycosides against corticosterone-induced cell death in PC12 cells through antioxidant action. J Ethnopharmacol. 2011;133(3):1121–1125. | ||
Hellewell SB, Bowen WD. A sigma-like binding site in rat pheochromocytoma (PC12) cells: decreased affinity for (+)-benzomorphans and lower molecular weight suggest a different sigma receptor form from that of guinea pig brain. Brain Res. 1990;527(2):244–253. | ||
Chuang DM. The antiapoptotic actions of mood stabilizers: molecular mechanisms and therapeutic potentials. Ann N Y Acad Sci. 2005;1053:195–204. | ||
Kasper S, McEwen BS. Neurobiological and clinical effects of the antidepressant tianeptine. CNS Drugs. 2008;22(1):15–26. | ||
Langford A. On Depression. Drugs, Diagnosis and Despair in the Modern World By Nassir Ghaemi. The Johns Hopkins University Press. 2013. British Journal of Psychiatry. 2014;205(1):80–80. | ||
Lee S, Jeong J, Kwak Y, Park SK. Depression research: where are we now? Mol Brain. 2010;3:8. | ||
Nemeroff CB, Vale WW. The neurobiology of depression: inroads to treatment and new drug discovery. J Clin Psychiatry. 2005;66(Suppl 7):5–13. | ||
Gillman P. Monoamine oxidase inhibitors, opioid analgesics and serotonin toxicity. Br J Anaesth. 2005;95(4):434–441. | ||
Raison CL, Demetrashvili M, Capuron L, Miller AH. Neuropsychiatric adverse effects of interferon-alpha: recognition and management. CNS Drugs. 2005;19(2):105–123. | ||
Zhang Y, Han M, Liu Z, Wang J, He Q, Liu J. Chinese herbal formula xiao yao san for treatment of depression: a systematic review of randomized controlled trials. Evid Based Complement Alternat Med. 2012;2012:931636. | ||
Chen WF, Xu L, Yu CH, et al. The in vivo therapeutic effect of free wanderer powder (Xiaoyaosan) on mice with 4T1 cell induced breast cancer model. J Tradit Complement Med. 2012;2(1):67–75. | ||
Qin F, Wu XA, Tang Y, Huang Q, Zhang ZJ, Yuan JH. Meta-analysis of randomized controlled trials to assess the effectiveness and safety of free and easy wanderer plus, a polyherbal preparation for depressive disorders. J Psychiatr Res. 2011;45(11):1518–1524. | ||
Fratkin J, Dharmananda S. Chinese Herbal Patent Medicines: The Clinical Desk Reference. Boulder, CO: Shya Publications; 2001. | ||
Dong N, Tang QS, Zhao RZ, Xu S, Yang XK, Mao YQ. Effects of Danzhi Xiaoyao San on cell apoptosis of Papez’s circuit in rat model of generalized anxiety disease (GAD). J Beijing Uni Tradit Chin Med. 2015;38(2):11. | ||
Li N, Tang QS, Zhao RZ, Guo SL. Ethological changes in rats with chronic stress-induced anxiety and interventional effect of danzhixiaoyao powder. J Beijing Uni Tradit Chin Med. 2009;32(12):826–829. | ||
Xu ZW, Wang WZ, Su JF, Yan C, Wu LL. Experimental study on antianxietic action of Xiaoyao San and Dan Zhi Xiaoyao San. J Guangzhou Uni Tradit Chin Med. 2006;23(4):6. | ||
Caudal D, Alvarsson A, Björklund A, Svenningsson P. Depressive-like phenotype induced by AAV-mediated overexpression of human α-synuclein in midbrain dopaminergic neurons. Exp Neurol. 2015;273:243–252. | ||
Jellinger KA. Lewy body/α-synucleinopathy in schizophrenia and depression: a preliminary neuropathological study. Acta Neuropathol. 2009;117(4):423–427. | ||
Jing LL, Zhu XX, Lv ZP, Sun XG. Effect of Xiaoyaosan on major depressive disorder. Chin Med. 2015;10:18. | ||
Cheng P, Chen K, Yu W, et al. Protein phosphatase 2A (PP2A) activation promotes axonal growth and recovery in the CNS. J Neurol Sci. 2015;359(1–2):48–56. | ||
Lou H, Montoya SE, Alerte TN, et al. Serine 129 phosphorylation reduces the ability of α-synuclein to regulate tyrosine hydroxylase and protein phosphatase 2A in vitro and in vivo. J Biol Chem. 2010;285(23):17648–17661. | ||
Lobner D. Comparison of the LDH and MTT assays for quantifying cell death: validity for neuronal apoptosis? J Neurosci Methods. 2000;96(2):147–152. | ||
Chen L, Feany MB. α-Synuclein phosphorylation controls neurotoxicity and inclusion formation in a Drosophila model of Parkinson disease. Nat Neurosci. 2005;8(5):657–663. | ||
Yan R, Li SL, Chung HS, Tam YK, Lin G. Simultaneous quantification of 12 bioactive components of Ligusticum chuanxiong Hort. by high-performance liquid chromatography. J Pharm Biomed Anal. 2005;37(1):87–95. | ||
Naito T, Sakata M, Ikeya Y, Okada M, Maruno M. Quantitative analysis of effective constituents for blood circulation in cnidii rhizoma and ligustici rhizoma–comparison of the contents of constituents in commercial cnidii rhizoma and ligustici rhizoma. Nat Med. 1995;49:425–430. | ||
Li SL, Chan SS, Lin G, et al. Simultaneous analysis of seventeen chemical ingredients of Ligusticum chuanxiong by on-line high performance liquid chromatography-diode array detector-mass spectrometry. Planta Med. 2003;69(5):445–451. | ||
Wang YH, Liang S, Xu DS, et al. Effect and mechanism of senkyunolide I as an anti-migraine compound from Ligusticum chuanxiong. J Pharm Pharmacol. 2011;63(2):261–266. | ||
Wang Y, Liu J, Chen M, et al. The novel mechanism of rotenone-induced α-synuclein phosphorylation via reduced protein phosphatase 2A activity. Int J Biochem Cell Biol. 2016;75:34–44. | ||
Yang W, Wang X, Duan C, Lu L, Yang H. Alpha-synuclein overexpression increases phospho-protein phosphatase 2A levels via formation of calmodulin/Src complex. Neurochem Int. 2013;63(3):180–194. | ||
Lorrio S, Romero A, González-Lafuente L, et al. PP2A ligand ITH12246 protects against memory impairment and focal cerebral ischemia in mice. ACS Chem Neurosci. 2013;4(9):1267–1277. | ||
Chen L, Periquet M, Wang X, et al. Tyrosine and serine phosphorylation of α-synuclein have opposing effects on neurotoxicity and soluble oligomer formation. J Clin Invest. 2009;119(11):3257–3265. | ||
Kragh CL, Lund LB, Febbraro F, et al. α-Synuclein aggregation and Ser-129 phosphorylation-dependent cell death in oligodendroglial cells. J Biol Chem. 2009;284(15):10211–10222. |
 © 2018 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2018 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.