Back to Journals » Drug Design, Development and Therapy » Volume 12
Semen Brassicae ameliorates hepatic fibrosis by regulating transforming growth factor-β1/Smad, nuclear factor-κB, and AKT signaling pathways in rats
Authors Cao S, Zheng B, Chen T, Chang X, Yin B, Huang ZH, Shuai P, Han L
Received 24 October 2017
Accepted for publication 12 March 2018
Published 11 May 2018 Volume 2018:12 Pages 1205—1213
DOI https://doi.org/10.2147/DDDT.S155053
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 2
Editor who approved publication: Dr Sukesh Voruganti
Si Cao,1,2,* Baoping Zheng,3,* Tao Chen,4 Xinfeng Chang,4 Bao Yin,1 Zhihua Huang,4 Ping Shuai,4 Limin Han2
1School of Integrated Traditional Chinese and Western Medicine, Hunan University of Chinese Medicine, Changsha, Hunan, China; 2Gannan Medical University, Ganzhou, Jiangxi, China; 3Department of Chinese Medicine, The First Affiliated Hospital, Gannan Medical University, Ganzhou, Jiangxi, China; 4School of Basic Medical Sciences, Gannan Medical University, Ganzhou, Jiangxi, China
*These authors contributed equally to this work
Purpose: There is no effective treatment for liver fibrosis, which is a common phase during the progression of many chronic liver diseases to cirrhosis. Previous studies found that Semen Brassicae therapy can effectively improve the clinical symptoms of patients with asthma, allergic rhinitis, and chronic lung diseases; however, its effects on liver fibrosis in rats and its possible mechanisms of action remain unclear.
Methods: Rats were injected intraperitoneally with 4% thioacetamide aqueous solution (5 mL·kg-1) at a dose of 200 mg·kg-1 twice a week for 8 consecutive weeks to establish the liver fibrosis model and were then treated with different concentrations of Semen Brassicae extract. After Semen Brassicae treatment, the morphology of the liver tissue was analyzed using hematoxylin and eosin and Masson’s trichrome staining, and liver index and liver fibrosis grade were calculated. Thereafter, the levels of collagen-I, collagen-III, α-SMA, transforming growth factor (TGF)-β1, p-Smad 2/3, Smad 2/3, Smad4, NF-κB-p65, p-NF-κB-p65, IL-1β, IL-6, AKT, and p-AKT were determined using Western blotting.
Results: Compared with the untreated model group, the Semen Brassicae-treated group showed significantly decreased liver function indices; expression levels of collagen-I, collagen-III, and α-SMA; and hepatic fibrosis. Further studies also showed that the expression of TGF-β1, Smad4, p-Smad 2/3/Smad 2/3, p-NF-κB-p65/NF-κB-p65, IL-1β, IL-6, and p-AKT/AKT significantly decreased after the treatment.
Conclusion: These results indicate that Semen Brassicae exhibits an anti-hepatic fibrosis effect, and the underlying mechanism of action may be related to the regulation of TGF-β1/Smad, NF-κB, and AKT signaling pathways and the reduction of extracellular matrix deposition.
Keywords: hepatic fibrosis, Semen Brassicae, NF-κB, AKT, TGF-β1
Introduction
Liver fibrosis refers to the pathological process of diffuse and excessive deposition of extracellular matrix (ECM) in the liver from abnormal connective tissue proliferation, caused by a variety of pathogenic factors. It is a necessary phase of the progression of various chronic liver diseases to cirrhosis. The liver fibrosis process is reversible, and early intervention can delay or even prevent its progression.1
The treatment for liver fibrosis has advanced considerably, but no effective anti-fibrotic drugs are available.2–4 In recent years, traditional Chinese medicine formulations have been shown to exhibit great efficacy in treating liver fibrosis, with good prospects for application.5–10 Traditional Chinese medicine practitioners believe that “phlegm to hinder collaterals” is the basic pathogenesis of liver fibrosis, and therefore, suggest “eliminating phlegm and freeing channels” as one of the potential treatments for liver fibrosis. Semen Brassicae, the seed of Brassica alba (L) Boiss, a plant of the family Brassicaceae, is widely used to treat diseases. The main components of Semen Brassicae are sinalbin, sinapine, and sinapic acid. Sinapine and mesylate are soluble in water. Previous studies found that Semen Brassicae therapy can effectively improve the clinical symptoms of patients with asthma, allergic rhinitis, and chronic lung diseases.11–14 Traditional Chinese medicine practitioners believe that Semen Brassicae has the “eliminating phlegm” property and that it is a potential drug for the treatment of liver fibrosis. Our previous study also found that water extracts of Semen Brassicae have preventive and therapeutic effects, including phlegm-dissolving effects on hepatic fibrosis.15 However, the anti-hepatic fibrosis effect and mechanisms of Semen Brassicae need further study. Therefore, in this study, we established a rat liver fibrosis model to study the anti-fibrotic effect of Semen Brassicae and its underlying mechanisms in rat liver.
Materials and methods
Preparation of Semen Brassicae
An amount of 500 g of Semen Brassicae (Zhangshu Qingren Chinese Drinkadie Medicine Co., Zhangshu, China; catalog number: 1311234) was extracted by boiling in distilled water three times for 1 h each time and then filtered using gauze. Then, all three extract filtrates were combined and concentrated to 500 mL using a rotary evaporator to obtain the final Semen Brassicae extract at a concentration of 1.0 g·mL−1, which was subsequently diluted with distilled water to working concentrations of 0.25, 0.5, and 1.0 g·mL−1 for use in the various experiments.
Animal husbandry
A total of 48 specific pathogen-free male Sprague-Dawley rats weighing 160–200 were used. All animals were purchased from Hunan SJA Laboratory Animal Co, Ltd. (Changsha, China; license number: SCXK [Xiang] 2013-0004, certification number: 43004700008607). They were allowed to adapt to the environment and were given free access to water and food for 7 days. Room temperature was maintained at 18°C–20°C with relative humidity at 50%–60%; the room was continuously ventilated. All animal experiments were approved by the Animal Care and Use Committee of Gannan Medical University, and conducted according to National Institutes of Health guidelines. The experiments were performed in accordance with the Guide for the Care and Use of Laboratory Animals published by the US National Institutes of Health (NIH publication number 85-23, revised 1985). According to these guidelines, efforts were made to minimize animal suffering and reduce the number of animals used.
Establishment of liver fibrosis model and treatment
Thioacetamide (TAA) is used to establish models of both acute and chronic experimental hepatic injury. In previous studies, 300 mg·kg−1 or 400 mg·kg−1 TAA was intraperitoneally injected for induction of acute experimental hepatic injury.16,17 Previous studies suggested the use of 200 mg·kg−1 TAA for the establishment of models of chronic experimental hepatic injury with advanced fibrosis.18,19 The rat liver fibrosis model was established according to a previously described method.20 The control group and the remaining 40 rats were all injected intraperitoneally with 4% TAA aqueous solution (5 mL·kg−1) at a dose of 200 mg·kg−1 twice a week for 8 consecutive weeks. The 40 Sprague-Dawley rats that developed liver fibrosis were randomly divided into the following five groups (n = 8): model, positive control drug treatment (50 mg·kg−1 silybin, batch number: 130115, Tasly Pharmaceutical Group Co., Tianjin, China), low-dose Semen Brassicae (BL), medium-dose Semen Brassicae (BM), and high-dose Semen Brassicae treatment (BH). Thirty-two Sprague-Dawley rats that were injected intraperitoneally with the same volume of normal saline were randomly divided into the following four groups (n = 8): control, control+BL, control+BM, and control+BH groups.
The doses of Semen Brassicae were determined and dose conversion was performed as described previously.21 Briefly, the usual dosage of Semen Brassicae in adults is 10 g·day−1, and according to the “human and animal body weight conversion chart” (the conversion factor for adult humans and rats is 6.25), the equivalent dosage of Semen Brassicae in rats is 1.0 g·kg−1. This was set as the medium dose, and considering the multiplication factors of 0.5, 1.0, and 2.0, the BL, BM, and BH dosages were determined to be 0.5, 1.0, and 2.0 g·kg−1, respectively. From day 2 of model establishment, the rats in each group were treated with the drugs at 2 mL·kg−1 body weight by gavage once a day for 8 weeks. The rats in the positive drug treatment group were administered 2.5 mg·mL−1 silybin solution at a dose of 50 mg·kg−1 body weight by gavage, while rats in the control and model groups were administered similar volumes of normal saline.
Morphological examination of liver tissue
After the last drug administration, the animals were fasted for 12 h, but they were allowed water, and then they were weighed and anesthetized with ether. After the animals were euthanized, their entire livers were dissected, washed with normal saline, weighed, and the general liver morphology was examined. The liver index was calculated using the following equation: liver index = liver weight (mg)/body weight (g).
The corresponding parts of the right lobes of the rat livers from each group were fixed with 4% formalin for 24 h, washed, dehydrated, hardened, cleared and wax infiltrated, and then the samples were embedded in paraffin and sectioned at 4 μm. Hematoxylin and eosin (H&E) and Masson’s trichrome staining were performed according to the kit manufacturers’ instructions (Beijing Solarbio Science and Technology Co., Ltd., Beijing, China). The degree of liver fibrosis and interstitial collagen morphology were observed under an optical microscope, and the liver fibrosis was graded as described previously.22 Fibrosis was scored as follows: grade 0, normal liver; grade 1, an increase in collagen matrix accumulation without the formation of septa (small stellate expansions of the portal fields); grade 2, formation of incomplete septa from the portal tract to the central vein (septa that do not interconnect with each other); grade 3, complete but thin septa interconnecting with each other to divide the parenchyma into separate fragments; grade 4, same as grade 3, except for the presence of thick septa (complete cirrhosis).
Western blot analysis
Western blot analysis was used to detect the protein expression in the liver tissue. Briefly, the liver tissue samples were minced on ice, the protein lysis buffer was added, and then the tissue was homogenized. The samples were mixed by rotating the tubes at 4°C for 1 h, and after centrifugation at 12,000 rpm at 4°C, the supernatant was collected. The protein concentrations of the liver tissue samples were quantified using the BCA protein quantification kit (Tiangen Biotech (Beijing) Co., Ltd., Beijing, China), and subsequently aliquoted into samples of 50 μg protein. The protein samples were electrophoresed using an 8% separation gel and transferred onto a nitrocellulose membrane (BioTrace™, Pensacola, FL, USA), which was incubated in Tris-buffered saline plus Tween (TBST) containing 5% skimmed milk and then blocked for 1 h at room temperature on a horizontal shaker. The membranes were incubated with antibodies against collagen-I, collagen-III, α-smooth muscle actin (SMA), phosphorylated (p)-Smad 2/3, Smad 2/3, Smad4, transforming growth factor (TGF)-β1 (Abcam, Cambridge, MA, USA; 1:800 dilution), Akt, p-Akt, p-nuclear factor (NF)-κB, NF-κB, and β-actin (Cell Signaling Technology, Danvers, MA, USA; 1:1,000 dilution) at 4°C on a rotating shaker overnight. The membranes were subsequently washed with TBST three times for 5 min each time, followed by incubation with horseradish peroxidase (HRP)-labeled goat anti-rabbit IgG (H+L; ZSGB Bio, Beijing, China; 1:5,000 dilution) for 1 h at room temperature on a horizontal shaker. Then, the membranes were washed with TBST twice for 5 min each time, followed by chemiluminescent detection. The protein band areas were quantified using the Image Lab 6.0 software, and the β-actin antibody was used as the internal reference.
Statistical analysis
The data were analyzed using the Statistical Package for the Social Sciences (SPSS) 20.0 (IBM Corporation, Armonk, NY, USA) statistical software. The measurement indices were presented as the mean ± standard deviation (SD). An analysis of variance (ANOVA) was used for the overall comparison of the measurement indices between groups. The Newman-Keuls t-test was used for multiple comparisons of the means of each group and the model group, and a P < 0.05 was considered statistically significant.
Results
Semen Brassicae improves morphology of the livers in a rat model of liver fibrosis
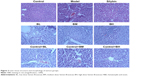
The liver index of each group is shown in Figure 1 and compared with that of the normal group, the liver index of the model group significantly increased (P < 0.05). Compared with that of the model group, the liver index of the treatment groups decreased to different degrees; the BM treatment group, in particular, showed the most significant decrease. Changes in the structure and morphology of the rat liver tissues were examined using H&E staining (Figure 2). The results showed that in the control group and control+BL/BM/BH group, the liver lobular structure was complete, the boundaries were clear, the liver cords radiated outward from the central vein in the middle, and the hepatic cells were in close contact while the cell nuclei were large and round with clear nucleoli and abundant cytoplasm. Furthermore, the portal areas showed no inflammatory cell infiltration, and the structure was clearly visible. The model group rats showed destruction of the liver tissue structure while the arrangement of the hepatic cells was disordered and exhibited steatosis, hepatocyte necrosis, fibrous tissue hyperplasia in the portal areas, and inflammatory cell infiltration in the portal and surrounding areas. The silybin group showed improvement of the damage to the lobular structure and steatosis of the rat liver tissues while the collagen fiber hyperplasia was reduced. The microscopic analyses of the BL, BM, and BH tissue samples revealed a reduction in damage of liver lobular structure as well as an improvement in liver cell edema and steatosis, inflammatory cell infiltration, and other aspects to varying degrees compared with levels in the model group. Furthermore, the BM and BH treatments showed better efficacy than BL. Additionally, BL, BM, and BH treatment in control rats had no hepatotoxic effect according to the results of liver index and H&E staining (Figures 1 and 2).
Treatment of a rat model of liver fibrosis with Semen Brassicae
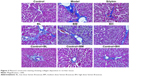
The Masson’s trichrome staining results are shown in Figure 3. The control group tissue samples showed stained fibers in the central vein wall of the hepatic lobules, and a small amount of fiber deposition in interlobular septum. In the model group, the collagen fibers significantly increased, and the fibrous septum of hepatic lobule was formed. The fiber deposition around the central vein and the portal area increased, and it interacted with the neighboring septa, and both were wrapped around each other. In the silybin group, the proliferation of collagen fibers decreased, the fibrosis was improved, and some blue fibers were found around the vessels. The BL, BM, and BH groups showed a significant reduction in the collagen fibrous tissue compared with the model group. The level of fibrosis decreased, and a small amount of thin blue fibers was found around the blood vessels. Furthermore, the anti-fibrotic effects of the BM and BH treatments were better than that of the BL. The liver fibrosis was scored in each group (Figure 4) and the results indicated that the silybin, BL, BM, and BH treatments reduced the liver fibrosis, and the efficacy of the BM and BH treatments was comparable to that of silybin but better than that of BL. The results were similar to those of the Masson’s trichrome staining. Additionally, BL, BM, and BH treatment in control rats had no hepatotoxic effect according to the results of Masson’s trichrome staining and liver fibrosis score (Figures 3 and 4).
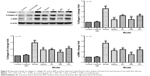
Semen Brassicae decreases the expression of collagen-I, collagen-III, and α-SMA in the liver tissue of rats with hepatic fibrosis
The expression levels of collagen-I, collagen-III, and α-SMA proteins were detected using Western blot analysis (Figure 5) and the model group levels were significantly higher than those of the control group. Compared with that in the control group, the expression levels of collagen-I, collagen-III, and α-SMA proteins showed no obvious change in the control+BM group. Furthermore, compared with the model group, the expression levels of these proteins decreased to varying degrees after treatment with BL, BM, and BH. Moreover, BM treatment decreased the collagen-I, collagen-III, and α-SMA proteins most significantly.
Semen Brassicae regulates the TGF-β1/p-Smad, NF-κB, and AKT signaling pathways in liver samples of a rat model of fibrosis
The expression levels of TGF-β1, p-Smad 2/3, Smad 2/3, Smad4, NF-κB-p65, p-NF-κB-p65, IL-1β, IL-6, AKT, and p-AKT were detected using Western blotting (Figure 6). Compared with those in the control group, the levels of TGF-β1, Smad4, p-Smad 2/3/Smad 2/3, p-NF-κB-p65/NF-κB-p65, IL-1β, IL-6, and p-AKT/AKT showed no obvious change in the control+BM group, while the levels in the model group were significantly enhanced. Furthermore, compared with those in the model group, the levels of TGF-β1, Smad4, p-Smad 2/3/Smad 2/3, p-NF-κB-p65/NF-κB-p65, IL-1β, IL-6, and p-AKT/AKT were altered to different degrees after treatment with Semen Brassicae. The BM treatment showed the most significant effects on the levels of TGF-β1, Smad4, p-Smad 2/3/Smad 2/3, p-NF-κB-p65/NF-κB-p65, IL-1β, IL-6, and p-AKT/AKT.
Discussion
The occurrence and development of liver fibrosis is a complex multi-factorial and multi-step process. It has been difficult to achieve a breakthrough in anti-fibrotic therapy by focusing on only a single target, pathway, or pathological aspect. Currently, there is no widely accepted effective anti-hepatic fibrosis drug available.23 The mechanisms of the anti-hepatic fibrosis effect of traditional Chinese medicines involve multiple aspects, levels, and targets in the pathophysiological process of liver fibrosis and, therefore, show unique advantages.24 The results of this study indicate that compared with the control group, the expression levels of collagen-I, collagen-III, and α-SMA in the liver tissues of the TAA-induced liver fibrosis rat model increased, and the collagen deposition was obvious and the degree of fibrosis increased. Activation of hepatic stellate cells (HSCs) is a central event in the development of hepatic fibrosis.25 Liver damage can lead to the activation of HSCs, which are subsequently transformed into α-SMA-positive cells that proliferate, migrate, contract, and secrete ECM. α-SMA is an important marker of HSC activation.
Compared with the model group, the expression levels of collagen-I, collagen-III, and α-SMA in the liver tissues from the hepatic fibrosis rat model decreased significantly after treatment with Semen Brassicae. Furthermore, the collagen deposition was also significantly reduced, and the degree of fibrosis was greatly improved, suggesting that Semen Brassicae inhibited the expression of collagen-I, collagen-III, and α-SMA, reduced liver fibrosis, and improved the liver tissue structure. Furthermore, BM treatment showed a better efficacy than that of BL, BH, and silybin treatments. The dose of Semen Brassicae was verified after many years in traditional Chinese medicine. The BM dose is based on the clinical dose for adults, same with the dose of clinical medication, which was a potential reason for BM to have had the most beneficial effect on liver fibrosis.
Activated HSCs interact with surrounding hepatic, endothelial, and immune cells, and secrete a variety of inflammatory factors and chemokines to promote fibrosis. The regulation of several cell signaling pathways is involved in this process, and the most important pathways are TGF-β1/Smad, NF-κB, and AKT.26–28 TGF-β1, which is mainly synthesized by HSCs, Kupffer cells, and sinusoidal endothelial cells promotes the proliferation of HSCs, and their myofibroblastic transformation is one of the strongest pro-fibrotic factors.26 TGF-β1 also strongly stimulates HSCs to produce collagen-I and collagen-III to produce a large amount of ECM, while inhibiting ECM degradation.29 TGF-β1 also binds to its receptors on the cell membrane and phosphorylates the receptor-related Smad 2/3 proteins. The conformation of activated Smad 2/3 changes, leading to its release from the receptor complex to reacting with Smad4 to form a Smad 2/3-Smad4 complex.28 The transportation of this complex to the nucleus enhances the expression of α-SMA by HSCs, which further activates the HSCs.28 NF-κB is an important transcription factor, which is involved in many physiological and pathological processes, such as inflammation, apoptosis, and liver fibrosis.30 NF-κB activation can contribute to hepatocyte injury and subsequent inflammation. Subsequently, massive hepatocyte death and inflammation may activate HSCs, leading to fibrosis.31 PI3K/AKT signaling is associated with cell growth, apoptosis, differentiation, and regulation. Silencing of the PI3K/AKT signaling enhanced the expression of α-SMA and vimentin, promoted the viability and migration of HSCs, and induced liver fibrosis.32 A previous study showed that quercetin, a traditional Chinese medicine, prevents hepatic fibrosis by inhibiting HSC activation via the TGF-β1/Smads and PI3K/Akt pathways.33 The results of this study show that the levels of TGF-β1, Smad4, p-Smad 2/3/Smad 2/3, p-NF-κB-p65/NF-κB-p65, IL-1β, IL-6, and p-AKT/AKT in the liver tissue of the TAA-induced hepatic fibrosis rat model significantly increased compared to the levels in the control group. Furthermore, compared with those in the model group, the levels of TGF-β1, Smad4, p-Smad 2/3/Smad 2/3, p-NF-κB-p65/NF-κB-p65, IL-1β, IL-6, and p-AKT/AKT in the liver tissues of the hepatic fibrosis rat model were significantly reduced after treatment with Semen Brassicae, suggesting that Semen Brassicae inhibited the expression of these proteins. This indicates that the anti-hepatic fibrosis effect of Semen Brassicae may be mediated by the TGF-β1/Smad, NF-κB, and AKT pathways. Treatment with BM reduced the levels of TGF-β1, Smad4, p-Smad 2/3/Smad 2/3, p-NF-κB-p65/NF-κB-p65, IL-1β, IL-6, and p-AKT/AKT more significantly than treatments with BL/BH and silybin.
Conclusion
The results of this study indicate that the underlying mechanisms of the anti-fibrotic effect of Semen Brassicae in the liver may be mediated by the regulation of TGF-β1/Smad, NF-κB, and AKT signaling pathways, which reduce the excessive deposition of ECM.
Acknowledgments
The present study was supported by grants from the National Natural Science Foundation (81360521) and Nature Science Foundation of Gannan Medical University (YB201608).
Disclosure
The authors report no conflicts of interest in this work.
References
Luangmonkong T, Suriguga S, Bigaeva E, et al. Evaluating the antifibrotic potency of galunisertib in a human ex vivo model of liver fibrosis. Br J Pharmacol. 2017;174(18):3107–3117. | ||
Luetkemeyer AF, Wyles DL. CROI 2017: highlights of advances in viral hepatitis and liver fibrosis. Top Antivir Med. 2017;25(2):84–92. | ||
Prestigiacomo V, Weston A, Messner S, Lampart F, Suter-Dick L. Pro-fibrotic compounds induce stellate cell activation, ECM-remodelling and Nrf2 activation in a human 3D-multicellular model of liver fibrosis. PLoS One. 2017;12(6):e0179995. | ||
Wang HW, Peng CY, Lai HC, et al. New noninvasive index for predicting liver fibrosis in Asian patients with chronic viral hepatitis. Sci Rep. 2017;7(1):3259. | ||
Li XM, Peng JH, Sun ZL, et al. Chinese medicine CGA formula ameliorates DMN-induced liver fibrosis in rats via inhibiting MMP2/9, TIMP1/2 and the TGF-beta/Smad signaling pathways. Acta Pharmacol Sin. 2016;37(6):783–793. | ||
Song YN, Zhang GB, Lu YY, et al. Huangqi decoction alleviates dimethylnitrosamine-induced liver fibrosis: an analysis of bile acids metabolic mechanism. J Ethnopharmacol. 2016;189:148–156. | ||
Wang S, Shi XL, Feng M, et al. Puerarin protects against CCl4-induced liver fibrosis in mice: possible role of PARP-1 inhibition. Int Immunopharmacol. 2016;38:238–245. | ||
Liu C, Yuan X, Tao L, et al. Xia-yu-xue decoction (XYXD) reduces carbon tetrachloride (CCl4)-induced liver fibrosis through inhibition hepatic stellate cell activation by targeting NF-kappaB and TGF-beta1 signaling pathways. BMC Complement Altern Med. 2015;15:201. | ||
Pan Q, Wang YQ, Li GM, Duan XY, Fan JG. Fuzheng Huayu recipe ameliorates liver fibrosis by restoring balance between epithelial-to-mesenchymal transition and mesenchymal-to-epithelial transition in hepatic stellate cells. Biomed Res Int. 2015;2015:935903. | ||
Cheng Q, Li N, Chen M, et al. Fuzheng Huayu inhibits carbon tetrachloride-induced liver fibrosis in mice through activating hepatic NK cells. J Ethnopharmacol. 2013;145(1):175–181. | ||
Zhuang LX, Zhao MH, Yang JJ, Deng QP. [A study on the time-effect relationship in the treatment of bronchial asthma with medicinal vesiculation therapy]. Zhen Ci Yan Jiu. 2007;32(1):53–57. Chinese. | ||
Hu Q, Gu P, Jiang X, et al. [Moderate and severe persistent allergic rhinitis treated with acupoint application therapy of the different intensity: a randomized controlled trial]. Zhongguo Zhen Jiu. 2017;37(11):1177–1182. Chinese. | ||
Wen BL, Liu BY, Jin P, et al. Clinical research of acupoint application for “treatment of winter disease in summer” used to prevent and treat bronchial asthma in children. J Tradit Chin Med. 2012;32(1):31–39. | ||
Peng J, Wu XQ, He LY, et al. [Effects of summer acupoint application therapy in reducing exacerbation frequency of chronic lung diseases: protocol of a retrospective and prospective study]. Zhong Xi Yi Jie He Xue Bao. 2012;10(1):39–47. Chinese. | ||
Zheng BP, Han LM, Lin TT, et al. [Effects of Semen Sinapis Albae related medicine groups on the expression of VEGF and bFGF in rats with hepatic fibrosis under the guidance of resolving phlegm]. Journal of Gannan Medical University. 2016;36(2):17–19. Chinese. | ||
Shapiro H, Ashkenazi M, Weizman N, et al. Curcumin ameliorates acute thioacetamide-induced hepatotoxicity. J Gastroenterol Hepatol. 2006;21(2):358–366. | ||
Margeli AP, Papadimitriou L, Ninos S, et al. Hepatic stimulator substance administration ameliorates liver regeneration in an animal model of fulminant hepatic failure and encephalopathy. Liver Int. 2003;23(3):171–178. | ||
Lebda MA, Sadek KM, Abouzed TK, Tohamy HG, El-Sayed YS. Melatonin mitigates thioacetamide-induced hepatic fibrosis via antioxidant activity and modulation of proinflammatory cytokines and fibrogenic genes. Life Sci. 2018;192:136–143. | ||
Hessin AF, Hegazy RR, Hassan AA, Yassin NZ, Kenawy SA. Resveratrol prevents liver fibrosis via two possible pathways: modulation of alpha fetoprotein transcriptional levels and normalization of protein kinase C responses. Indian J Pharmacol. 2017;49(4):282–289. | ||
Abdulaziz Bardi D, Halabi MF, Abdullah NA, et al. In vivo evaluation of ethanolic extract of Zingiber officinale rhizomes for its protective effect against liver cirrhosis. Biomed Res Int. 2013;2013:918460. | ||
Cao S, Chang XF, Yin B, et al. [The therapeutic effect and mechanism of Semen Brassicae group on liver fibrosis rats]. Lishizhen Medicine And Materia Medica Research. 2017;28(2):265–268. Chinese. | ||
Jiang Y, Wang C, Li YY, et al. Mistletoe alkaloid fractions alleviates carbon tetrachloride-induced liver fibrosis through inhibition of hepatic stellate cell activation via TGF-beta/Smad interference. J Ethnopharmacol. 2014;158 Pt A:230–238. | ||
Kamal S, Khan MA, Seth A, et al. Beneficial effects of statins on the rates of hepatic fibrosis, hepatic decompensation, and mortality in chronic liver disease: a systematic review and meta-analysis. Am J Gastroenterol. 2017;112(10):1495–1505. | ||
Hamed AM, El-Kharashi OA, Boctor SS, Abd-Elaziz LF. Potential involvement of PPAR alpha activation in diminishing the hepatoprotective effect of fenofibrate in NAFLD: accuracy of non-invasive panel in determining the stage of liver fibrosis in rats. Biomed Pharmacother. 2017;85:68–78. | ||
Huang Y, Deng X, Liang J. Modulation of hepatic stellate cells and reversibility of hepatic fibrosis. Exp Cell Res. 2017;352(2):420–426. | ||
Xu F, Liu C, Zhou D, Zhang L. TGF-beta/SMAD pathway and its regulation in hepatic fibrosis. J Histochem Cytochem. 2016;64(3):157–167. | ||
Sunami Y, Leithauser F, Gul S, et al. Hepatic activation of IKK/NFkappaB signaling induces liver fibrosis via macrophage-mediated chronic inflammation. Hepatology. 2012;56(3):1117–1128. | ||
Ma T, Cai X, Wang Z, et al. miR-200c accelerates hepatic stellate cell-induced liver fibrosis via targeting the FOG2/PI3K pathway. Biomed Res Int. 2017;2017:2670658. | ||
Yang JH, Kim SC, Kim KM, et al. Isorhamnetin attenuates liver fibrosis by inhibiting TGF-beta/Smad signaling and relieving oxidative stress. Eur J Pharmacol. 2016;783:92–102. | ||
Wang Y, Wang R, Wang Y, et al. Ginkgo biloba extract mitigates liver fibrosis and apoptosis by regulating p38 MAPK, NF-kappaB/IkappaBalpha, and Bcl-2/Bax signaling. Drug Des Devel Ther. 2015;9:6303–6317. | ||
Shen H, Sheng L, Chen Z, et al. Mouse hepatocyte overexpression of NF-kappaB-inducing kinase (NIK) triggers fatal macrophage-dependent liver injury and fibrosis. Hepatology. 2014;60(6):2065–2076. | ||
Zhu L, Cheng M, Liu Y, et al. Thymosin-beta4 inhibits proliferation and induces apoptosis of hepatic stellate cells through PI3K/AKT pathway. Oncotarget. 2017;8(40):68847–68853. | ||
Wu L, Zhang Q, Mo W, et al. Quercetin prevents hepatic fibrosis by inhibiting hepatic stellate cell activation and reducing autophagy via the TGF-beta1/Smads and PI3K/Akt pathways. Sci Rep. 2017;7(1):9289. |
 © 2018 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2018 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.