Back to Journals » Drug Design, Development and Therapy » Volume 12
Schisandrin B ameliorated chondrocytes inflammation and osteoarthritis via suppression of NF-κB and MAPK signal pathways
Authors Ran J, Ma C, Xu K, Xu L, He Y, Moqbel SAA, Hu P, Jiang L, Chen W, Bao J, Xiong Y, Wu L
Received 9 January 2018
Accepted for publication 28 February 2018
Published 9 May 2018 Volume 2018:12 Pages 1195—1204
DOI https://doi.org/10.2147/DDDT.S162014
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 4
Editor who approved publication: Dr Qiongyu Guo
Jisheng Ran,* Chiyuan Ma,* Kai Xu,* Langhai Xu, Yuzhe He, Safwat Adel Abdo Moqbel, Pengfei Hu, Lifeng Jiang, Weiping Chen, Jiapeng Bao, Yan Xiong, Lidong Wu
Department of Orthopedic Surgery, The Second Affiliated Hospital, School of Medicine, Zhejiang University, Hangzhou, China
*These authors contributed equally to this work
Introduction: Osteoarthritis (OA) is the most prevalent joint disorder in the elderly population, and inflammatory mediators like IL-1βwere thought to play central roles in its development. Schisandrin B, the main active component derived from Schisandra chinensis, exhibited antioxidative and antiinflammatory properties.
Methods: In the present study, the protective effect and the underlying mechanism of Schisandrin B on OA was investigated in vivo and in vitro.
Results: The results showed that Schisandrin B decreased IL-1β-induced upregulation of matrix metalloproteinase 3 (MMP3), MMP13, IL-6, and inducible nitric oxide synthase (iNOS) and increased IL-1β-induced downregulation of collagen II, aggrecan, and sox9 as well. Schisandrin B significantly decreased IL-1β-induced p65 phosphorylation and nuclear translocation of p65 in rat chondrocytes. Mitogen-activated protein kinase (MAPK) activation was also inhibited by Schisandrin B, as evidenced by the reduction of p38, extracellular signal-regulated kinase (Erk), and c-Jun amino-terminal kinase (Jnk) phosphorylation. In addition, Schisandrin B prevented cartilage degeneration in rat OA model with significantly lower Mankin’s score than the control group.
Conclusion: Our study demonstrated that Schisandrin B ameliorated chondrocytes inflammation and OA via suppression of nuclear factor-κB (NF-κB) and MAPK signal pathways, indicating a therapeutic potential in OA treatment.
Keywords: osteoarthritis, Schisandrin B, chondrocytes, MMPs, NF-κB pathway, MAPK pathway
Introduction
Osteoarthritis (OA), characterized by progressive articular cartilage loss, chronic pain, and subsequent joint failure, is a major cause of disability worldwide.1 The overall prevalence of OA continued to increase with increasing age, and the lifetime risk of knee OA was reported to be 14%–45% in western countries.2,3 Though OA is considered to be a noninflammatory disease, recent evidence demonstrated that chronic low-grade inflammation contributes to disease symptoms and OA progression.4 Inflammatory mediators, particularly IL-1β and tumor necrosis factor α, could compromise chondrocytes viability, change their differential fate, and induce proinflammatory and procatabolic responses.5,6 Enhanced catabolic response would exacerbate cartilage matrix degradation by the suppression of cartilage-related genes expression and promotion of matrix-degrading genes like collagenases and matrix metalloproteinases (MMPs).7 Signal pathways like NK-κB and mitogen-activated protein kinase (MAPK) signaling are well recognized to participate in this progress and could be effective targets in OA treatment.8,9 Targeting the inflammatory response in OA progress may lead to new transformative therapies.
Schisandrin B, the main active component derived from Schisandra chinensis, has been reported to exhibit antioxidative and antiinflammatory properties. Studies focusing on the molecular mechanism revealed that Schisandrin B could effectively reduce the activation of inflammatory signal pathways including nuclear factor-κB (NF-κB) pathway and MAPK/extracellular signal-regulated kinase (Erk)/p38/c-Jun amino-terminal kinase (Jnk) pathway.10–13 Several in vivo studies have also demonstrated the protective role of Schisandrin B in inflammatory bowel disease,14 neuroinflammatory damage,15 acute lung injuries,16 etc. However, the effect of Schisandrin B on chondrocyte inflammation and OA progression has not been reported yet. In the present study, we report first that Schisandrin B can inhibit IL-1β-induced chondrocytes inflammation in vitro and ameliorate rat OA in vivo via suppression of NF-κB and MAPK signal pathways.
Materials and methods
Materials
Schisandrin B was obtained from Shanghai PureOne Biotechnology. DMEM, penicillin, streptomycin, fetal bovine serum (FBS), and 0.25% trypsin were all purchased from Gibco RRL, Grand Island, NY, USA. Type II collagenase and bull serum albumin (BSA) were purchased from Sigma-Aldrich, St. Louis, MO, USA. Recombinant rat IL-1β was obtained from R&D Systems, Abingdon, UK. Trizol reagent, Pierce™ ECL Western Blotting Substrate, protease, and phosphatase inhibitors were purchased from Invitrogen, Carlsbad, CA, USA.
Chondrocyte isolation, culture, and treatment
Chondrocyte isolation was conducted as previously described.17 Briefly, knee and hip cartilage harvested from 6-week-old Sprague Dawley rats was cut into 1 mm3 particles. Then, the cartilage particles were digested with 0.25% tyrosine for 30 minutes followed by 0.2% type II collagenase on a horizontal shaker at 37°C for 4 hours. The isolated chondrocytes were seeded and grown in low-glucose DMEM supplemented with 10% FBS, 100 U/mL penicillin, and 100 mg/mL streptomycin at 37°C with 5% CO2. Cells seeded in 96-well plates or 24-well plates were treated with Schisandrin B at different concentrations for 24 or 48 hours to detect cell viability and chondrocyte phenotype changes. For chondrocyte inflammation and signal pathway involvement analysis, 6-well seeded cells were pretreated with Schisandrin B at different concentrations for 1 hour and then incubated with or without IL-1β (10 ng/mL) for 48 hours or 30 minutes according to the study design.
Cell viability analysis
To analyze the cytotoxicity of Schisandrin B on chondrocytes, CCK-8 assay was conducted according to the manufacturer’s instruction. Five thousand chondrocytes were seeded in 96-well plates and treated with different concentrations (0, 25, 50, 75, 100, and 150 μM) of Schisandrin B for 24 and 48 hours. Then, the pretreated cells were incubated with 10 μL CCK-8 reagent per well for 4 hours and the optical density was read at a wavelength of 450 nm with a microplate spectrophotometer.
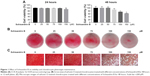
To detect the chondrocyte phenotype changes, safranin O staining were carried out in this study. Chondrocytes seeded in 12-well plates were treated with different concentrations (0, 25, 50, 75, 100, and 150 μM) of Schisandrin B for 48 hours. The cells were fixed with 4% paraformaldehyde solution for 20 minutes and then stained with 0.1% safranin O solution for 5 minutes. The cells were washed with PBS for three times and then imaged by gross camera and Leica light microscope.
RNA extraction and real-time polymerase chain reaction (RT-PCR)
Total RNA was extracted by lysis in Trizol reagent followed by a one-step phenol chloroform isoamyl alcohol extraction according to the manufacturer’s instruction. RNA concentrations were detected and adjusted to the same before the reverse transcription with PrimeScript™ RT Master Mix purchased from TAKARA. RT-PCR was conducted with SYBR® Premix Ex Taq™ II (TAKARA) by Applied Biosystems StepOnePlus™ according to the protocol. The expressions of MMP3, MMP13, IL-6, inducible nitric oxide synthase (iNOS), collagen II, aggrecan, and sox9 were detected. 18S was used as the endogenous control. All primers used in this study are listed in Table 1. Gene expression was analyzed for fold difference using 2−ΔΔCT.
  | Table 1 Primer sequences used in this study |
Protein extraction and Western blot
After treatment, equal amounts of cells were lysed for whole protein extraction by RIPA Lysis Buffer containing protease and phosphatase inhibitors. The samples were then separated by 10% or 12% sodium dodecyl sulfate-polyacrylamide gels and then transferred onto nitrocellulose membranes. After blocking with 5% BSA for 1 hour at room temperature, the membranes were incubated with primary antibodies at 4°C overnight and then with secondary antibodies at room temperature for 2 hours. The protein bands were luminesced using Pierce ECL Western Blotting Substrate and detected with Bio-Rad ChemiDoc system. Antibodies against MMP3, MMP13, IL-6, iNOS, collagen II, aggrecan, sox9, p65, phosphor-p65, Jnk, phosphor-Jnk, Erk, phosphor-Erk, p38, and phosphor-p38 were used in this study. Glyceraldehyde 3-phosphate dehydrogenase (GAPDH) was used as endogenous control. The relative amount of proteins was analyzed with Quantity One software (Bio-Rad) and normalized to GAPDH.
Immunofluorescence
Chondrocytes cultured on glass coverslips were pretreated with 50 μM Schisandrin B for 1 hour and then incubated with 10 ng/mL IL-1β for 30 minutes. After fixation with cold methanol for 20 minutes, the cells were permeabilized by PBS containing 0.3% v/v Triton X-100 for 10 minutes and blocked with 5% BSA for 1 hour at room temperature. Afterward, the cells were incubated with primary antibody against p65 at 4°C overnight and then with fluorescein isothiocyanate-conjugated secondary antibody for 1 hour. Cell nucleuses were stained with DAPI for 5 minutes, and then the coverslips were mounted on glass slides for immunofluorescence analysis with a Leica fluorescence microscope.
Animal experiments
Twenty Sprague Dawley rats were used to develop OA by surgical resection of medial meniscus in knee joints as previously described.18 Briefly, the rats were anesthetized by pentobarbital (40 mg/kg), and knee joints were opened with a medial parapatellar approach. Then, the medial meniscuses were carefully resected without cartilage and ligament injuries. One week after surgery, intra-articular injection of 50 μM Schisandrin B or equal volume of vehicle was conducted every 7 days. Rats were sacrificed after 4 weeks of treatment, and knee samples were fixed with 4% paraformaldehyde solution. The study was conducted in accordance with NIH guidelines (NIH Pub No 85-23, revised 1996), and the protocol was approved by the Ethics Committee of the Second Affiliated Hospital, School of Medicine, Zhejiang University, Hangzhou, China.
Tissue sample preparation, histological analysis, and immunohistochemistry
Fixed rat knee joints were decalcified with 10% formic acid until the samples became soft. The samples were then gradiently dehydrated and embedded within paraffin blocks. Histological sections (5 mm) were prepared using a microtome, and subsequently deparaffinized with xylene, hydrated gradiently, and then subjected to safranin O staining and immunohistochemistry. For immunohistochemistry, the hydrated sections were first blocked with hydrogen peroxide before pepsin treatment for 20 minutes. Afterward, the sections were blocked with 5% BSA for 1 hour at room temperature and incubated with primary antibodies overnight at 4°C. Then, the sections were incubated with horseradish peroxidase-linked secondary antibodies for 1 hour at room temperature, and 3,30-diaminobenzidine was used as a chromogenic agent. Histological evaluation of OA was performed by three individuals using modified Mankin’s score.19
Statistical analysis
All quantitative data sets are presented as mean±SD. Student’s t-test was performed to assess statistically significant differences in the results of different experimental groups. Statistical differences were performed with SPSS 20.0 (IBM Corporation, Armonk, NY, USA) and values of p<0.05 were considered to be significantly different.
Results
Effect of Schisandrin B on cell viability and chondrocyte phenotype maintenance
The cytotoxicity of different concentrations (25, 50, 75, 100, and 150 μM) of Schisandrin B on chondrocytes was accessed by the CCK-8 assay. As shown in Figure 1A, Schisandrin B had no obvious cytotoxicity on chondrocytes with concentrations ≤100 μM at 24 hours and ≤75 μM at 48 hours. The effect of Schisandrin B on chondrocyte phenotype was also detected by safranin O staining, and the results showed that 75 μM Schisandrin B caused slight safranin O stain loss and concentrations ≥100 μM lead to mild to severe safranin O stain loss. Chondrocyte phenotype was not apparently affected by Schisandrin B with concentrations ≤50 μM (Figure 1B and C). Based on the results above, 25 and 50 μM concentrations of Schisandrin B were used for in vitro tests, and a concentration of 50 μM was used for in vivo test.
Effect of Schisandrin B on IL-1β-induced inflammation and cartilage-gene expression change in rat chondrocytes
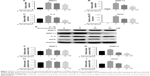
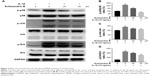
The effect of Schisandrin B on IL-1β-induced chondrocyte inflammation and matrix-degrading genes expression was evaluated by RT-PCR and Western blot. Pretreatment with Schisandrin B significantly decreased IL-1β-induced upregulation of inflammatory genes (IL-6 and iNOS) and matrix-degrading genes (MMP3, MMP13) in rat chondrocytes in a concentration-dependent manner in both mRNA level and protein level (Figure 2). Western blot analysis also revealed that 50 μM Schisandrin B could significantly reverse IL-1β-induced downregulation of collagen II, aggrecan, and sox9 in protein level (Figure 3). In addition, safranin O stain loss induced by IL-1β was effectively restored by 50 μM Schisandrin B in rat chondrocytes (Figure 3). Thus, Schisandrin B could protect rat chondrocytes by inhibiting the IL-1β-induced matrix-degrading genes expression and promoting cartilage-gene expression in vitro.
Effect of Schisandrin B on IL-1β-induced NF-κB signal activation in chondrocytes
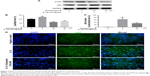
To explore the underlying mechanism of cartilage protection effect of Schisandrin B, the NF-κB signal activation was investigated by detecting the status and location of its key effector p65. The activated status of p65 (phosphor-p65, p-p65) and total p65 was detected by Western blot. The results demonstrated that pretreatment with 25 and 50 μM Schisandrin B significantly reduced the elevation of p-p65/p65 ratio induced by IL-1β stimulation (Figure 4A and B). Further, the immunofluorescence analysis revealed that IL-1β-induced nuclear translocation of p65 was significantly blocked by pretreatment with 50 μM Schisandrin B in rat chondrocytes (Figure 4C and D). Taking together, our results demonstrated Schisandrin B could effectively inhibit IL-1β-induced NF-κB signal activation in rat chondrocytes in vitro.
Effect of Schisandrin B on IL-1β-induced MAPK activation in chondrocytes
The activation of MAPK was investigated by detecting the phosphorylation of p38, Erk, and Jnk through Western blot analysis. IL-1β stimulation could significantly activate MAPK pathway by increasing the p-p38, p-Erk, and p-Jnk levels, which were reduced by Schisandrin B in a concentration-dependent manner (Figure 5). The results indicated that Schisandrin B could also inhibit IL-1β-induced MAPK activation in rat chondrocytes.
Effect of Schisandrin B on cartilage degeneration in rat OA model
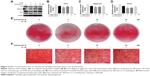
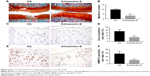
To evaluate the effect of Schisandrin B on cartilage degeneration in vivo, rat OA model was developed by surgical resection of medial meniscus. Knee joint cartilage from rats with intra-articular injection of vehicle exhibited typical OA features with wearing surface, fissuring in matrix, decreased chondrocytes, and loss of safranin O stain. However, few fissuring and safranin O stain loss were observed in cartilage from rats with intra-articular injection of 50 μM Schisandrin B (Figure 6A). The average Mankin’s score of Schisandrin B group was lower than the OA group with significant difference (Figure 6B). Immunohistochemistry also revealed that cox-2 and MMP13 expressions were significantly reduced in the Schisandrin B group in vivo (Figure 6C–F). These data demonstrated Schisandrin B could ameliorate OA progress in the rat model.
Discussion
OA is the most prevalent form of joint disease in elderly population, which often leads to chronic pain and joint disability. Currently, the main purpose of OA treatment in clinical practice is to control the symptoms, and a replacement surgery is always needed in end-stage disease. Nonsteroidal antiinflammatory drugs (NSAIDs) is used extensively in the treatment of OA to relieve chronic pain and swelling, whereas the cartilage degeneration could not be effectively ameliorated. However, long-term use of NSAIDs may result in serious side effects. Thus, effective and safe therapeutic agents are required in the treatment of OA. Schisandrin B, the main active component derived from S. chinensis, has been reported to exhibit anti-inflammatory properties in inflammatory bowel disease, neuroinflammatory damage, and acute lung injuries.14–16 For the first time, to the best of our knowledge, we report the protective effect of Schisandrin B on cartilage degeneration via suppression of NF-κB and MAPK pathways in this study.
OA is now considered a disease of the whole joint with cartilage degeneration, synovial inflammation, and subchondral sclerosis.1,20 Among these, the degeneration of cartilage plays central role in OA development. The cartilage matrix synthesis and degradation are dynamic balanced in normal joints, whereas the inflammatory factors like IL-1β can break the homeostasis by upregulation of matrix-degrading genes including the MMPs and downregulation of matrix-synthesizing genes like collagen II, aggrecan, and sox9.4,21,22 Inhibition of MMPs expression and chondrocyte inflammation was a demonstrated therapeutic effect in OA.23–25 Our data revealed that Schisandrin B could significantly reduce the IL-1β-induced inflammatory and matrix-degrading genes expression including IL-6, iNOS, MMP3, and MMP13. Moreover, IL-1β-induced downregulation of collagen II, aggrecan, and sox9 could be restored by Schisandrin B as well. These data indicated that Schisandrin B had an antidegenerative role in chondrocytes in vitro. The effect of Schisandrin B on cartilage degeneration in vivo was also investigated, and the results further confirmed the protective role of Schisandrin B by ameliorating cartilage erosion and reducing matrix degeneration.
The mechanism of cartilage degeneration is complicated, and many signal pathways are involved. Because of the vital role in chondrocyte inflammation, cartilage degradation, and MMPs regulation,8,9 NF-κB and MAPK pathways were focused in our study. In rest status, p65 resides with its inhibitor IKB in cytoplasm. When stimulated by inflammatory factors like IL-1β, p65 is freed with increased phosphorylation and quickly translocates to nucleus, where p65 promotes plenty of inflammatory gene expressions such as MMPs, iNOS, cytochrome c oxidase subunit 2, and IL-6.26 Our results revealed that Schisandrin B inhibited p65 phosphorylation and nuclear translocation, indicating a suppressive role of NF-κB pathway in chondrocytes. This is in accordance with other researches which demonstrated Schisandrin B inhibited NF-κB pathway in microglia cells15 and lymphocytes.27 Conventional MAPKs mainly include the ERK1/2, Jnk, and p38, which can be activated by phosphorylation from upstream signals.28 According to the Western blot data, we demonstrated Schisandrin B could decrease the IL-1β-induced phosphorylation of Erk, p38, and Jnk, thus inhibiting MAPK signal. Therefore, the underlying mechanism of Schisandrin B in preventing cartilage degeneration is associated with the inhibition of NF-κB and MAPK pathways. However, it is still unknown whether NF-κB and MAPK are direct targets of Schisandrin B. Further studies are needed to elucidate the exact mechanism by which Schisandrin B regulates NF-κB and MAPK signal pathways.
Conclusion
Our study is the first to demonstrate that Schisandrin B inhibited chondrocytes inflammation in vitro and ameliorated cartilage degeneration in vivo via suppression of NF-κB and MAPK signal pathways. Our findings indicate a therapeutic potential of Schisandrin B in OA treatment.
Acknowledgment
This study was supported by the National Natural Science Foundation of China (81371996 and 81572173) and Natural Science Foundation of Zhejiang Province (LQ18H060001).
Disclosure
The authors report no conflicts of interest in this work.
References
Roos EM, Arden NK. Strategies for the prevention of knee osteoarthritis. Nat Rev Rheumatol. 2016;12(2):92–101. | ||
Losina E, Weinstein AM, Reichmann WM, et al. Lifetime risk and age at diagnosis of symptomatic knee osteoarthritis in the US. Arthritis Care Res. 2013;65(5):703–711. | ||
Murphy L, Schwartz TA, Helmick CG, et al. Lifetime risk of symptomatic knee osteoarthritis. Arthritis Rheum. 2008;59(9):1207–1213. | ||
Liu-Bryan R, Terkeltaub R. Emerging regulators of the inflammatory process in osteoarthritis. Nat Rev Rheumatol. 2015;11(1):35–44. | ||
Kapoor M, Martel-Pelletier J, Lajeunesse D, Pelletier JP, Fahmi H. Role of proinflammatory cytokines in the pathophysiology of osteoarthritis. NatRev Rheumatol. 2011;7(1):33–42. | ||
Charlier E, Relic B, Deroyer C, et al. Insights on molecular mechanisms of chondrocytes death in osteoarthritis. Int J Mol Sci. 2016;17(12):2146. | ||
Heinegard D, Saxne T. The role of the cartilage matrix in osteoarthritis. Nat Rev Rheumatol. 2011;7(1):50–56. | ||
Saklatvala J. Inflammatory signaling in cartilage: MAPK and NF-kappaB pathways in chondrocytes and the use of inhibitors for research into pathogenesis and therapy of osteoarthritis. Curr Drug Targets. 2007;8(2):305–313. | ||
Sondergaard BC, Schultz N, Madsen SH, Bay-Jensen AC, Kassem M, Karsdal MA. MAPKs are essential upstream signaling pathways in proteolytic cartilage degradation–divergence in pathways leading to aggrecanase and MMP-mediated articular cartilage degradation. Osteoarthritis Cartilage. 2010;18(3):279–288. | ||
Park SY, Bae YS, Ko MJ, Lee SJ, Choi YW. Comparison of anti-inflammatory potential of four different dibenzocyclooctadiene lignans in microglia; action via activation of PKA and Nrf-2 signaling and inhibition of MAPK/STAT/NF-kappaB pathways. Mol Nutr Food Res. 2014;58(4):738–748. | ||
Guo M, An F, Wei X, Hong M, Lu Y. Comparative effects of Schisandrin A, B, and C on acne-related inflammation. Inflammation. 2017;40(6):2163–2172. | ||
Zhu N, Cai C, Zhou A, Zhao X, Xiang Y, Zeng C. Schisandrin B prevents hind limb from ischemia-reperfusion-induced oxidative stress and inflammation via MAPK/NF-kappaB pathways in rats. Bio Med Res Int. 2017;2017:4237973. | ||
Chen Q, Zhang H, Cao Y, et al. Schisandrin B attenuates CCl4-induced liver fibrosis in rats by regulation of Nrf2-ARE and TGF-beta/Smad signaling pathways. Drug Design Devel Ther. 2017;11:2179–2191. | ||
Liu W, Liu Y, Wang Z, Yu T, Lu Q, Chen H. Suppression of MAPK and NF-kappa B pathways by schisandrin B contributes to attenuation of DSS-induced mice model of inflammatory bowel disease. Die Pharmazie. 2015;70(9):598–603. | ||
Zeng KW, Zhang T, Fu H, Liu GX, Wang XM. Schisandrin B exerts anti-neuroinflammatory activity by inhibiting the Toll-like receptor 4-dependent MyD88/IKK/NF-kappaB signaling pathway in lipopolysaccharide-induced microglia. Eur J Pharmacol. 2012;692(1–3):29–37. | ||
Cai Z, Liu J, Bian H, Cai J, Zhu G. Suppression of P2X7/NF-kappaB pathways by Schisandrin B contributes to attenuation of lipopolysaccharide-induced inflammatory responses in acute lung injury. Arch Pharm Res. 2016;39(4):499–507. | ||
Bao JP, Jiang LF, Li J, Chen WP, Hu PF, Wu LD. Visceral adipose tissue-derived serine protease inhibitor inhibits interleukin-1beta-induced catabolic and inflammatory responses in murine chondrocytes. Mol Med Rep. 2014;10(4):2191–2197. | ||
Iijima H, Aoyama T, Ito A, et al. Destabilization of the medial meniscus leads to subchondral bone defects and site-specific cartilage degeneration in an experimental rat model. Osteoarthritis Cartilage. 2014;22(7):1036–1043. | ||
van der Sluijs JA, Geesink RG, van der Linden AJ, Bulstra SK, Kuyer R, Drukker J. The reliability of the Mankin score for osteoarthritis. J Orthop Res. 1992;10(1):58–61. | ||
Goldring SR, Goldring MB. Changes in the osteochondral unit during osteoarthritis: structure, function and cartilage-bone crosstalk. NatRev Rheumatol. 2016;12(11):632–644. | ||
Stove J, Huch K, Gunther KP, Scharf HP. Interleukin-1beta induces different gene expression of stromelysin, aggrecan and tumor-necrosis-factor-stimulated gene 6 in human osteoarthritic chondrocytes in vitro. Pathobiology. 2000;68(3):144–149. | ||
Burrage PS, Mix KS, Brinckerhoff CE. Matrix metalloproteinases: role in arthritis. Front Biosci. 2006;11:529–543. | ||
Sabatini M, Lesur C, Thomas M, et al. Effect of inhibition of matrix metalloproteinases on cartilage loss in vitro and in a guinea pig model of osteoarthritis. Arthritis Rheum. 2005;52(1):171–180. | ||
Kobayashi M, Squires GR, Mousa A, et al. Role of interleukin-1 and tumor necrosis factor alpha in matrix degradation of human osteoarthritic cartilage. Arthritis Rheum. 2005;52(1):128–135. | ||
Chin KY. The spice for joint inflammation: anti-inflammatory role of curcumin in treating osteoarthritis. Drug Des Devel Ther. 2016;10:3029–3042. | ||
Rigoglou S, Papavassiliou AG. The NF-kappaB signalling pathway in osteoarthritis. Int J Biochem Cell Biol. 2013;45(11):2580–2584. | ||
Checker R, Patwardhan RS, Sharma D, et al. Schisandrin B exhibits anti-inflammatory activity through modulation of the redox-sensitive transcription factors Nrf2 and NF-kappaB. Free Radic Biol Med. 2012;53(7):1421–1430. | ||
Cargnello M, Roux PP. Activation and function of the MAPKs and their substrates, the MAPK-activated protein kinases. Microbiol Mol Biol Rev. 2011;75(1):50–83. |
 © 2018 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2018 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.