Back to Journals » Drug Design, Development and Therapy » Volume 10
Saussurea tridactyla Sch. Bip.-derived polysaccharides and flavones reduce oxidative damage in ultraviolet B-irradiated HaCaT cells via a p38MAPK-independent mechanism
Authors Guo Y, Sun J, Ye J, Ma W, Yan H, Wang G
Received 17 September 2015
Accepted for publication 21 October 2015
Published 20 January 2016 Volume 2016:10 Pages 389—403
DOI https://doi.org/10.2147/DDDT.S96581
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 2
Editor who approved publication: Prof. Dr. Wei Duan
Yan Guo,1 Juan Sun,2 Juan Ye,1 Wenyu Ma,1 Hualing Yan,1 Gang Wang1
1Department of Dermatology and Venereology, Affiliated Hospital of Qinghai University, 2Department of Anatomy, Qinghai University Medical College, Xining, People’s Republic of China
Objective: To investigate whether Saussurea tridactyla Sch. Bip.-derived polysaccharides and flavones exert apoptosis-inhibiting effects in ultraviolet B (UVB)-irradiated HaCaT cells.
Methods: We divided HaCaT cells into low radiation UVB and high radiation UVB groups. Low radiation UVB and high radiation UVB groups were further divided into a control group, UVB radiation group (UVB group), S. tridactyla Sch. Bip.-derived polysaccharides and flavones low-dose group, and S. tridactyla Sch. Bip.-derived polysaccharides and flavones high-dose group. Cell viability and morphology were assayed by MTT and trypan blue staining. Superoxide dismutase activity, glutathione content, malondialdehyde content, and catalase activity test kits were used to detect superoxide dismutase activity, glutathione content, malondialdehyde content, and catalase activity, respectively. Cell apoptosis, intracellular Ca2+ levels, and mitochondrial membrane potential (ΔΨ) were detected by flow cytometry. Protein levels were analyzed by Western blotting and immunofluorescence.
Results: S. tridactyla Sch. Bip.-derived polysaccharides and flavones were found to increase the absorbance of MTT, decrease cell death, alleviate the degree of cell edema, restore the cell morphology, reduce cell death fragments and chip phenomenon, increase superoxide dismutase activity, glutathione content, and catalase activity while decreasing the content of malondialdehyde, lowering the population of apoptotic cells, reducing the intracellular Ca2+ fluorescence, increasing the mitochondrial membrane potential (ΔΨ), increasing the expressions of p-38, p-53, Bcl-2, and decreasing the expressions of Bax and active-caspase-3.
Conclusion: S. tridactyla Sch. Bip.-derived polysaccharides and flavones can reduce cell apoptosis to protect HaCaT cells from oxidative damage after UVB irradiation; however, this effect does not occur via the p38MAPK pathway.
Keywords: Saussurea tridactyla Sch. Bip.-derived polysaccharides, flavones, oxidative damage, p38MAPK-independent mechanism
Introduction
The solar ultraviolet (UV) spectrum can be divided into three segments: ultraviolet C, 200–290 nm; ultraviolet B (UVB), 290–320 nm, and ultraviolet A, 320–400 nm.1 UVB is more genotoxic and approximately 1,000 times more capable of causing sunburn than ultraviolet A,2,3 which causes a number of detrimental effects to the skin, such as inflammation, immunosuppression, premature skin aging (photoaging), and skin cancer.4,5 UVB primarily causes direct DNA damage via the generation of reactive oxygen species (ROS)4–9 such as hydrogen peroxide and the hydroxyl radical, which have harmful effects on the skin and may lead to oxidative DNA damage, lipid peroxidation, and protein modification.10,11 Other studies have demonstrated that accumulation of ROS within UVB-treated keratinocytes may promote apoptosis.12 An enzymatic antioxidant defense system, composed of catalase (CAT) and superoxide dismutase (SOD),13 plays an important role in the repair of ROS-induced DNA damage.
UVB-induced apoptosis is mediated by apoptotic signals activating two main pathways: intrinsic (mitochondrial) and extrinsic (death receptor-dependent).14 ROS alters the mitochondrial permeability transition.15 The ratio between antiapoptotic Bcl-2 and pro-apoptotic Bax has been suggested as a marker of determining the susceptibility to apoptosis, through the maintenance of mitochondrial integrity and inhibition of the activation of the caspase cascade.16 UVB can increase the population of apoptotic cells, decrease mitochondrial membrane potential (Δψ), and increase intracellular Ca2+.17 It has been shown JNK, ERK1/2, and p38 MAPK levels increase in keratinocytes after 30 minutes of UVB irradiation.18
“Snow lotus” is a well-known herbal medicine in People’s Republic of China, widely prescribed for the treatment of rheumatoid arthritis, stomachache, and dysmenorrhea.19 There are many types of snow lotuses available in People’s Republic of China, of which 12 species and one variety are often used as crude drugs. The chemical compositions of Saussurea involucrata Kar. et Kir., Saussurea Hand. Mazz., Saussurea medusa Maxim., and Saussurea tridactyla Sch. Bip. are being actively investigated.20 S. tridactyla Sch. Bip., which grows in the Qinghai-Tibetan plateau,21 is the only Asteraceae species with normal growth and reproduction at altitudes of 5,000 m or higher. Additionally, this plant is a source of several compounds that play an important role in the detoxification and promotion of blood circulation,22 which include polysaccharides, flavonoids, alkaloids, phenols, tannins, and essential oils.23 S. tridactyla Sch. Bip.-derived polysaccharides contain seven kinds of monosaccharide: xylose, arabinose, rhamnose, galactose, glucose, galactose uronic acid, and mannose, which show obvious scavenging effects on anion free radicals and hydroxyl-free radicals.24–26 Studies have also shown that the S. tridactyla Sch. Bip. water extract exhibits antioxidant effects, which may increase SOD activity and glutathione (GSH) content, and reduce malondialdehyde (MDA) content in mouse tissue.27–29
The present study is the first to investigate whether S. tridactyla Sch. Bip.-derived polysaccharides and flavones reduce cell apoptosis in human keratinocyte (HaCaT) cells via the p38MAPK pathway, with protective effects against UVB irradiation-induced damage.
Materials and methods
S. tridactyla Sch. Bip.-derived polysaccharides and flavones
According to a previously used protocol,30,31 95% ethanol was added to 50 g of S. tridactyla Sch. Bip. (solid to liquid ratio of 1:12). The Soxhlet extraction method with a filter and volatile organic solvent was used. The filter residue was added to distilled water (solid to liquid ratio of 1:12) in a water bath at 85°C, with heating for 2 hours and stirring every 15 minutes. After removing the supernatant from the precipitate with 95% ethanol in a 1:3 ratio, static set overnight to recycling supernatant ethanol, the precipitate was washed with anhydrous ethanol and dried to obtain S. tridactyla Sch. Bip.-derived polysaccharides. Low and high concentration (10 and 40 mg/mL, respectively) solutions of S. tridactyla Sch. Bip.-derived polysaccharides were prepared by dissolving the extract in 10% fetal bovine serum Dulbecco’s Modified Eagle’s Medium (DMEM) culture medium, and filtering using a 0.22 μm pore size filter.
According to a previously used protocol,32 70% ethanol was added to 100 g of S. tridactyla Sch. Bip. (liquid to solid ratio of 13:1 [mL/g]). The solution was twice sonicated at 320 W, 60°C with extraction for 50 minutes, and the extract was concentrated by freeze-drying to obtain S. tridactyla Sch. Bip.-derived rough flavones. Low and high concentration (2 and 6 mg/mL, respectively) solutions of S. tridactyla Sch. Bip.-derived flavones were prepared by dissolving the extract in 10% fetal bovine serum DMEM culture medium and filtering using a 0.22 μm pore size filter.
Cell culture
Cell culture was performed according to previous literature.9,33 HaCaT cells were obtained from the Nanjing KeyGen Biotech Development Co. Ltd. Cells were maintained at 37°C in an incubator (Shanghai Science Instrument Co. Ltd.) with a humidified atmosphere of 5% CO2. The cells were grown in DMEM containing 10% fetal calf serum (Biological Industries) with streptomycin (10 units/mL) and penicillin (10 units/mL). The study protocols were approved by the Affiliated Hospital of Qinghai University Institutional Ethical Committee, Xining, People’s Republic of China.
UVB irradiation
UVB irradiation was performed as previously described.3 Cells were washed with phosphate-buffered saline (PBS) and irradiated with UVB light (30 and 60 mJ/cm2). In parallel, nonirradiated cells were treated similarly and kept in the dark. For irradiation, TL20W/12 ultraviolet UVB light tube (Shanghai JIETU Trading Company, Shanghai, People’s Republic of China) was used. The UVB output was measured by an UVB irradiance monitor (UV-340A; Beijing Precise Instruments Co. Ltd., Beijing, People’s Republic of China).
Experimental groups and treatment
HaCaT cells were cultured in vitro and divided into a low radiation UVB (L-UVB) group (30 mJ/cm2 radiation for 1 hour) and a high radiation UVB (H-UVB) group (60 mJ/cm2 radiation for 1 hour).
S. tridactyla Sch. Bip.-derived polysaccharides L-UVB and H-UVB groups were subdivided into a control group, UVB radiation group (UVB), S. tridactyla Sch. Bip.-derived polysaccharides low-dose group (10 mg/mL), and S. tridactyla Sch. Bip.-derived polysaccharides high-dose group (40 mg/mL).
S. tridactyla Sch. Bip.-derived flavones L-UVB and H-UVB groups were subdivided into a control group, UVB group, S. tridactyla Sch. Bip.-derived flavones low-dose group (2 mg/mL), and S. tridactyla Sch-Bip.-derived flavones high-dose group (6 mg/mL).
Different concentrations of S. tridactyla Sch-Bip.-derived polysaccharides and flavones were added to the cells for 1 hour before exposure to UVB light at doses of 30 and 60 mJ/cm2. After 1 hour of irradiation, cells were incubated at 37°C for 18 hours in 10% fetal bovine serum DMEM culture medium.
First, we investigated whether treatment with S. tridactyla Sch. Bip.-derived polysaccharides and flavones reduced UVB-induced oxidative damage in HaCaT cells. Next, we investigated the protective effect of S. tridactyla Sch-Bip.-derived polysaccharides and flavones on HaCaT cells exposed to UVB irradiation and studied the mechanisms involved.
Cell viability assay
The effect of treatment with S. tridactyla Sch. Bip.-derived polysaccharides and flavones on the viability of HaCaT cells was assessed as previously described.34 Cells were seeded in a 96-well plate at a density of 1×106 cells/well. MTT stock solution (Promega, Madison, WI, USA) was added to each well to yield a total reaction volume of 20 μL. After the cells were incubated for 3 hours, the absorbance at 490 nm was read on a full wavelength microplate reader (X-Mark; Bio-Rad Laboratories Inc., Hercules, CA, USA).
Trypan blue staining
Cells were seeded in a 96-well plate at a density of 1×106 cells/well; each well was washed three times with 0.01 mol/L PBS, and 0.4% trypan blue was added to each well in each group to a total volume of 50 μL for 3 minutes. The cells were then visualized with fluorescence microscopy (IX71; Olympus Corporation, Tokyo, Japan), and the photomicrographs were saved and merged with Image Pro Plus 6.0 software (Olympus America Inc., Melville, NY, USA).
Determination of SOD, GSH, CAT, and MDA levels
After 18 hours of incubation in DMEM medium containing 10% fetal bovine serum, the SOD activity, GSH content, MDA content, and CAT activity of HaCaT cells were evaluated using SOD kit, GSH kit, MDA kit, and CAT kit (Jiancheng Biotechnology, Nanjing, People’s Republic of China), respectively, according to the manufacturer’s instructions.
Apoptosis assays and determination of intracellular Ca2+ levels and mitochondrial membrane potential (Δψ) by flow cytometry
The apoptosis assays were performed and intracellular Ca2+ levels and mitochondrial membrane potential (Δψ) of HaCaT cells were determined by flow cytometry, as described previously.17,35,36 First, apoptosis was detected by Dead Cell Apoptosis Kit with Annexin V Alexa Fluor® 488 & PI for Flow Cytometry (replacement for PHN1008, PHN1010; Thermo Fisher Scientific, Waltham, MA, USA), cells in 6-well plates were digested and washed with PBS, and 106 cells/pipe were centrifuged and the supernatant discarded. A 100 μL volume of 1× annexin-binding buffer was added; cells were pulsed with 5 μL annexin V and 1 μL PI working fluid and incubated for 15 minutes. Finally, 400 μl of 1×annexin-binding×Buffer was added and detected using flow cytomety. Next, HaCaT cells were digested in a 6-well plate and washed with Hanks Balanced Salt Solution (HBSS), 107 cells/pipe were added to 4 μL fluo-am4 (Thermo Fisher Scientific, Waltham, MA, USA), incubated at 37°C for 30 minutes, and washed twice with HBSS. Cells were suspended and detected by flow cytometry. Finally, cells were digested and washed with HBSS 6-well plate, 107 cells/pipe were added to 0.5 mL of culture medium and 0.5 mL of JC-1 (Life Technologies) working fluid, incubated for 20 minutes, and centrifuged at 600× g at 4°C for 3 minutes by low temperature centrifuge (Sigma, St Louis, MO, USA). The supernatant was discarded and cells were washed twice with HBSS. Cells were then suspended and detected by flow cytometry.
Western blotting
Western blotting was performed as described previously.37 Each group of HaCaT cells was extracted in radio immunoprecipitation assay (RIPA) lysis buffer (Beijing apply Gene Technology Co., Ltd., Beijing, People’s Republic of China), and centrifuged at 12,000× g at 4°C for 10 minutes by using a low temperature centrifuge (Sigma). The protein concentration of the supernatant was determined using a full wavelength microplate reader (X-Mark, Bio-Rad Laboratories Inc.).
The lysates (40 μg) were separated by 12% sodium dodecyl sulfate-polyacrylamide gel electrophoresis, and proteins were transferred onto a polyvinylidene fluoride membrane. The membrane was blocked with 3% bovine serum albumin, incubated with the primary antibody overnight at 4°C, and then incubated with horseradish peroxidase-conjugated secondary antibodies for 2 hours at room temperature. The primary antibodies and horseradish peroxidase-conjugated secondary antibodies are shown in Table 1. The bands were detected using a Chemiluminescent Kit (Beijing apply Gene Technology Co., Ltd.), followed by exposure to X-ray film and densitometry detection using Image J 5.0 software (National Institutes of Health, Bethesda, MD, USA). β-actin was used as a loading control.
  | Table 1 Antibodies and conditions used in Western blotting |
Immunofluorescence staining
Each group of HaCaT cells was washed with 0.01 mol/L PBS followed by fixing by 4% paraformaldehyde (pH 7.4) in a 24-well plate. Cells were incubated with 0.3% Triton X-100 for 30 minutes at 37°C, then washed with 0.01 mol/L PBS three times for 5 minutes, and treated with 3% bovine serum albumin at 37°C for 1 hour at room temperature. Cells were incubated with the following primary antibodies: rabbit polyclonal antibody p-38 (1:80, Abcam, Cambridge, UK), rabbit polyclonal antibody p-53 (1:50, Abcam), rabbit polyclonal antibody Bcl-2 (1:100, Abcam), rabbit polyclonal antibody Bax (1:50, Abcam), and rabbit polyclonal antibody active-caspase-3 (1:100, Abcam) overnight at 4°C. The cells were then incubated with corresponding fluorescence dye-conjugated secondary antibodies (Zhong Shan Jinqiao Biological Technology Co., Ltd., Beijing, People’s Republic of China) for 1 hour at room temperature, protected from light. To provide a negative control, control serum was used instead of the primary antibody. The cells were visualized by fluorescence microscopy (BX5; Olympus Corporation); the photomicrographs were saved and merged using Image Pro Plus 6.0 software (Olympus America Inc.).
Statistical analysis
All data were expressed as mean ± standard deviation and analyzed by one-way analysis of variance with Tukey–Kramer post hoc tests. A P-value of less than 0.05 was considered statistically significant.
Results
Antioxidative protective effects of S. tridactyla Sch. Bip. polysaccharides and S. tridactyla Sch. Bip. flavones on HaCaT cells after UVB
Cell viability assay
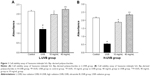
The effects of S. tridactyla Sch. Bip.-derived polysaccharides and flavones on cell viability, as indicated by the results of the cell viability assays, are shown in Figures 1 and 2. In the L-UVB and H-UVB groups, the absorbance was significantly lower in the UVB groups than in the control group (P<0.05), and the absorbance was significantly higher in the polysaccharides 10 mg/mL, polysaccharides 40 mg/mL, flavones 2 mg/mL, and flavones 6 mg/mL groups than in the UVB group (P<0.05). In L-UVB and H-UVB groups, the absorbance significantly increased in polysaccharides 40 mg/mL compared with polysaccharides 10 mg/mL group (P<0.05) and the absorbance significantly increased in the flavones 6 mg/mL compared with the flavones 2 mg/mL group (P<0.05).
Trypan blue staining
The results of trypan blue staining for S. tridactyla Sch. Bip.-derived polysaccharides and flavones are shown in Figures 3 and 4. In the L-UVB group, the number of blue cells was increased as compared to the control group. The cells showed mild swelling and floating, indicating that some of these cells had died in the UVB group; however, cell morphology could still be discerned. In the polysaccharides 10 mg/mL, polysaccharides 40 mg/mL, flavones 2 mg/mL, and flavones 6 mg/mL groups, the number of blue cells was low compared with the UVB group, and the degree of cell edema was alleviated. In the polysaccharides 40 mg/mL group, cell morphology resembled that of the control group, and the number of blue cells was lower than in the polysaccharides 10 mg/mL group. In the flavones 6 mg/mL group, cell morphology resembled the control group, and the number of blue cells was lower than in the flavones 2 mg/mL group. In the H-UVB group compared with control group, cells showed obvious swelling, cell death fragments and chip phenomenon were clearly observed, and cell morphology was blurred. In the polysaccharides 10 mg/mL, polysaccharides 40 mg/mL, flavones 2 mg/mL, and flavones 6 mg/mL groups, the morphology of cells was restored, the number of death cells had reduced, and cell death fragments and chip phenomenon was obviously improved. In the polysaccharides 40 mg/mL group, cell morphology resembled that in the control group, and the number of blue cells was significantly lower than in the polysaccharides 10 mg/mL group; and in the flavones 6 mg/mL group, cell morphology resembled the control group, and the number of blue cells was significantly lower than in the flavones 2 mg/mL group.
  | Figure 3 Trypan blue staining of Saussurea tridactyla Sch. Bip.-derived polysaccharides. |
  | Figure 4 Trypan blue staining of Saussurea tridactyla Sch. Bip.-derived flavones. |
Determination of SOD, GSH, CAT, and MDA levels
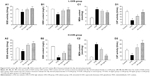
The effects of S. tridactyla Sch. Bip.-derived polysaccharides and flavones on SOD activity, GSH content, CAT activity, and MDA content are shown in Figures 5 and 6. In L-UVB and H-UVB groups, activity of SOD, content of GSH, and activity of CAT were significantly decreased, and content of MDA was significantly increased in the UVB compared with the control group (P<0.05); activity of SOD, content of GSH, activity of CAT were significantly increased, and content of MDA was significantly decreased in the polysaccharides 10 mg/mL, polysaccharides 40 mg/mL, flavones 2 mg/mL, and flavones 6 mg/mL groups compared with the UVB group (P<0.05), and SOD activity, GSH content, and activity of CAT were significantly increased, and content of MDA was significantly decreased in the polysaccharides 40 mg/mL compared with polysaccharides 10 mg/mL group (P<0.05). In the L-UVB group, activity of SOD, content of MDA, and activity of CAT were significantly decreased, while the content of GSH was significantly increased in the flavones 6 mg/mL compared with flavones 2 mg/mL group (P<0.05). In the H-UVB group, activity of SOD, content of GSH, content of MDA, and activity of CAT were significantly decreased in flavones 6 mg/mL compared with flavones 2 mg/mL group (P<0.05).
Protective mechanisms of S. tridactyla Sch.-Bip.-derived polysaccharides and flavones in UVB-irradiated HaCaT cells
Apoptosis assays and determination of intracellular Ca2+ levels and mitochondrial membrane potential (Δψ) by flow cytometry
S. tridactyla Sch. Bip.-derived flavones: through these three experiments, we found that in the H-UVB group, HaCaT cells were obviously swollen, cell death fragments and chip phenomenon were obvious, and cell morphology was blurred in the UVB group as indicated by trypan blue staining; therefore, we chose the L-UVB group for the following experiments. In the L-UVB group, the absorbance significantly increased in the 6 mg/mL compared with 2 mg/mL group, as detected by MTT (P<0.05). Also, the activity of SOD, content of MDA, and activity of CAT were significantly decreased, while the content of GSH was significantly increased in the 6 mg/mL compared with 2 mg/mL group (P<0.05); therefore we chose the S. tridactyla Sch. Bip.-derived flavones 6 mg/mL group for the following experiments.
The levels of apoptosis, intracellular Ca2+ levels, and mitochondrial membrane potential (Δψ) for S. tridactyla Sch. Bip.-derived polysaccharides and flavones, as measured by flow cytometry, are shown in Figures 7 and 8.
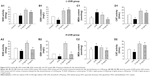
The annexin V/propidium iodide staining (Figures 7A and 8A) revealed that UVB irradiation of HaCaT cells leads to an increase in the population of apoptotic cells as compared to the control group, while in the L-UVB and H-UVB groups, the population of apoptotic cells decreased in the polysaccharides 10 mg/mL and polysaccharides 40 mg/mL groups compared with UVB group. And in the S. tridactyla Sch. Bip.-derived flavones group, the population of apoptotic cells decreased in the 6 mg/mL group compared with the UVB group.
The intracellular Ca2+ measurements (Figures 7B and 8B) revealed that intracellular Ca2+ fluorescence increased, indicating that the content of intracellular Ca2+ had increased in the UVB group compared with the control group. The level of intracellular Ca2+ fluorescence decreased, indicating that the content of intracellular Ca2+ had decreased in the polysaccharides 10 mg/mL and polysaccharides 40 mg/mL groups compared with the UVB group in the L-UVB and H-UVB groups. And in the S. tridactyla Sch. Bip.-derived flavones group, intracellular Ca2+ fluorescence decreased indicating that the content of intracellular Ca2+ was decreased in the 6 mg/mL compared with the UVB group.
The mitochondrial membrane potential (Δψ) (Figures 7C and 8C) revealed that green fluorescence was increased indicating that the mitochondrial membrane potential (Δψ) was decreased in the UVB compared with control group; while in the groups of L-UVB and H-UVB, green fluorescence was decreased, and the mitochondrial membrane potential (Δψ) was increased in the polysaccharides 10 mg/mL and polysaccharides 40 mg/mL groups compared with the UVB group. And in the S. tridactyla Sch. Bip.-derived flavones group, green fluorescence was decreased, and the mitochondrial membrane potential (Δψ) was increased in the 6 mg/mL compared with UVB group.
Western blotting
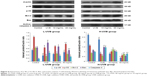
The results of Western blotting for p-38, p-53, Bcl-2, Bax, and active-caspase-3 in each group, after treatment with S. tridactyla Sch. Bip.-derived polysaccharides and flavones, are shown in Figures 9 and 10. In the L-UVB group, the expressions of p-38, p-53, and Bcl-2 were significantly decreased, and the expressions of Bax and active-caspase-3 were significantly increased in the UVB compared with control group (P<0.05); the expression of Bcl-2 was significantly increased, and the expressions of Bax and active-caspase-3 were significantly decreased in the 10 mg/mL compared with UVB group (P<0.05). However, the expressions of p-38 and p-53 were decreased in the 10 mg/mL compared with UVB group (P>0.05); the expressions of p-38, p-53, and Bcl-2 were significantly increased, and the expressions of Bax and active-caspase-3 were significantly decreased in the 40 mg/mL compared with UVB group; the expressions of p-53 was significantly increased, and the expressions of Bax were significantly decreased in the 40 mg/mL compared with 10 mg/mL group (P<0.05). In the H-UVB group, the expressions of p-38, p-53, and Bcl-2 were significantly decreased, and the expressions of Bax and active-caspase-3 were significantly increased in the UVB compared with control group (P<0.05); the expressions of p-53 and Bcl-2 were significantly increased, and the expressions of p-38, Bax, and active-caspase-3 were significantly decreased in the 10 and 40 mg/mL groups compared with UVB group (P<0.05); the expression of Bcl-2 was significantly increased, and the expressions of p-38, p-53, Bax, and active-caspase-3 were significantly decreased in the 40 mg/mL compared with 10 mg/mL group (P<0.05). And in the S. tridactyla Sch. Bip.-derived flavones group, the expressions of p-38, p-53, and Bcl-2 were significantly decreased, and the expressions of Bax and active-caspase-3 were significantly increased in the UVB compared with control group (P<0.05); the expressions of p-38, p-53, and Bcl-2 were significantly increased, and the expressions of Bax and active-caspase-3 were significantly decreased in the 6 mg/mL compared with UVB group significantly.
Immunofluorescence staining
The results of immunofluorescence staining for p-38, p-53, Bcl-2, Bax, and active-caspase-3 in each group after treatment with S. tridactyla Sch. Bip.-derived polysaccharides and flavones are shown in Figures 11 and 12. In the L-UVB and H-UVB groups, the expressions of p-38, p-53, and Bcl-2 were lower, and the expression levels of Bax and active-caspase-3 were higher in the UVB than in the control group; the expressions of p-38, p-53, and Bcl-2 were higher, and the expressions of Bax and active-caspase-3 were lower in the 10 mg/mL and 40 mg/mL groups than in the UVB group.
  | Figure 12 The expressions of p-38, p-53, Bcl-2, Bax, and active-caspase-3 indicated by immunofluorescence staining of Saussurea tridactyla Sch. Bip.-derived flavones. |
And in the S. tridactyla Sch. Bip.-derived flavones group, the expressions of p-38, p-53, and Bcl-2 were lower, and the expressions of Bax and active-caspase-3 were higher in the UVB than in the control group; the expressions of p-38, p-53, and Bcl-2 were increased, and the expressions of Bax and active-caspase-3 were decreased in the 6 mg/mL compared with the UVB group.
Discussion
Several previous studies have demonstrated suppression of UV irradiation- and/or peroxide-induced cell injury and melanogenesis in human skin cells.38,39 UVB irradiation injures cellular components of skin tissues and cells via the generation of ROS, which stimulates the oxidative stress response of the skin, resulting in the depletion of cellular antioxidants, increased damage to cellular components (eg, lipid membrane, protein, and DNA), photoaging, inflammation, immunosuppression, and apoptosis.40,41 An enzymatic antioxidant defense system composed of CAT and SOD13 and GSH protein repairs the skin by reducing photoaging due to the most common free radical, MDA, which is a frequently used marker of lipid peroxidation. Therefore, we assessed the degree of injury in HaCaT cells after UVB irradiation by using SOD activity, GSH content, CAT activity, and MDA content as indicators of such damage. In this study, we observed that S. tridactyla Sch. Bip.-derived polysaccharides and flavones increased cell viability by MTT, decreased the number of apoptotic cells, alleviated the degree of cell edema, restored the morphology of cells (as indicated by trypan blue staining), and increased SOD activity, GSH content, CAT activity, and decreased MDA content. These results suggest that S. tridactyla Sch. Bip.-derived polysaccharides and flavones exhibit protective effects against oxidative damage in UVB-irradiated HaCaT cells.
Other studies have demonstrated that accumulation of ROS in UVB-treated keratinocytes may promote apoptosis, resulting in mitochondrial permeability transition.12,15 In our study, we confirmed that S. tridactyla Sch. Bip.-derived polysaccharides and flavones can alleviate apoptosis in HaCaT cells through a mitochondrial pathway that decreases intracellular Ca2+ fluorescence and increases the mitochondrial membrane potential (Δψ).
It has been shown that the JNK, ERK1/2, and p38 MAPK pathways are activated in response to UVB irradiation in keratinocytes.18 The present study focused on the p38MAPK pathway, which inactivates p-53, triggering the apoptotic cascade by the formation of the pro-apoptotic caspase-3 complex. In our study, we confirmed using Western blotting and immunofluorescence staining that polysaccharides and flavones from S. tridactyla Sch. Bip. reduce apoptosis in HaCaT cells by increasing the expression of Bcl-2, and decreasing the expressions of Bax and active-caspase-3. However, the increase in the expressions of p-38 and p-53, as observed by Western blotting and immunofluorescence staining, suggests that this antiapoptotic effect is not achieved through the p38MAPK pathway. To our knowledge, this is the first study demonstrating that while S. tridactyla Sch. Bip.-derived polysaccharides and flavones can reduce cell apoptosis and exert protective effects against UVB irradiation-induced cell damage, these effects do not involve the p38MAPK pathway. These data provide new directions for the application of S. tridactyla Sch. Bip.-derived polysaccharides and flavones in drug development and for future investigation of the mechanisms by which these compounds exert their protective effects.
Acknowledgment
The study is supported by Basic Research Project of Qinghai Province (application) (grant 2013-Z-730).
Disclosure
The authors report no conflicts of interest in this work.
References
Chan CF, Huang WY, Guo HY, Wang BR. Potent antioxidative and UVB protective effect of water extract of Eclipta prostrata L. ScientificWorldJournal. 2014;2014:759039. | ||
Svobodova A, Zdarilova A, Vostalova J. Lonicera caerulea and Vaccinium myrtillus fruit polyphenols protect HaCaT keratinocytes against UVB-induced phototoxic stress and DNA damage. J Dermatol Sci. 2009;56(3):196–204. | ||
Vostalova J, Zdarilova A, Svobodova A. Prunella vulgaris extract and rosmarinic acid prevent UVB-induced DNA damage and oxidative stress in HaCaT keratinocytes. Arch Dermatol Res. 2010;302(3):171–181. | ||
Saitoh Y, Miyanishi A, Mizuno H, et al. Super-highly hydroxylated fullerene derivative protects human keratinocytes from UV-induced cell injuries together with the decreases in intracellular ROS generation and DNA damages. J Photochem Photobiol B. 2011;102(1):69–76. | ||
Hyun YJ, Piao MJ, Ko MH, et al. Photoprotective effect of Undaria crenata against ultraviolet B-induced damage to keratinocytes. J Biosci Bioeng. 2013;116(2):256–264. | ||
Piao MJ, Lee NH, Chae S, Hyun JW. Eckol inhibits ultraviolet B-induced cell damage in human keratinocytes via a decrease in oxidative stress. Biol Pharm Bull. 2012;35(6):873–880. | ||
Filip A, Daicoviciu D, Clichici S, Mocan T, Muresan A, Postescu ID. Photoprotective effects of two natural products on ultraviolet B-induced oxidative stress and apoptosis in SKH-1 mouse skin. J Med Food. 2011;14(7–8):761–766. | ||
Park HM, Hwang E, Lee KG, Han SM, Cho Y, Kim SY. Royal jelly protects against ultraviolet B-induced photoaging in human skin fibroblasts via enhancing collagen production. J Med Food. 2011;14(9):899–906. | ||
Piao MJ, Kang KA, Kim KC, et al. Diphlorethohydroxycarmalol attenuated cell damage against UVB radiation via enhancing antioxidant effects and absorbing UVB ray in human HaCaT keratinocytes. Environ Toxicol Pharmacol. 2013;36(2):680–688. | ||
Kim KC, Kim D, Kim SC, Jung E, Park D, Hyun JW. Empetrum nigrum var. japonicum extract suppresses ultraviolet B-induced cell damage via absorption of radiation and inhibition of oxidative stress. Evid Based Complement Alternat Med. 2013;2013:983609. | ||
Shin SW, Jung E, Kim S, Lee KE, Youm JK, Park D. Antagonist effects of veratric acid against UVB-induced cell damages. Molecules. 2013;18(5):5405–5419. | ||
Piao MJ, Hyun YJ, Cho SJ, et al. An ethanol extract derived from Bonnemaisonia hamifera scavenges ultraviolet B (UVB) radiation-induced reactive oxygen species and attenuates UVB-induced cell damage in human keratinocytes. Mar Drugs. 2012;10(12):2826–2845. | ||
Piao MJ, Yoon WJ, Kang HK, et al. Protective effect of the ethyl acetate fraction of Sargassum muticum against ultraviolet B-irradiated damage in human keratinocytes. Int J Mol Sci. 2011;12(11):8146–8160. | ||
Fischer TW, Zmijewski MA, Wortsman J, Slominski A. Melatonin maintains mitochondrial membrane potential and attenuates activation of initiator (casp-9) and effector caspases (casp-3/casp-7) and PARP in UVR-exposed HaCaT keratinocytes. J Pineal Res. 2008;44(4):397–407. | ||
Lee SJ, Park JW. Enhancement of UVB radiation-mediated apoptosis by knockdown of cytosolic NADP+-dependent isocitrate dehydrogenase in HaCaT cells. BMB Rep. 2014;47(4):209–214. | ||
Afaq F, Syed DN, Malik A, et al. Delphinidin, an anthocyanidin in pigmented fruits and vegetables, protects human HaCaT keratinocytes and mouse skin against UVB-mediated oxidative stress and apoptosis. J Invest Dermatol. 2007;127(1):222–232. | ||
Farrukh MR, Nissar UA, Afnan Q, et al. Oxidative stress mediated Ca(2+) release manifests endoplasmic reticulum stress leading to unfolded protein response in UV-B irradiated human skin cells. J Dermatol Sci. 2014;75(1):24–35. | ||
Huang CC, Wu WB, Fang JY, et al. (-)-Epicatechin-3-gallate, a green tea polyphenol is a potent agent against UVB-induced damage in HaCaT keratinocytes. Molecules. 2007;12(8):1845–1858. | ||
Yi T, Lo H, Zhao Z, Yu Z, Yang Z, Chen H. Comparison of the chemical composition and pharmacological effects of the aqueous and ethanolic extracts from a Tibetan “Snow Lotus” (Saussurea laniceps) herb. Molecules. 2012;17(6):7183–7194. | ||
Wang Y, Zhang BY, Tao YY, Shao Y, Mei LJ, Wang QL. Advances in research of chemical constituents and pharmacological effects of Saussurea involucrata. Chin J Spectrosc Lab. 2013;30(2):530–535. | ||
Wen J, Zhang JQ, Nie ZL, Zhong Y, Sun H. Evolutionary diversifications of plants on the Qinghai-Tibetan Plateau. Front Genet. 2014;5:4. | ||
Zeng L, Chong L, Tersing T, Bu N, Zhao J, Zhong Y. Microsatellite markers for Saussurea gnaphalodes (Asteraceae), a native Himalayan mountain species. Am J Bot. 2012;99(8):e326– e329. | ||
Yuan XF, Zhao B, Wang YC. Advances in studies on Saussurea involucrata. Chin Tradit Herb Drugs. 2004;35(12):1424–1426. | ||
Xiao W, Li N, Bolati M, et al. Advances in studies on chemical components and pharmacological activities of Saussureae Involucratae Herba. Drugs & Clinic. 2011;26(5):344–348. | ||
Liu CL, Deng YH, Zhong TT, Du N, Liu Y, Yang L. Study on preliminary purification and scavenging free radical activity of polysaccharide of Tibetan Saussurea. J Beijing Univ Agric. 2007;22(1):4–7. | ||
Zhang X, Yu M, Chen J. Simultaneous determination of seven compounds in snow lotus herb using high-performance liquid chromatography. J Chromatogr Sci. 2003;41(5):241–244. | ||
Ning P, Zhang S, Yang TD, et al. Anti-oxidizing efficacy of Tibetan Saussurea water extract, on mice. J Qinghai Univ (Nat Sci). 2009;27(2):63–65. | ||
Ning P, Yang XM. Effects of Tibetan Saussurea water extract on the activity of GSH-Px in mouse tissues. J Qinghai Univ (Nat Sci). 2008;26:58–60. | ||
Li ZH. Effects of Tibetan Saussurea water extract on the MDA content in mice tissues. J Anhui Agric Sci. 2008;36:15510–15534. | ||
Liu CL, Deng YH, Du N, et al. Isolation purification and composition analysis of water-soluble polysaccharide from Xinjiang Sausurea involuctate. Nat Prod Res Dev. 2009;21:99–103. | ||
Liu CL, Deng YH, Yang L, Du N. Study on extraction process of water soluble polysaccharide from Saussurea involucrata. J Food Sci Biotechnol. 2007;26:37–40. | ||
Ma FL, Zhang Y, Wang L, et al. Ultrasonic-assisted extraction and antioxidation of total flavonoids from Saussurea involucrate. Central South Pharmacy. 2014;12:1088–1092. | ||
Zheng J, Piao MJ, Kim KC, et al. Photo-protective effect of americanin B against ultraviolet B-induced damage in cultured human keratinocytes. Environ Toxicol Pharmacol. 2014;38(3):891–900. | ||
Hyun YJ, Piao MJ, Zhang R, Choi YH, Chae S, Hyun JW. Photo-protection by 3-bromo-4,5-dihydroxybenzaldehyde against ultraviolet B-induced oxidative stress in human keratinocytes. Ecotoxicol Environ Saf. 2012;83:71–78. | ||
Arora S, Bhardwaj A, Srivastava SK, et al. Honokiol arrests cell cycle, induces apoptosis, and potentiates the cytotoxic effect of gemcitabine in human pancreatic cancer cells. PloS One. 2011;6(6):e21573. | ||
Piao MJ, Zhang R, Lee NH, Hyun JW. Protective effect of triphlorethol-A against ultraviolet B-mediated damage of human keratinocytes. J Photochem Photobiol B. 2012;106:74–80. | ||
Park G, Kim HG, Sim Y, Sung SH, Oh MS. Sauchinone, a lignan from Saururus chinensis, protects human skin keratinocytes against ultraviolet B-induced photoaging by regulating the oxidative defense system. Biol Pharm Bull. 2013;36(7):1134–1139. | ||
Kato S, Aoshima H, Saitoh Y, Miwa N. Defensive effects of fullerene-C60 dissolved in squalane against the 2,4-nonadienal-induced cell injury in human skin keratinocytes HaCaT and wrinkle formation in 3D-human skin tissue model. J Biomed Nanotechnol. 2010;6(1):52–58. | ||
Kato S, Kikuchi R, Aoshima H, Saitoh Y, Miwa N. Defensive effects of fullerene-C60/liposome complex against UVA-induced intracellular reactive oxygen species generation and cell death in human skin keratinocytes HaCaT, associated with intracellular uptake and extracellular excretion of fullerene-C60. J Photochem Photobiol B. 2010;98(2):144–151. | ||
Santos CX, Nabeebaccus AA, Shah AM, Camargo LL, Filho SV, Lopes LR. Endoplasmic reticulum stress and Nox-mediated reactive oxygen species signaling in the peripheral vasculature: potential role in hypertension. Antioxid Redox Signal. 2014;20(1):121–134. | ||
Narayanapillai S, Agarwal C, Tilley C, Agarwal R. Silibinin is a potent sensitizer of UVA radiation-induced oxidative stress and apoptosis in human keratinocyte HaCaT cells. Photochem Photobiol. 2012;88(5):1135–1140. |
 © 2016 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2016 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.