Back to Journals » Clinical Interventions in Aging » Volume 15
Safety and Efficacy of Tirofiban During Mechanical Thrombectomy for Stroke Patients with Preceding Intravenous Thrombolysis
Authors Huo X, Yang M, Ma N, Gao F, Mo D, Li X, Wang A, Wang Y, Miao Z
Received 15 November 2019
Accepted for publication 29 May 2020
Published 23 July 2020 Volume 2020:15 Pages 1241—1248
DOI https://doi.org/10.2147/CIA.S238769
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 3
Editor who approved publication: Dr Zhi-Ying Wu
Xiaochuan Huo,1,2,* Ming Yang,1,2,* Ning Ma,1,2 Feng Gao,1,2 Dapeng Mo,1,2 Xiaoqing Li,1,2 Anxin Wang,2,3 Yongjun Wang,2,3 Zhongrong Miao1,2
1Department of Interventional Neuroradiology, Beijing Tiantan Hospital, Capital Medical University, Beijing, People’s Republic of China; 2China National Clinical Research Center for Neurological Diseases, Beijing, People’s Republic of China; 3Department of Neurology, Beijing Tiantan Hospital, Capital Medical University, Beijing, People’s Republic of China
*These authors contributed equally to this work
Correspondence: Zhongrong Miao
Beijing Tiantan Hospital, Capital Medical University, No. 119, the South Fourth Ring West Road, Beijing, Fengtai District 100070, People’s Republic of China
Email [email protected]
Purpose: Whether tirofiban is safe and effective for acute ischemic stroke (AIS) patients undergoing mechanical thrombectomy (MT) with preceding intravenous thrombolysis (IVT) remains unclear. We aim to evaluate the safety and efficacy of tirofiban during MT for patients with preceding IVT.
Patients and Methods: Patients who underwent MT and preceding IVT were derived from the ANGEL registry and were dichotomized into tirofiban and non-tirofiban group according to whether rescue tirofiban was performed. The safety endpoints were sICH, total ICH and distal embolization. The efficacy endpoints were arterial recanalization, three-month functional independence (modified Rankin Scale [mRS]: 0– 2) and mortality.
Results: We included 207 MT patients with preceding IVT from the entire registry. Among them, there were 55 in tirofiban group and 152 in non-tirofiban group, and 17 (8.2%) patients suffered sICH and 36 (17.4%) suffered ICH within 24 hours post-MT; 11 (5.3%) distal embolization of thrombus; 111 (53.6%) achieved functional independence and 34 (16.4%) died after three-month follow-up. No significant differences in safety outcomes on sICH, ICH and distal embolization of thrombus and efficacy outcomes on recanalization and long-term functional independence were found between tirofiban and non-tirofiban group for the entire cohort (p> 0.05 for all groups). Tirofiban was correlated with long-term mortality reduction for patients underwent MT and preceding IVT (adjusted hazard ratio 0.28 [0.08– 0.94], adjusted p=0.03).
Conclusion: In AIS patients who underwent MT and preceding IVT, rescue tirofiban was not correlated with increased risk of safety endpoints on sICH, ICH or distal embolization of thrombus, and might be associated with a lower risk of long-term mortality. Further study is needed to confirm the effect of early antiplatelet therapy with tirofiban for patients underwent MT and preceding IVT.
Keywords: acute ischemic stroke, mechanical thrombectomy, intravenous thrombolysis, tirofiban, bridging therapy
Introduction
Mechanical thrombectomy (MT) has substantially improved functional outcomes and reduce mortality in patients with large artery occlusive stroke, compared with medical therapy.1–3 However, early reocclusion after successful MT recanalization occurs in approximately 20% of patients.4 Most of which complicated with underlying severe atherosclerotic stenosis or endothelial damage.5,6 Researchers have concentrated on studying the efficacy and safety of rescue therapies for patients refractory to recanalization during MT, of which the non-peptide GP IIb/IIIa receptor inhibitor is an important alternative option. The non-peptide GP IIb/IIIa receptor inhibitor tirofiban, which reversibly and efficiently blocks the final pathway of activated platelet aggregation and subsequent thrombus formation, has attracted the most attention.
Tirofiban has been used as rescue therapy during MT in a series of preliminary clinical trials, aiming to prevent early arterial reocclusion and thromboembolic complications. Most of them showed acceptable safety and efficacy profiles of tirofiban,7,8 although controversies exist.9 One of the important concerns is if the benefits of tirofiban on clinical outcomes will prevail upon the bleeding risks for patients who have undergone intravenous thrombolysis (IVT) before MT, also known as bridging therapy with IVT. Besides, the use of antiplatelet agents is not recommended within 24 hours following IVT in the American Heart Association/American Stroke Association (AHA/ASA) guidelines because of the concern of increased hemorrhagic complications.10 However, the effects of intensive antiplatelet therapy with GP IIb/IIIa receptor inhibitor during MT for patients with bridging IVT have not been studied up to now.
Thus, we aim to evaluate the safety of rescue tirofiban during MT on sICH, ICH, distal embolization of thrombus, as well as its efficacy in improving artery recanalization and long-term functional outcomes for patients with preceding IVT in a multi-center, prospective study.
Patients and Methods
Patient Enrollment
All patients included in this study were retrieved from the ANGEL (Acute Ischemic Stroke Cooperation Group of Endovascular Treatment) registry, which is a multi-centric, nationwide, prospective registry study launched in June 2015 and terminated in December 2017. Details of the study design have been reported.11 Consecutive AIS patients secondary to large-artery occlusion treated with endovascular methods were selected according to the inclusion and exclusion criteria from the ANGEL registry study.11
All candidates underwent emergency cranial non-contrast CT before treatment. In cases with unknown or prolonged time window (>6 hours), DWI/PWI or CTV/CTP mismatch was introduced for the interventional recanalization. The protocol and data collection of the ANGEL Registry was approved by the ethics committee of Beijing Tiantan Hospital and all other participating centers. This study was conducted in accordance with the Declaration of Helsinki. Written informed consent was provided by all patients or their representatives.
Baseline Data Collection
Baseline information was collected and then sent to the central core laboratory in digital case report forms by trained coordinators. All imaging data, including pretreatment non-contrast head computerized tomography (CT) and CT angiography (CTA), MRI and MRA, DSA images during the endovascular therapy, and follow-up CT or MR imaging, were anonymized and reviewed centrally by two readers (X.C.H and M.Y).
Endovascular Interventions
All eligible patients underwent MT immediately after imaging and clinical assessment of indications according to current guidelines. Subjects who underwent MT employed stent retriever (Solitaire AB/FR, Covidien/ev3, Irvine, CA; Trevo Proview, Stryker, CA), aspiration device (Penumbra, Alameda, CA) as the first recanalization option according to protocol. For cases failed to first MT attempt, additional thrombectomy attempts and alternative rescue therapies were adopted at the discretion of the operator, including intra-arterial/intravenous tirofiban administration, intra-arterial thrombolysis (urokinase: increments of 100000 IU to a maximum dose of 1000000 IU, or rtPA: 5–30mg), balloon angioplasty and emergent stenting.
Tirofiban Administration During Mechanical Thrombectomy
All patients underwent endovascular treatment immediately after the indication assessment. Rescue tirofiban therapy was prescribed according to intraoperative vascular recanalization status and procedures. In general, tirofiban (Grand Pharmaceutical Co. Ltd., China; 5mg of tirofiban diluted with 100 mL normal saline) was considered when confronting following revascularization refractory conditions: (1) emergency stenting for severe residual stenosis or instant reocclusion (2) balloon angioplasty for severe residual stenosis or instant reocclusion (3) successful recanalization with ≥ 3 passes with stent retriever for presumed endothelial damage or instant reocclusion; (4) severe in situ atherosclerosis with high risk of early re-occlusion. Unless an ICH was suspected, a low-dose intra-arterial bolus (0.25mg-1mg) followed by continuous intravenous infusion (0.1μg/kg/min) was administrated for 12 to 24 hours as a standard procedure. After that, intravenous tirofiban was bridged with dual antiplatelet (100mg aspirin and 75mg clopidogrel once daily) and overlapped for 4 hours before tirofiban cessation if ICH was excluded by 24 hours post-MT CT. Based on stroke pathogenesis and head CT findings 24 hours post-MT, oral antiplatelet treatment (aspirin 100mg or clopidogrel 75mg once daily) or dual antiplatelet therapy were prescribed. All operation and medication details were digitally documented for further analysis.
Safety and Efficacy Outcomes
The primary safety endpoint was sICH evaluated on CT or T2*MR images within post-MT 24 hours. sICH was defined as an ICH associated with clinical deterioration (increase ≥ 4 points in NIHSS) according to ECASS-III.12 The secondary safety outcome was any ICH detected by follow-up CT or T2*MR images and distal embolization of thrombus during MT. Distal embolization was referred to thrombus escape or shift during revascularization procedures that led to downstream arterial thromboembolism distal to the original occlusion site.
The primary efficacy endpoints were three-month functional independence and mortality, which was assessed with mRS (range 0–6) by trained research coordinators who were blinded to subjects’ baseline characters. Functional independence was defined as mRS 0–2. The secondary efficacy outcome was successful vascular recanalization, defined as a modified Tissue Thrombolysis in Cerebral Ischemia (mTICI) grade of 2b/3 on the final angiogram.
Statistical Analysis
Baseline characteristics and operation details were compared between patients treated with and without tirofiban. Likewise, all endpoints, including artery recanalization, ICH, and long-term functional outcome, were compared between groups. We used χ2 test for categorical variables, one-way analysis of variance, or Kruskal–Wallis test for continuous variables. The logistic regression model was adopted to explore the correlation between primary/secondary endpoint and rescue tirofiban. Confounding factors were selected based on theoretical considerations and baseline characteristic statistical differences by univariate analysis (including demographics, cerebrovascular disease risk factors, operation procedure details, and TOAST stroke classification).
All statistical analyses were conducted with SAS software version 9.4 (SAS Institute Inc, Cary, NC). Two-tailed P values <0.05 were considered as statistically significant.
Results
Demographics and Baseline Characteristics
A total of 917 eligible patients who underwent endovascular treatment were recruited in the ANGEL registry. Consequently, 662 patients were analyzed in the present study after excluded patients for admission NIHSS score <6 (n=132) and intra-arterial thrombolysis alone (n=123). (Figure 1) The average age of included patients was 64.1±13.6 years; 432 (65.3%) were male. IVT bridging was performed in 207 (31.3%) patients, and 55 (26.6%) of them received rescue tirofiban. IVT bridging patients treated with tirofiban carried significantly heavier atherosclerotic burden such as smoking (49.1% versus 30.3%, P=0.01) and were more likely to undergo intra-arterial thrombolysis (30.9% versus 15.8%, P=0.02), balloon angioplasty (23.6% versus 7.9%, P<0.01) and permanent stenting during MT (18.2% versus 7.9%, P=0.03). On the other hand, patients who did not receive rescue tirofiban (non-tirofiban group) were more likely to have atrial fibrillation (23.0% versus 9.1%, P=0.03). Patients with tirofiban also had more baseline NIHSS score than non-tirofiban group (18 versus 15, P=0.03). (Table 1)
 |
Table 1 Baseline Characteristics of Patients Underwent MT Therapy and Preceding Intravenous Thrombolysis |
 |
Figure 1 Flowchart. |
Safety Outcomes
Overall, 17 (8.2%) patients suffered sICH within 24 hours post-MT, including 6 (10.9%) patients in the tirofiban group and 11 (7.2%) in the non-tirofiban group (Table 2); however, no significant differences between groups were observed in the entire cohort. In addition, there were 10 (18.2%), and 26 (17.1%) patients who experienced any ICH and distal embolization of thrombus, respectively; and again, no significant differences were found between groups. Distal embolization of thrombus occurred in 11 (5.3%) patients under IVT bridging, and there was no difference between tirofiban and non-tirofiban group (10.9% versus 7.2%, P=0.15).
 |
Table 2 Safety and Efficacy Endpoints of MT Patients with Preceding Intravenous Thrombolysis Grouped by Tirofiban Use |
Efficacy Outcome
Overall, 181 (87.4%) patients with IVT bridging achieved successful recanalization, 46 (83.6%) in the tirofiban group, and 135 (88.8%) in the non-tirofiban group. No significant difference was found between tirofiban and non-tirofiban group on vascular recanalization rate (p=0.32).
After three months’ follow-up, 111 (53.6%) patients reached functional independence (mRS 0–2), 27 (49.1%) in the tirofiban group and 84 (55.3%) in the non-tirofiban group. Multivariate regression analysis did not demonstrate a significant correlation of rescue tirofiban with long-term functional independence for IVT bridging patients (p>0.05 for all groups).
On the other hand, 34 (16.4%) patients died at three-month follow-up, 4 (7.3%) patients from the tirofiban group and 30 (19.7%) patients from the non-tirofiban group. Of note, rescue tirofiban was significantly correlated with long-term mortality reduction for IVT bridging patients (adjusted hazard ratio 0.28 [0.08–0.94], adjusted p=0.03). (Figure 2)
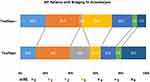 |
Figure 2 Distributions of the 3-month modified Rankin Scale score (mRS) of patients who underwent mechanical thrombectomy and preceding intravenous thrombolysis. |
Discussion
In this prospective registry study, we found that rescue tirofiban during MT with bridging IVT provided acceptable safety over sICH, ICH, and distal embolization of thrombus, as well as efficacy over three-month mortality. Accordingly, this may qualify rescue tirofiban a preferable alternative rescue therapy during MT for patients with bridging IVT, especially for those complicated with severe in situ atherosclerotic stenosis, permanent stenting, or obvious endothelial damage.
Tirofiban has been selectively administrated intravenously or intra-arterially as rescue therapy for cases with endothelial damage or in situ atherosclerotic stenosis at the site of occlusion during MT in a number of case series and preliminary clinical trials.7,9 These studies shared similar indications for rescue tirofiban that involves stent implantation and endothelial damage suspected cases, but end up with controversial results.7–9 We assume that IVT preceding MT may partly account for the variable results among these studies. However, the effects of intensive antiplatelet therapy with GP IIb/IIIa receptor inhibitor during MT for patients with bridging IVT have not been studied up to now. We believe that this is the first multi-center study that evaluates the safety and efficacy of rescue tirofiban during MT for patients with bridging IVT.
The intravenous thrombolytic agent used for AIS, such as alteplase, could induce prolonged platelet activation and platelet-related inflammation, which can undermine the initial thrombolytic effect and lead to secondary thrombogenesis, distal microcirculation obstruction, or even large-vessel reocclusion.13 Theoretically, early administration of antiplatelet agents after IVT may prevent the secondary platelet aggregation, and result in improved clinical outcomes. However, the ARTIS trial (Antiplatelet Therapy in Combination With rt-PA Thrombolysis in Ischemic Stroke) showed that early intravenous administration of aspirin 300mg shortly after rt-PA significantly associated with a higher risk of sICH, instead of improved clinical outcomes at 3-month follow-up.14 In addition, CLEAR trial did not indicate the net benefits of combined IVT with rt-PA plus eptifibatide.15 Based on this, the use of antiplatelet agents is not recommended within 24 hours following IVT in the American Heart Association/American Stroke Association (AHA/ASA) guidelines because of the concern of increased hemorrhagic complications.10 But current evidence should not indiscriminately negate the possible efficacy of early antiplatelet therapy after IVT. The SaTIS trial and several pilot studies have confirmed the safety of tirofiban on antiplatelet aggregation.16 In clinical practice for patients with large cerebral artery occlusion, GP IIb/IIIa receptor inhibitor was frequently used during MT when rescue angioplasty or permanent stenting was performed to maintain forward blood flow. In the current study, after careful screening for indications of rescue tirofiban, we preliminarily confirmed the safety of rescue tirofiban during MT over sICH, ICH, distal embolization, as well as its efficacy over three-month mortality for patients with preceding IVT. On the other hand, this study indicated the potential feasibility of early antiplatelet treatment, but only for patients undergoing MT, and may serve as a resource for future studies on the safety of tirofiban use during MT for patients with preceding IVT.
Our results were partially different from the previous controlled studies on the efficacy of tirofiban administration during MT.7,9 We postulated that some of the following mechanisms may account for the discrepancy. First and foremost is the dosage of tirofiban administration during MT. Tirofiban produces dose-dependent inhibiting effects on platelet aggregation within 5 minutes, which restored approximately 50% in 4 hours and reached near-baseline levels in 8 hours after cessation of administration.17,18 We reviewed all the studies on tirofiban dosage during endovascular treatment of large cerebrovascular occlusion,19 and based on this, we introduced the low-dose intra-arterial bolus of tirofiban 0.25~1.0 mg for fast-acting and real-time feedback on angiographic changes,20 followed by continuous intravenous infusion at a lower rate of 0.1μg/kg/min lasting 24 hours for improved microvascular patency and prevention of delayed arterial re-occlusion.4 Secondly, based on GP IIb/IIIa inhibitors’ specific inhibition effect on platelet aggregation and atherothrombosis, we prespecified the indications of tirofiban administration during MT in the protocol.11 In this study, rescue tirofiban was more selectively adopted for large artery atherosclerosis infarction rather than cardio-embolic stroke (72.7% versus 16.4%), which might also contribute to the benefits of tirofiban. Thirdly, 41.5% of patients in the present study received heparin during MT, in contrast to Zhao et al’s study,7 which mandatory prescribed intravenous heparin for all subjects to maintain the activated clotting time ranging 250 to 300s. Several preliminary studies suggested that administration of tirofiban coupled with heparin (2000–4000IU) during MT was associated with an improved recanalization rate (75.1% to 84.6%), but with a higher sICH (14.3% to 37.5%) and mortality rate (18.8% to 28.6%).21–23
Limitations
Several limitations of the present study must be taken into consideration when interpreting these results. First and foremost, we enrolled patients from an observational study, and rescue tirofiban was decided at the discretion of the operator according to arterial recanalization status, which might cause selection bias. Thus, we included all these potential confounders into the multivariable logistic regression model and got the same results. Secondly, all subjects were from China, which has a high prevalence of intracranial atherosclerosis (ICAS).24 Thus, the findings from the present study may not be generalizable to the overall population.
Conclusion
In summary, low-dose tirofiban during MT was not correlated with increased risk of safety endpoints on sICH, ICH or distal embolization, and may be associated with a lower risk of three-month mortality for patients with preceding IVT. Further studies are needed to confirm it.
Grant Support
The National Key Research and Development Program of China (2015BAI12B04, 2015BAI12B02 and 2016YFC1301501); the Ministry of Science and Technology of the People’s Republic of China (2016YFC0901002, 2016YFC0901001, 2017YFC1310901 and 2017YFC1307905).
Data Sharing Statement
The data that support the findings of this study are available from the corresponding author on reasonable request.
Acknowledgments
Special thanks to ANGEL investigators.
Author Contributions
All authors contributed to data analysis, drafting or revising the article, gave final approval of the version to be published, and agree to be accountable for all aspects of the work.
Disclosure
The authors report no conflicts of interest in this work.
References
1. Campbell BC, Mitchell PJ, Kleinig TJ, et al. Endovascular therapy for ischemic stroke with perfusion-imaging selection. N Engl J Med. 2015;372(11):1009–1018. doi:10.1056/NEJMoa1414792
2. Goyal M, Menon BK, van Zwam WH, et al. Endovascular thrombectomy after large-vessel ischaemic stroke: a meta-analysis of individual patient data from five randomised trials. Lancet. 2016;387(10029):1723–1731. doi:10.1016/S0140-6736(16)00163-X
3. Nogueira RG, Jadhav AP, Haussen DC, et al. Thrombectomy 6 to 24 hours after stroke with a mismatch between deficit and infarct. N Engl J Med. 2018;378(1):11–21. doi:10.1056/NEJMoa1706442
4. Kang DH, Kim YW, Hwang YH, Park SP, Kim YS, Baik SK. Instant reocclusion following mechanical thrombectomy of in situ thromboocclusion and the role of low-dose intra-arterial tirofiban. Cerebrovasc Dis. 2014;37(5):350–355. doi:10.1159/000362435
5. Teng D, Pannell JS, Rennert RC, et al. Endothelial trauma from mechanical thrombectomy in acute stroke: in vitro live-cell platform with animal validation. Stroke. 2015;46(4):1099–1106. doi:10.1161/STROKEAHA.114.007494
6. Abraham P, Scott Pannell J, Santiago-Dieppa DR, et al. Vessel wall signal enhancement on 3-t mri in acute stroke patients after stent retriever thrombectomy. Neurosurg Focus. 2017;42(4):E20. doi:10.3171/2017.1.FOCUS16492
7. Zhao W, Che R, Shang S, et al. Low-dose tirofiban improves functional outcome in acute ischemic stroke patients treated with endovascular thrombectomy. Stroke. 2017;48(12):3289–3294. doi:10.1161/STROKEAHA.117.019193
8. Seo JH, Jeong HW, Kim ST, Kim EG. Adjuvant tirofiban injection through deployed solitaire stent as a rescue technique after failed mechanical thrombectomy in acute stroke. Neurointervention. 2015;10(1):22–27. doi:10.5469/neuroint.2015.10.1.22
9. Kellert L, Hametner C, Rohde S, et al. Endovascular stroke therapy: tirofiban is associated with risk of fatal intracerebral hemorrhage and poor outcome. Stroke. 2013;44(5):1453–1455. doi:10.1161/STROKEAHA.111.000502
10. Powers WJ, Rabinstein AA, Ackerson T, et al. Guidelines for the early management of patients with acute ischemic stroke: a guideline for healthcare professionals from the american heart association/american stroke association. Stroke. 2018;2018(49):e46–e110.
11. Huo X, Ma N, Mo D, et al. Acute ischaemic stroke cooperation group of endovascular treatment (angel) registry: study protocol for a prospective, multicentre registry in china. Stroke Vasc Neurol. 2019;4(1):57–60. doi:10.1136/svn-2018-000188
12. Hacke W, Kaste M, Bluhmki E, et al. Thrombolysis with alteplase 3 to 4.5 hours after acute ischemic stroke. N Engl J Med. 2008;359(13):1317–1329. doi:10.1056/NEJMoa0804656
13. Rubiera M, Alvarez-Sabin J, Ribo M, et al. Predictors of early arterial reocclusion after tissue plasminogen activator-induced recanalization in acute ischemic stroke. Stroke. 2005;36(7):1452–1456. doi:10.1161/01.STR.0000170711.43405.81
14. Zinkstok SM, Roos YB. Early administration of aspirin in patients treated with alteplase for acute ischaemic stroke: a randomised controlled trial. Lancet. 2012;380(9843):731–737. doi:10.1016/S0140-6736(12)60949-0
15. Pancioli AM, Broderick J, Brott T, et al. The C ombined Approach to L ysis Utilizing E ptifibatide and r t-PA in Acute Ischemic Stroke. Stroke. 2008;39(12):3268–3276. doi:10.1161/STROKEAHA.108.517656
16. Siebler M, Hennerici MG, Schneider D, et al. Safety of tirofiban in acute ischemic stroke: the satis trial. Stroke. 2011;42(9):2388–2392. doi:10.1161/STROKEAHA.110.599662
17. McClellan KJ, Goa KL. Tirofiban. A review of its use in acute coronary syndromes. Drugs. 1998;56(6):1067–1080. doi:10.2165/00003495-199856060-00017
18. Abumiya T, Fitridge R, Mazur C, et al. Integrin α IIb β 3 inhibitor preserves microvascular patency in experimental acute focal cerebral ischemia. Stroke. 2000;31(6):1402–1409. doi:10.1161/01.STR.31.6.1402
19. Yang M, Huo X, Miao Z, Wang Y. Platelet glycoprotein iib/iiia receptor inhibitor tirofiban in acute ischemic stroke. Drugs. 2019;79(5):515–529. doi:10.1007/s40265-019-01078-0
20. Kwon OK, Lee KJ, Han MH, Oh CW, Han DH, Koh YC. Intraarterially administered abciximab as an adjuvant thrombolytic therapy: report of three cases. AJNR Am J Neuroradiol. 2002;23(3):447–451.
21. Mangiafico S, Cellerini M, Nencini P, Gensini G, Inzitari D. Intravenous glycoprotein iib/iiia inhibitor (tirofiban) followed by intra-arterial urokinase and mechanical thrombolysis in stroke. AJNR Am J Neuroradiol. 2005;26(10):2595–2601.
22. Ihn YK, Sung JH, Kim BS. Intravenous glycoprotein iib/iiia inhibitor (tirofiban) followed by low-dose intra-arterial urokinase and mechanical thrombolysis for the treatment of acute stroke. Neuroradiol J. 2011;24(6):907–913. doi:10.1177/197140091102400614
23. Kwon JH, Shin SH, Weon YC, Hwang JC, Baik SK. Intra-arterial adjuvant tirofiban after unsuccessful intra-arterial thrombolysis of acute ischemic stroke: preliminary experience in 16 patients. Neuroradiology. 2011;53(10):779–785. doi:10.1007/s00234-011-0939-y
24. Wang Y, Zhao X, Liu L, et al. Prevalence and outcomes of symptomatic intracranial large artery stenoses and occlusions in china: the chinese intracranial atherosclerosis (CICAS) study. Stroke. 2014;45(3):663–669. doi:10.1161/STROKEAHA.113.003508
 © 2020 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2020 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
