Back to Archived Journals » Research and Reports in Biology » Volume 6
Role of small GTPases in polarized vesicle transport to primary cilium
Received 19 October 2014
Accepted for publication 10 December 2014
Published 18 February 2015 Volume 2015:6 Pages 17—24
DOI https://doi.org/10.2147/RRB.S57087
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 3
Editor who approved publication: Professor Zvi Kelman
Kollu Nageswara Rao, Hemant Khanna
Department of Ophthalmology, Albert Sherman Center, University of Massachusetts Medical School, Worcester, MA, USA
Abstract: Small GTPases play crucial roles in regulating polarized vesicle trafficking in a cell. These molecules are involved in diverse pathways and regulate cell shape, organelle integrity, and cell signaling. One of the most important cellular signaling centers is the primary or sensory cilium. Small GTPases orchestrate a diverse set of events to not only generate these structures but also mediate their function and maintenance during the life of the cell. In this review, we will discuss the various small GTPases involved in regulating cilia biogenesis and function and their involvement in human diseases.
Keywords: ciliopathies, RPGR, RP2, retina, kidney, photoreceptor, neuronal cilia
Introduction
The cilium is an evolutionarily conserved microtubule based structure that protrudes from the cell surface of almost all cell types.1 There are two types of cilia that are structurally fairly similar but show distinct functional involvement: motile cilia and immotile cilia. Motile cilia (other than flagella) are involved in mucociliary transport and are composed of 9 outer doublets of microtubules and a pair of central microtubules (9+2 arrangement).2 On the other hand, nonmotile or primary (sensory) cilium is usually present as a single entity per cell and is involved in sensing extracellular environment. It is also composed of 9 microtubule doublets but lacks the central pair (9+0 arrangement).3 A stringently controlled and elaborate program of coordinated trafficking of membrane and microtubule assemblies regulates early ciliogenic events, which are orchestrated by a process called intraflagellar transport (IFT). Elegant studies and review articles have covered their role and involvement in cilia formation.4–7 The aim of this article is to discuss the mechanisms involved in regulating trafficking of membrane proteins to the cilia, specifically focusing on the role of small GTPases involved in polarized trafficking of vesicles from the Golgi network to the base of cilia for entry and incorporation into the ciliary membrane.
Primary cilia are involved in dynamic cellular processes such as signaling cascades (sonic hedgehog signaling, planar cell polarity signaling) and sensory transduction (olfaction, mechanosensation, and photoreception).8–13 Cilia carry out such processes by concentrating key receptor and signaling moieties in the ciliary membrane while excluding others.6 It is a considerable feat on the part of cilia to regulate their membrane composition given the fact that the ciliary membrane is continuous with the plasma membrane. This suggests that cilia maintain a barrier-like structure called membrane diffusion barrier, first characterized in the unicellular green alga Chlamydomonas.14,15 However, membrane diffusion barriers have been described in other cell types (eg, those between apical and basolateral membranes of polarized epithelial cells and in neurons).16,17
Delivery of membrane proteins to cilia
Polarized vesicle trafficking has emerged as a fundamental mechanism to deliver proteins and membranes to their proper cellular compartments. Abnormalities in this process can lead to significant impacts on normal cell function.18–21 Trafficking to the cilium consist of two steps: 1) sorting of the proteins destined to the cilium and 2) transport to the cilium. Both these steps require a coordinated action of multiple protein assemblies. In the first step, protein sorting from the trans-Golgi network (TGN) is based upon the presence of a ciliary targeting sequence (CTS). Such a sequence was first described in the G-protein-coupled receptor rhodopsin present in rod photoreceptor cilia.22,23 It was shown that disruption of the CTS of rhodopsin results in its mistrafficking and severe retinal degeneration.24,25 Following these studies, additional ciliary membrane proteins were found to possess functional CTS.26–29 A notable observation in the case of photoreceptors is that due to immense load of protein trafficking to cilia, a majority of the cellular machinery is dedicated to sorting of proteins to outer segment (OS), which results in the ciliary targeting being a default pathway in photoreceptors. Proteins that do not possess a targeting signal seem to piggyback on rhodopsin transport machinery and are targeted to the OS.30 Such observations reinforce the need to critically evaluate protein-targeting mechanisms in a cell-type specific manner. It is possible that multiple mechanisms are involved in ciliary targeting in different cell types depending upon the load of cilia function.19
The next step in ciliary targeting is the machinery that recognizes the CTS and targets those vesicles to the cilium. Although vesicular trafficking is essential for several cellular functions, the regulation of vesicular trafficking is still poorly understood. Small GTPases are highly conserved proteins that play an important role in regulating polarized vesicle trafficking. These GTPases include proteins belonging to the RAB family, along with the Arf/Arl family of small GTPases, which regulate the vesicle formation, movement, and fusion.31–35 Here, we will discuss the role of these small GTPases in vesicular transport.
Small GTPases in ciliary protein trafficking
Much of the information about polarized protein trafficking to cilia has come from elegant studies using vertebrate photoreceptors. Photoreceptors are polarized and one of the highly metabolically active cell types, second only to cancer cells.36 The polar distribution of key proteins is maintained by stringently regulated trafficking of proteins from their site of synthesis in the inner segment to the sensory ciliary compartment called the OS.37 In addition, photoreceptors periodically shed their distal ciliary tips containing the bleached photopigment.38 Such shedding triggers replenishment of the ciliary membrane and associated proteins at the proximal end. It is estimated that approximately 2,000 molecules of rhodopsin are transported per minute in a normal human retina.39–41 Post-Golgi vesicles containing the most abundant photoreceptor protein rhodopsin fuse with the plasma membrane near the base of the cilium.42 Several GTPases have been identified that participate in the trafficking of rhodopsin.43
On the basis of sequence and function, small GTPases can be divided into five major subfamilies: Ras, Rho, RAB, Arf/Arl, and Ran.44 These enzymes undergo cycling reactions between the active GTP-bound and the inactive GDP-bound states. Guanine nucleotide exchange factors (GEFs) stimulate GDP (guanosine diphosphate) to GTP (guanosine triphosphate) conversion whereas GTPase activating proteins (GAPs) mediate GTP hydrolysis.44 Elegant analyses have revealed the involvement of Arf/Arl, RAB, and Ran GTPases in ciliary function (Table 1).31
  | Table 1 Role of GTPases in cilia function |
Arf/Arl GTPases
Arf (ADP ribosylation factor) proteins were originally identified as cofactors for cholera toxin-catalyzed ADP-ribosylation.45 There are 6 types of mammalian Arfs (Arf1–Arf6).46 Of these, Arf4 is the only known Arf family GTPase to function in ciliary targeting.24 Recent studies have shown that these proteins regulate vesicular trafficking pathways. The CTS of rhodopsin recruits Arf4 at the Golgi/TGN in inner segment of photoreceptors.43 Arf4 activity is modulated by its GEF GBF1 and GAP ASAP1. ASAP1 is also involved in ciliary targeting of rhodopsin and in the coordinated action of Arf and RAB GTPases (see “RAB GTPases” section). In addition to Arfs, there are Arf-like GTPases called Arls. There are >20 members in the Arl family of proteins.47,48 A notable feature of Arls is a characteristic lack of biochemical activity.49 Nonetheless, Arl proteins play key roles in membrane trafficking and human ciliopathies.34 Three Arl proteins, Arl3, Arl6, and Arl13b have been implicated in ciliary targeting.50
Arl3
The involvement of Arl3 in cilia development and function was revealed in studies using Leishmania donovani and Caenorhabditis elegans.51 In these investigations, it was revealed that Arl3 likely acts by modulating the integrity of the IFT complex thereby regulating cilia formation. Although its exact role in cilia formation is still unclear, Arl3 has been shown to modulate cilia-dependent signaling events. This is achieved by participating in the trafficking of ciliary receptor proteins, such as polycystin-1 and polycystin-2.52 Interestingly, Arl3–/– mice exhibit ciliopathy phenotypes, including cystic kidney disease and photoreceptor development.53 Consistently, rhodopsin mistrafficking was observed in Arl3–/– mouse retina. Further involvement of Arl3 in photoreceptor function came from studies indicating that Arl3 activity could be modulated in vitro by RP2 (retinitis pigmentosa 2), which is mutated in X-linked retinitis pigmentosa.54–57 It was found that RP2 acts as a GAP for Arl3, which is predicted to trigger the release of lipid-modified cargo, such as PDE6d and UNC119 in the cilia.57 Arl3 interacts with these proteins in GTP-bound form. According to the current model, Arl3-GTP binds to UNC119 complexed with myristoylated NPHP3 (nephrocystin-3) to traffic to cilia. When inside cilia, RP2 acts to release UNC119 and NPHP3 from Arl3 by converting Arl3-GTP to Arl3-GDP.58,59 Although such a hypothesis is attractive, additional experimental support is necessary to further test this model. For example, although RP2 is involved in retinal degeneration there was no evidence of mislocalization of NPHP3 in the Rp2null mice.60 Moreover, RP2, Arl3, and NPHP3 have not been found in a complex in the retina. Such discrepancies underscore the need to interpret results in a cell-type dependent manner.
Arl6
Also known as BBS3, Arl6 was the first small GTPase protein that was linked to the human ciliopathy, Bardet–Biedl syndrome (BBS).61,62 Using a protein pull-down assay with homogenized bovine retina, Jin et al63 showed that Arl6 bound a ciliary membrane protein complex containing 7 BBS proteins (BBSome) and BBIP10. Ciliary localization of BBSome is dependent on ARL6 in its GTP bound form. Studies have shown that SSTR3 (somatostatin receptor 3) is a cargo for BBSome and is lost from cilia in hippocampal neurons of Bbs2–/– and Bbs4–/–.64 Arl6 and the BBSome bind to the CTS of SSTR3 to transport to the ciliary membrane. This finding supports the hypothesis that the abnormal Arl6 activity leads to the compromised ciliary entry of critical cargo proteins essential for cilia assembly and signaling and, thus, causes the variety of BBS symptoms. A recent study by Su et al,65 showed that polycystin-1 interacts with the BBSome complex and expression of a mutant form of Arl6 results in loss of ciliary localization suggesting that polycystin-1 may be a cargo for the BBSome.
Arl13b
Arl13b is another small GTPase protein connected to the human ciliopathy, Joubert syndrome, an inherited neurodevelopmental disorder with midbrain–hindbrain malformations, retinal dystrophy and, occasionally, nephronophthisis.66 Duldulao et al,67 first demonstrated that Arl13b is a protein that is highly enriched in the cilium and is required for cilia formation in multiple organs in zebrafish, and that loss of Arl13b leads to multiple cilia-associated phenotypes and thus ciliary localization is crucial for the in vivo function of Arl13b. Arl13b localizes to a proximal ciliary compartment, where it associates with ciliary membranes via palmitoylation modification motifs. Defects in ciliary morphology and ultrastructure and destabilization of IFT complex have been observed in C. elegans Arl13 mutants. Li et al68 observed shortened cilia with various ultrastructural deformities and a disrupted association between IFT subcomplexes A and B in Arl13 mutants and that these abnormalities were eliminated by depletion of Arl3, another ciliary small GTPase. Recently, it was shown that SUMOylation of Arl13 is crucial for proper ciliary targeting of various sensory receptors such as polycystin-2 suggesting SUMOylation modification of GTPase ARL13b regulates proper ciliary targeting of various sensory receptors.69 Humbert et al28 identified that Arl13b is in complex with other proteins and helps regulate ciliary trafficking of phospholipid phosphatase INPP5E, which is mutated in Joubert Syndrome.
RAB GTPases
RAB GTPases represent a large family of small GTPases, and in humans more than 60 members have been identified.70 Several RAB proteins have been shown to be involved in regulation of ciliary functions and these include RAB8, RAB11, RAB10, RAB17, and RAB23.33 Identification of RAB8 in photoreceptors was the first study to link cilia and RAB proteins.42 In that study, it was demonstrated that a small fraction of RAB8 is associated with post-Golgi vesicles. Microscopic analysis showed that post-Golgi vesicles, having rhodopsin, were localized at the base of the OS where colocalization of RAB8 and actin were observed suggesting that RAB8 may be involved in trafficking of rhodopsin. Later, it was shown that RAB8 was also involved in trafficking of several other proteins, such as fibrocystin (involved in autosomal recessive polycystic kidney disease), to cilia.26 Nachury et al,71 showed that the BBSome associates with the RAB8 GEF RABIN8 and promotes docking and fusion of vesicles to the ciliary membrane. Elegant live-cell imaging experiments by Westlake et al,72 revealed the earliest steps in RAB8 membrane assembly during cilia formation. RAB8 is targeted to the primary cilium during early ciliogenesis followed by a gradual loss from the cilium as the organelle matures. RAB8 is activated by its GEF RABIN8, which is recruited to the centrosome and activated by a mechanism involving RAB11 and homologs of the yeast transport protein particle II (TRAPPII) complex subunits.73 RABIN8 binds to the TRAPPII complex and this interaction is essential for RABIN8 localization to centrosome, trafficking, and ciliogenesis. In addition to specific proteins, cell cytoskeleton is also known to play a role in modulating RAB8–RAB11 activity during cilia formation. It was found that branched actin network negatively regulates RAB8 activation likely by inhibiting the association of RAB8 with RAB11-containing vesicles.74 Knödler et al,75 showed a functional connection between RAB11, RABIN8, and RAB8. RAB11 binds directly to RABIN8 and stimulates the GEF activity of RABIN8 toward RAB8. In another study it was shown that RAB8, RAB5, and RAB23 have distinct functions in ciliary transport.76 RAB8 is involved in the transport of Smo, EB1, and kim1, but only RAB5 plays a role in the trafficking of the apical-membrane-localized Kim1, whereas RAB23 is localized in the cilia and regulates ciliary entry of Smo. It was also shown that full length RABIN8 has a self-inhibitory sequence.77 RAB11 binds to this region and activates RABIN8 toward RAB8. In its activated conformation, RABIN8 also interacts with Sec15, a subunit of the exocyst and downstream effector of RAB8. Expression of constitutively activated RAB8 promotes the association of Sec15 with RABIN8. Using immunofluorescence microscopy, it was shown that Sec15 colocalized with RAB8 along the primary cilium and that localization was necessary for ciliary functions. Hence, the RABIN8–RAB8–Sec15 interaction may couple the activation of RAB8 to the recruitment of the RAB8 effector and is involved in the regulation of vesicular trafficking for primary cilium formation.77
Small GTPases and motor proteins in vesicle trafficking
Primary cilia are built and maintained by IFT, whereby the two IFT complexes, IFT-A and IFT-B, carry cargo via kinesin and dynein motors for anterograde and retrograde transport, respectively.78 The interactions between IFT motors and IFT complexes are essential for the transport of ciliary cargo.79 IFT complexes likely function as adaptors that mediate interactions between anterograde/retrograde motors and ciliary cargoes, facilitating cargo transport between the base and tip of the cilium.79
The microtubule and actin network plays an important role in motor protein driven ciliary trafficking. Actin based myosin motors are usually involved in slower and short range local transport events.80 Kinesin and dyneins mediate microtubules based long-range transport. Kinesin motors transport cargo toward the plus end of the microtubules and have a domain structure relatively similar to myosins. Kinesin motors consist of a heavy chain and a light chain.81 There are two dynein motor isoforms, cytoplasmic dynein 1 and cytoplasmic dynein 2. Dyneins are minus end-directed motor proteins which mediate cargo transport toward the centrosome.82 Dynein motors are huge multimeric complexes composed of two heavy chains and associate with intermediate, light intermediate, and several light chains. Motor domains are located in the heavy chain, whereas the accessory subunits are involved in the interaction with the cargo and regulatory proteins.83
Small GTPases play an important role in regulating the interaction of cargo with actin- and microtubule-based motor proteins.84 ARL13 regulates the binding of IFT-A and IFT-B, while ARL3 acts antagonistically with ARL-13 to regulate IFT integrity and ciliogenesis.68,85 Defects in Arl13 mutants are rescued by depletion of ARL3 through an HDAC6-dependent pathway suggesting that ARL13 acts antagonistically with ARL3 in cilia formation.68 Like ARL3, activation of HDAC6 was also found to promote cilia disassembly while loss of HDAC6 activity selectively stabilizes cilia in human epithelial cells.86 Interestingly, HDAC6 was shown to interact with BBIP10, a subunit of the BBSome that binds to both IFT-A and IFT-B, suggesting a potential functional crosstalk between IFT, ARL3, ARL13, and ARL6-BBSome in cilia.87
Recent studies have shown that a kinesin family member KIF13A binds to the active form of RAB11 to regulate endosomal sorting and recycling of endosomal cargo.88 In photoreceptors, actin cytoskeleton at the base of the ciliary OS is involved in the targeted delivery and fusion of RAB8-positive vesicles. This is organized by a systematic and concerted process involving RAB8, Sec8 (part of the exocyst complex), actin motors, and the actin cytoskeleton.89–91
Working model of GTPase-regulated ciliary protein trafficking
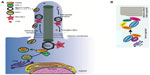
As evident from the aforementioned discussion, there are significant parallels between different cell types for regulating ciliary biogenesis and membrane protein trafficking. The basic machinery for vesicle sorting, targeting, delivery, and fusion are shared among different cell types. Figure 1A depicts a brief illustration of the basic model of ciliary membrane protein trafficking. RAB GTPases and its effectors, the BBSome and IFT are involved in cargo vesicle targeting to the ciliary membrane as well as exit from the cilium. Post-Golgi cargo vesicles are coated with RAB8-GDP and trafficked to the base of the cilium. RABIN8, a GEF for RAB8A, trafficked independently by RAB11 activates RAB8 to RAB8-GTP, which results in the fusion of the cargo vesicles with the ciliary membrane. The BBSome complex is involved in the fusion as well as tethering of the cargo to the IFT for trafficking. This is mediated by the action of BBS3/ARL6. Figure 1B demonstrates the working model of the targeting of rhodopsin to the photoreceptor OS. First step in rhodopsin sorting involves the action of Arf4 at the TGN (Figure 1). These rhodopsin containing post-Golgi vesicles are called rhodopsin transport carriers. At the TGN, activated Arf4 interacts directly with the rhodopsin ciliary targeting signal VxPx.92 Arf4 dependent budding of the vesicles are regulated by ASAP1, RAB11, and RAB11-Arf effector. ASAP1 selectively binds RAB11a and the RAB11-Arf effector FIP3. Following GTP hydrolysis and dissociation of Arf4, ASAP1 and RAB11a remain associated at the TGN where they recruit RAB8 and its GEF RABIN8. RAB8 then regulates the final stages of polarized membrane trafficking at the cilium.43 Some ciliary and centrosomal proteins involved in retinal degeneration, such as RPGR, CEP290, PCM-1, and OCRL bind to RAB8 and likely affect its localization and/or function in cilia assembly or maintenance.93–95 Of these, RPGR is the only protein other than RABIN8, which possess a GEF activity toward RAB8.93 The molecular mechanisms underlying such effects are under investigation.
Future directions
Owing to their biochemical activities, small GTPases are an attractive candidate for drug design to treat disorders associated with them. However, a roadblock in such studies is the ubiquitous and developmentally crucial role of these GTPases in organisms. Therefore, wide-range targeting of such GTPases is not relevant to devise therapeutic paradigms. Tissue and cell-type specific investigation of GTPases in cilia related functions and the involvement of compensating role of GTPases are critical to develop a detailed understanding of a context-dependent function of these proteins. Given that the load of ciliary function is different in distinct cell types depending upon their function, such investigations have a high probability of success and should provide novel information on their activity which can then be utilized to develop cell-type specific therapeutic intermediates.
Acknowledgments
We thank Manisha Anand for help with figure preparation. We apologize to those authors whose contributions could not be included in this article due to space limitations. The research is supported by grants from the National Institutes of Health (EY022372), Foundation Fighting Blindness, and University of Massachusetts Center for Clinical and Translational Sciences (UMCCTS).
Disclosure
The authors report no conflicts of interest in this work.
References
Fliegauf M, Benzing T, Omran H. When cilia go bad: cilia defects and ciliopathies. Nat Rev Mol Cell Biol. 2007;8(11):880–893. | |
Mitchell DR. The evolution of eukaryotic cilia and flagella as motile and sensory organelles. Adv Exp Med Biol. 2007;607:130–140. | |
Satir P, Pedersen LB, Christensen ST. The primary cilium at a glance. J Cell Sci. 2010;123(Pt 4):499–503. | |
Rosenbaum JL, Witman GB. Intraflagellar transport. Nat Rev Mol Cell Biol. 2002;3(11):813–825. | |
Wang J, Deretic D. Molecular complexes that direct rhodopsin transport to primary cilia. Prog Retin Eye Res. 2014;38:1–19. | |
Nachury MV, Seeley ES, Jin H. Trafficking to the ciliary membrane: how to get across the periciliary diffusion barrier? Annu Rev Cell Dev Biol. 2010;26:59–87. | |
Hao L, Scholey JM. Intraflagellar transport at a glance. J Cell Sci. 2009;122(Pt 7):889–892. | |
Goetz SC, Anderson KV. The primary cilium: a signalling centre during vertebrate development. Nat Rev Genet. 2010;11(5):331–344. | |
Davis EE, Brueckner M, Katsanis N. The emerging complexity of the vertebrate cilium: new functional roles for an ancient organelle. Dev Cell. 2006;11(1):9–19. | |
Davenport JR, Yoder BK. An incredible decade for the primary cilium: a look at a once-forgotten organelle. Am J Physiol Renal Physiol. 2005;289(6):F1159–F1169. | |
Perkins LA, Hedgecock EM, Thomson JN, Culotti JG. Mutant sensory cilia in the nematode Caenorhabditis elegans. Dev Biol. 1986;117(2):456–487. | |
Satir P, Christensen ST. Overview of structure and function of mammalian cilia. Annu Rev Physiol. 2007;69:377–400. | |
Tan PL, Barr T, Inglis PN, et al. Loss of Bardet Biedl syndrome proteins causes defects in peripheral sensory innervation and function. Proc Natl Acad Sci U S A. 2007;104(44):17524–17529. | |
Hunnicutt GR, Kosfiszer MG, Snell WJ. Cell body and flagellar agglutinins in Chlamydomonas reinhardtii: the cell body plasma membrane is a reservoir for agglutinins whose migration to the flagella is regulated by a functional barrier. J Cell Biol. 1990;111(4):1605–1616. | |
Pazour GJ, Bloodgood RA. Targeting proteins to the ciliary membrane. Curr Top Dev Biol. 2008;85:115–149. | |
Caudron F, Barral Y. Septins and the lateral compartmentalization of eukaryotic membranes. Dev Cell. 2009;16(4):493–506. | |
Nakada C, Ritchie K, Oba Y, et al. Accumulation of anchored proteins forms membrane diffusion barriers during neuronal polarization. Nat Cell Biol. 2003;5(7):626–632. | |
Sung CH, Leroux MR. The roles of evolutionarily conserved functional modules in cilia-related trafficking. Nat Cell Biol. 2013;15(12):1387–1397. | |
Hsiao YC, Tuz K, Ferland RJ. Trafficking in and to the primary cilium. Cilia. 2012;1(1):4. | |
Reiter JF, Mostov K. Vesicle transport, cilium formation, and membrane specialization: the origins of a sensory organelle. Proc Natl Acad Sci U S A. 2006;103(49):18383–18384. | |
Leroux MR. Taking vesicular transport to the cilium. Cell. 2007;129(6):1041–1043. | |
Concepcion F, Mendez A, Chen J. The carboxyl-terminal domain is essential for rhodopsin transport in rod photoreceptors. Vision Res. 2002;42(4):417–426. | |
Deretic D, Schmerl S, Hargrave PA, Arendt A, McDowell JH. Regulation of sorting and post-Golgi trafficking of rhodopsin by its C-terminal sequence QVS(A)PA. Proc Natl Acad Sci U S A. 1998; 95(18):10620–10625. | |
Deretic D, Williams AH, Ransom N, Morel V, Hargrave PA, Arendt A. Rhodopsin C terminus, the site of mutations causing retinal disease, regulates trafficking by binding to ADP-ribosylation factor 4 (ARF4). Proc Natl Acad Sci U S A. 2005;102(9):3301–3306. | |
Tam BM, Moritz OL, Hurd LB, Papermaster DS. Identification of an outer segment targeting signal in the COOH terminus of rhodopsin using transgenic Xenopus laevis. J Cell Biol. 2000;151(7):1369–1380. | |
Follit JA, Li L, Vucica Y, Pazour GJ. The cytoplasmic tail of fibrocystin contains a ciliary targeting sequence. J Cell Biol. 2010;188(1):21–28. | |
Dishinger JF, Kee HL, Jenkins PM, et al. Ciliary entry of the kinesin-2 motor KIF17 is regulated by importin-beta2 and RanGTP. Nat Cell Biol. 2010;12(7):703–710. | |
Humbert MC, Weihbrecht K, Searby CC, et al. ARL13B, PDE6D, and CEP164 form a functional network for INPP5E ciliary targeting. Proc Natl Acad Sci U S A. 2012;109(48):19691–19696. | |
Follit JA, San Agustin JT, Jonassen JA, et al. Arf4 is required for Mammalian development but dispensable for ciliary assembly. PLoS Genet. 2014;10(2):e1004170. | |
Baker SA, Haeri M, Yoo P, et al. The outer segment serves as a default destination for the trafficking of membrane proteins in photoreceptors. J Cell Biol. 2008;183:485–498. | |
Li Y, Hu J. Small GTPases and cilia. Protein Cell. 2011;2(1):13–25. | |
Ward HH, Brown-Glaberman U, Wang J, et al. A conserved signal and GTPase complex are required for the ciliary transport of polycystin-1. Mol Biol Cell. 2011;22(18):3289–3305. | |
Lim YS, Chua CE, Tang BL. Rabs and other small GTPases in ciliary transport. Biol Cell. 2011;103(5):209–221. | |
Li Y, Ling K, Hu J. The emerging role of Arf/Arl small GTPases in cilia and ciliopathies. J Cell Biochem. 2012;113(7):2201–2207. | |
Deretic D. Crosstalk of Arf and Rab GTPases en route to cilia. Small GTPases. 2013;4(2):70–77. | |
Bramall AN, Wright AF, Jacobson SG, McInnes RR. The genomic, biochemical, and cellular responses of the retina in inherited photoreceptor degenerations and prospects for the treatment of these disorders. Annu Rev Neurosci. 2010;33:441–472. | |
Pearring JN, Salinas RY, Baker SA, Arshavsky VY. Protein sorting, targeting and trafficking in photoreceptor cells. Prog Retin Eye Res. 2013;36:24–51. | |
Basinger S, Hoffman R, Matthes M. Photoreceptor shedding is initiated by light in the frog retina. Science. 1976;194(4269):1074–1076. | |
Wolfrum U, Schmitt A. Rhodopsin transport in the membrane of the connecting cilium of mammalian photoreceptor cells. Cell Motil Cytoskeleton. 2000;46(2):95–107. | |
Williams DS. Transport to the photoreceptor outer segment by myosin VIIa and kinesin II. Vision Res. 2002;42(4):455–462. | |
Krock BL, Perkins BD. The intraflagellar transport protein IFT57 is requiredfor cilia maintenance and regulates IFT-particle-kinesin-II dissociation in vertebrate photoreceptors. J Cell Sci. 2008;121(Pt 11):1907–1915. | |
Deretic D, Huber LA, Ransom N, Mancini M, Simons K, Papermaster DS. Rab8 in retinal photoreceptors may participate in rhodopsin transport and in rod outer segment disk morphogenesis. J Cell Sci. 1995;108(Pt 1):215–224. | |
Wang J, Morita Y, Mazelova J, Deretic D. The Arf GAP ASAP1 provides a platform to regulate Arf4- and Rab11-Rab8-mediated ciliary receptor targeting. EMBO J. 2012;31(20):4057–4071. | |
Wennerberg K, Rossman KL, Der CJ. The Ras superfamily at a glance. J Cell Sci. 2005;118(Pt 5):843–846. | |
Kahn RA, Gilman AG. Purification of a protein cofactor required for ADP-ribosylation of the stimulatory regulatory component of adenylate cyclase by cholera toxin. J Biol Chem. 1984;259(10):6228–6234. | |
Kahn RA, Volpicelli-Daley L, Bowzard B, et al. Arf family GTPases: roles in membrane traffic and microtubule dynamics. Biochem Soc Trans. 2005;33(Pt 6):1269–1272. | |
Donaldson JG, Jackson CL. ARF family G proteins and their regulators: roles in membrane transport, development and disease. Nat Rev Mol Cell Biol. 2011;12(6):362–375. | |
Pretorius PR, Baye LM, Nishimura DY, et al. Identification and functional analysis of the vision-specific BBS3 (ARL6) long isoform. PLoS Genet. 2010;6(3):e1000884. | |
Tamkun JW, Kahn RA, Kissinger M, et al. The arf like gene encodes an essential GTP-binding protein in Drosophila. Proc Natl Acad Sci U S A. 1991;88(8):3120–3124. | |
Zhang Q, Hu J, Ling K. Molecular views of Arf-like small GTPases in cilia and ciliopathies. Exp Cell Res. 2013;319(15):2316–2322. | |
Cuvillier A, Redon F, Antoine JC, Chardin P, DeVos T, Merlin G. LdARL-3A, a Leishmania promastigote-specific ADP-ribosylation factor-like protein, is essential for flagellum integrity. J Cell Sci. 2000;113(Pt 11):2065–2074. | |
Hurd T, Zhou W, Jenkins P, et al. The retinitis pigmentosa protein RP2 interacts with polycystin 2 and regulates cilia-mediated vertebrate development. Hum Mol Genet. 2010;19(22):4330–4344. | |
Schrick JJ, Vogel P, Abuin A, Hampton B, Rice DS. ADP-ribosylation factor-like 3 is involved in kidney and photoreceptor development. Am J Pathol. 2006;168(4):1288–1298. | |
Schwahn U, Lenzner S, Dong J, et al. Positional cloning of the gene for X-linked retinitis pigmentosa 2. Nat Genet. 1998;19(4):327–332. | |
Evans RJ, Schwarz N, Nagel-Wolfrum K, Wolfrum U, Hardcastle AJ, Cheetham ME. The retinitis pigmentosa protein RP2 links pericentriolar vesicle transport between the Golgi and the primary cilium. Hum Mol Genet. 2010;19(7):1358–1367. | |
Bartolini F, Bhamidipati A, Thomas S, Schwahn U, Lewis SA, Cowan NJ. Functional overlap between retinitis pigmentosa 2 protein and the tubulin-specific chaperone cofactor C. J Biol Chem. 2002;277(17):14629–14634. | |
Veltel S, Gasper R, Eisenacher E, Wittinghofer A. The retinitis pigmentosa 2 gene product is a GTPase-activating protein for Arf-like 3. Nat Struct Mol Biol. 2008;15(4):373–380. | |
Wright KJ, Baye LM, Olivier-Mason A, et al. An ARL3-UNC119-RP2 GTPase cycle targets myristoylated NPHP3 to the primary cilium. Genes Dev. 2011;25(22):2347–1360. | |
Schwarz N, Hardcastle AJ, Cheetham ME. Arl3 and RP2 mediated assembly and traffic of membrane associated cilia proteins. Vision Res. 2012;75:2–4. | |
Li L, Khan N, Hurd T, et al. Ablation of the X-linked retinitis pigmentosa 2 (Rp2) gene in mice results in opsin mislocalization and photoreceptor degeneration. Invest Ophthalmol Vis Sci. 2013;54(7):4503–4511. | |
Fan Y, Esmail MA, Ansley SJ, et al. Mutations in a member of the Ras superfamily of small GTP-binding proteins causes Bardet-Biedl syndrome. Nat Genet. 2004;36(9):989–993. | |
Chiang AP, Nishimura D, Searby C, et al. Comparative genomic analysis identifies an ADP-ribosylation factor-like gene as the cause of Bardet-Biedl syndrome (BBS3). Am J Hum Genet. 2004;75:475–484. | |
Jin H, White SR, Shida T, et al. The conserved Bardet-Biedl syndrome proteins assemble a coat that traffics membrane proteins to cilia. Cell. 2010;141(7):1208–1219. | |
Berbari NF, Lewis JS, Bishop GA, Askwith CC, Mykytyn K. Bardet-Biedl syndrome proteins are required for the localization of G protein-coupled receptors to primary cilia. Proc Natl Acad Sci U S A. 2008;105:4242–4246. | |
Su X, Driscoll K, Yao G, et al. Bardet-Biedl syndrome proteins 1 and 3 regulate the ciliary trafficking of polycystic kidney disease 1 protein. Hum Mol Genet. 2014;23(20):5441–5451. | |
Cantagrel V, Silhavy JL, Bielas SL, et al. Mutations in the cilia gene ARL13B lead to the classical form of Joubert syndrome. Am J Hum Genet. 2008;83(2):170–179. | |
Duldulao NA, Lee S, Sun Z. Cilia localization is essential for in vivo functions of the Joubert syndrome protein Arl13b/Scorpion. Development. 2009;136(23):4033–4042. | |
Li Y, Wei Q, Zhang Y, Ling K, Hu J. The small GTPases ARL-13 and ARL-3 coordinate intraflagellar transport and ciliogenesis. J Cell Biol. 2010;189(6):1039–1051. | |
Li Y, Zhang Q, Wei Q, Zhang Y, Ling K, Hu J. SUMOylation of the small GTPase ARL-13 promotes ciliary targeting of sensory receptors. J Cell Biol. 2012;199(4):589–598. | |
Stenmark H. Rab GTPases as coordinators of vesicle traffic. Nat Rev Mol Cell Biol. 2009;10(8):513–525. | |
Nachury MV, Loktev AV, Zhang Q, et al. A core complex of BBS proteins cooperates with the GTPase Rab8 to promote ciliary membrane biogenesis. Cell. 2007;129(6):1201–1213. | |
Westlake CJ, Baye LM, Nachury MV, et al. Primary cilia membrane assembly is initiated by Rab11 and transport protein particle II (TRAPPII) complex-dependent trafficking of Rabin8 to the centrosome. Proc Natl Acad Sci U S A. 2011;108(7):2759–2764. | |
Schou KB, Morthorst SK, Christensen ST, Pedersen LB. Identification of conserved, centrosome-targeting ASH domains in TRAPPII complex subunits and TRAPPC8. Cilia. 2014;3:6. | |
Hattula K, Furuhjelm J, Tikkanen J, TanhuanpÄÄ K, Laakkonen P, PerÄnen J. Characterization of the Rab8-specific membrane traffic route linked to protrusion formation. J Cell Sci. 2006;119:4866–4877. | |
Knödler A, Feng S, Zhang J, et al. Coordination of Rab8 and Rab11 in primary ciliogenesis. Proc Natl Acad Sci U S A. 2010;107(14):6346–6351. | |
Boehlke C, Bashkurov M, Buescher A, et al. Differential role of Rab proteins in ciliary trafficking: Rab23 regulates smoothened levels. J Cell Sci. 2010;123(Pt 9):1460–1467. | |
Feng S, Knödler A, Ren J, et al. A Rab8 guanine nucleotide exchange factor-effector interaction network regulates primary ciliogenesis. J Biol Chem. 2012;287(19):15602–15609. | |
Scholey JM. Intraflagellar transport motors in cilia: moving along the cell’s antenna. J Cell Biol. 2008;180(1):23–29. | |
Baldari CT, Rosenbaum J. Intraflagellar transport: it’s not just for cilia anymore. Curr Opin Cell Biol. 2010;22(1):75–80. | |
Tuxworth RI, Titus MA. Unconventional myosins: anchors in the membrane traffic relay. Traffic. 2000;1:11–18. | |
Verhey KJ, Dishinger J, Kee HL. Kinesin motors and primary cilia. Biochem Soc Trans. 2011;39(5):1120–1125. | |
Kardon JR, Vale RD. Regulators of the cytoplasmic dynein motor. Nat Rev Mol Cell Biol. 2009;10(12):854–865. | |
Schroer TA. Dynactin. Annu Rev Cell Dev Biol. 2004;20:759–779. | |
Jordens I, Marsman M, Kuijl C, Neefjes J. Rab proteins, connecting transport and vesicle fusion. Traffic. 2005;6(12):1070–1077. | |
Cevik S, Hori Y, Kaplan OI, et al. Joubert syndrome Arl13b functions at ciliary membranes and stabilizes protein transport in Caenorhabditis elegans. J Cell Biol. 2010;188(6):953–969. | |
Pugacheva EN, Jablonski SA, Hartman TR, Henske EP, Golemis EA. HEF1-dependent Aurora A activation induces disassembly of the primary cilium. Cell. 2007;129(7):1351–1363. | |
Loktev AV, Zhang Q, Beck JS, et al. A BBSome subunit links ciliogenesis, microtubule stability, and acetylation. Dev Cell. 2008;15(6):854–865. | |
Delevoye C, Miserey-Lenkei S, Montagnac G, et al. Recycling endosome tubule morphogenesis from sorting endosomes requires the kinesin motor KIF13A. Cell Rep. 2014;6(3):445–454. | |
Chaitin MH, Coelho N. Immunogold localization of myosin in the photoreceptor cilium. Invest Ophthalmol Vis Sci. 1992;33(11):3103–3108. | |
Williams DS, Linberg KA, Vaughan DK, Fariss RN, Fisher SK. Disruption of microfilament organization and deregulation of disk membrane morphogenesis by cytochalasin D in rod and cone photoreceptors. J Comp Neurol. 1988;272(2):161–176. | |
Mazelova J, Ransom N, Astuto-Gribble L, Wilson MC, Deretic D. Syntaxin 3 and SNAP-25 pairing, regulated by omega-3 docosahexaenoic acid, controls the delivery of rhodopsin for the biogenesis of cilia-derived sensory organelles, the rod outer segments. J Cell Sci. 2009;122(Pt 12):2003–20213. | |
Mazelova J, Astuto-Gribble L, Inoue H, et al. Ciliary targeting motif VxPx directs assembly of a trafficking module through Arf4. EMBO J. 2009;28(3):183–192. | |
Murga-Zamalloa CA, Atkins SJ, Peranen J, Swaroop A, Khanna H. Interaction of retinitis pigmentosa GTPase regulator (RPGR) with RAB8A GTPase: implications for cilia dysfunction and photoreceptor degeneration. Hum Mol Genet. 2010;19(18):3591–3598. | |
Kim J, Krishnaswami SR, Gleeson JG. CEP290 interacts with the centriolar satellite component PCM-1 and is required for Rab8 localization to the primary cilium. Hum Mol Genet. 2008;17(23):3796–3805. | |
Hagemann N, Hou X, Goody RS, Itzen A, Erdmann KS. Crystal structure of the Rab binding domain of OCRL1 in complex with Rab8 and functional implications of the OCRL1/Rab8 module for Lowe syndrome. Small GTPases. 2012;3(2):107–110. |
 © 2015 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2015 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.