Back to Journals » Drug Design, Development and Therapy » Volume 11
Riluzole 5 mg/mL oral suspension: for optimized drug delivery in amyotrophic lateral sclerosis
Received 4 October 2016
Accepted for publication 25 November 2016
Published 22 December 2016 Volume 2017:11 Pages 59—64
DOI https://doi.org/10.2147/DDDT.S123776
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 3
Editor who approved publication: Dr James Janetka
Ann Margaret Dyer, Alan Smith
PharmaSci Consulting Limited, Nottingham, UK
Abstract: The aim of the present work is to extensively evaluate the pharmaceutical attributes of currently available riluzole presentations. The article describes the limitations and risks associated with the administration of crushed tablets, including the potential for inaccurate dosing and reduced rate of absorption when riluzole is administered with high-fat foods, and the advantages that a recently approved innovative oral liquid form of riluzole confers on amyotrophic lateral sclerosis (ALS) patients. The article further evaluates the patented and innovative controlled flocculation technology used in the pseudoplastic suspension formulation to reduce the oral anesthesia seen with crushed tablets, resulting in optimized drug delivery for riluzole. Riluzole is the only drug licensed for treating ALS, which is the most common form of motor neurone disease and a highly devastating neurodegenerative condition. The licensed indication is to extend life or the time to mechanical ventilation. Until recently, riluzole was only available as an oral tablet dosage form in the UK; however, an innovative oral liquid form, Teglutik® 5 mg/mL oral suspension, is now available. An oral liquid formulation provides an important therapeutic option for patients with ALS, >80% of who may become unable to swallow solid oral dosage forms due to disease-related dysphagia. Prior to the launch of riluzole oral suspension, the only way for many patients to continue to take riluzole as their disease progressed was through crushed tablets. A novel suspension formulation enables more accurate dosing and consistent ongoing administration of riluzole. There are clear and important advantages such as enhanced patient compliance compared with crushed tablets administered with food or via an enteral feeding tube and the potential for an improved therapeutic outcome and enhanced quality of life for ALS patients.
Keywords: amyotrophic lateral sclerosis, riluzole, dysphagia, patient compliance, oral liquid
Introduction
Background
The aim of the present work is to extensively evaluate the pharmaceutical attributes of currently available riluzole presentations.
Riluzole is the only drug licensed for use in the management of amyotrophic lateral sclerosis (ALS), the most common form of motor neurone disease.1
ALS is a progressive, ultimately fatal neurodegenerative disease, marked by a gradual degeneration of nerve cells of the central nervous system that control voluntary muscle movement. Degeneration of motor neurones is characterized by muscle weakness, typically impacting arms and legs, speech, swallowing and breathing. Impairment of swallowing (dysphagia) is a feature of ALS resulting from weakness or spasticity of muscles affecting the tongue, lips, palate, jaw, pharynx, larynx and upper trunk, causing difficulties for patients in the oral consumption of dry, tough-textured or crumbly food and thin liquids, although the specific nature of the difficulties depends on the patient’s individual clinical pathology.1
Destruction of nerve cells in motor neurone disease may be caused by excess glutamate (neurotransmitter) in the brain and spinal cord. Riluzole is a glutamate antagonist that reduces the release of glutamate, thus slowing the progression of the early disease.2 The burden of disease associated with poor patient compliance and a more rapid degeneration of motor neurones upon patients, family members and caregivers is substantial, with increasing cost associated with increasing disability and the need for assisted medical care.2 Riluzole 100 mg daily is a disease-specific therapy that has been shown to slow disease progression in patients with probable and definitive ALS with symptoms of <5 years. A median survival prolongation of up to 3 months has been shown in some patients in clinical studies.2 The same review article cited earlier observational studies, which suggested that treatment with riluzole may be associated with a delay in disease progression of up to 21 months.2
The National Institute for Health and Care Excellence (NICE) approved riluzole for use in motor neurone disease in 2001, and the drug is freely available for diagnosed patients in the UK.3 Riluzole is also approved in the European Union and US for the treatment of ALS.
Challenges for ALS patients
More than 80% of ALS patients develop dysphagia, rendering the oral administration of riluzole tablets impossible.1,4 The inability of a patient to swallow a tablet formulation may result in poor patient compliance and early discontinuation of a life-prolonging treatment. Swallowing dysfunction and dysphagia however is a challenge for oral drug therapy.5 Swallowing is a complex function involving several nerves and muscles acting in a synchronized reflex mode upon voluntary initiation.
Manipulation of a solid oral drug product by a patient or caregiver, for example, by crushing of tablets to aid administration or ingestion, increases the likelihood of medication errors and promotes the possibility of incorrect and incomplete dosing, changes in drug product performance and safety concerns. It is important to recognize the potential consequences of manipulating a medicinal product. Changes to the way a dosage form is presented can impact its absorption characteristics, cause product instability, produce local irritant or anesthetic effects (particularly for riluzole), cause failure to reach the site of absorption, may produce occupational health and safety issues and could result in a preparation with an unacceptable taste and/or unpleasant mouthfeel.6 From a safety perspective, crushed tablets may expose carers or health care professionals to health risks due to dust inhalation, ingestion or non-oral absorption. Irritation may also arise as a consequence of powdered drug substance coming into contact with the skin, eyes or other mucous membranes.
National Health Service guidance for riluzole tablets states that tablets can be crushed and given in a spoonful of sugar, food puree or yoghurt, however that crushed tablets can have an anesthetic effect on the tongue.7 Such an undesirable and untoward side effect may result in a patient refusing or being unable to take his/her prescribed medication.
Crushing tablets for the purpose of administration via an enteral feeding tube typically falls outside of the scope of a marketing authorization. Under such circumstances, the prescriber and practitioner accept liability for any adverse events arising from administration. Potential issues of drug administration via an enteral feeding tube include cleanliness and infection control, tube blockage, incorrect or incomplete dosing and particularly in a community setting, safety of the carer. Applicable guidance states that tablet crushing should be considered as a last resort.8 Furthermore administration of crushed tablets to ALS patients exhibiting swallowing dysfunction or dysphagia may result in aspiration pneumonia and an increased risk of silent aspiration.4
A recent article highlights the many deficiencies of administering crushed tablets to patients.9
Until recently, riluzole was only available as an oral tablet dosage form; however, the first oral liquid form of riluzole (Teglutik®, novel riluzole oral suspension 5 mg/mL), has now been launched in the UK (Martindale Pharma Limited, Romford, Essex).10,11
Advantages and potential challenges of an oral liquid presentation of riluzole
The availability of an oral suspension of riluzole precludes the need for manipulation of tablets to facilitate administration. Riluzole oral suspension is presented as a 5 mg/mL palatable oral liquid formulation comprising finely divided particles of drug substance suspended in a thixotropic vehicle. The oral suspension utilizes controlled flocculation technology and exhibits pseudoplastic flow (shear thinning), meaning that the apparent viscosity of the vehicle is relatively high when the applied shear stress is low (ie, upon standing), but that the apparent viscosity decreases as the applied shear stress increases (ie, upon shaking). The novel composition of the aqueous vehicle is designed to minimize the local anesthetic effect of the drug in the mouth and the unpleasant metallic taste.
Riluzole oral suspension is supplied with a plastic graduated oral dosing syringe for accurate and reproducible dose administration for ALS patients. The syringe barrel is graduated in milliliters up to 10 mL, to provide enhanced flexibility of dosing. Each 1 mL of riluzole oral suspension contains 5 mg of active drug, allowing accurate incremental dosing up to 50 mg riluzole per 10 mL dose.12 Riluzole oral suspension is indicated for twice-daily administration, with a 10 mL dose exhibiting the consistency of single cream, which is thus easily ingested by ALS patients who may experience difficulties in managing thin liquids.1 In contrast, riluzole film-coated tablets are only available as a single-dose strength of 50 mg.13
Potential challenges of riluzole oral suspension may include the perception that oral liquids are intended “for children” and that some patients may not initially be expecting to be offered an oral liquid. In addition, some patients may not like having to manage 2×300 mL glass bottles of their medication each month. Conversely, these possible negatives are more than offset by having the utility of riluzole in a dosage form that patients can easily take and that caregivers can easily administer with no compromise on the effectiveness of the drug.
Product overview
Rationale for an aqueous suspension
Riluzole is a lipophilic drug substance with a low aqueous solubility. The drug is very slightly soluble in water at neutral pH (~0.3 mg/ml at pH 7), and although the solubility of the drug substance increases with decreasing pH (~12 mg/ml at pH 1.2), chemical stability decreases significantly under acidic conditions.14,15 An aqueous solution formulation of riluzole is therefore not feasible.
The solubility of riluzole may be increased by means of co-solvents or solubilizers to produce a physically and chemically stable drug solution. However, palatability is found to be significantly compromised, and the prolonged local anesthetic effect in the mouth (>20–30 minutes) is exacerbated as a consequence of the intrinsic properties of this drug substance in solution.14,15
Suspensions as drug delivery systems
A pharmaceutical suspension is a relatively complex disperse system in which insoluble particles, generally >1 μm in diameter, are dispersed in a liquid medium, usually aqueous. The small particle size and large surface area of dispersed drug particles ensures a high availability for dissolution and hence absorption.
An acceptable suspension should typically exhibit the following properties: the suspended material should not settle too rapidly; particles that do settle must not form a hard mass and should be readily dispersed upon shaking; and the suspension must not be too viscous to pour freely and provide uniform dosing.16
However, many insoluble solids are not easily wetted by water and exhibit varying degrees of hydrophobicity, especially when finely divided. Particles may form large porous clumps within the liquid, while others remain on the surface and may become attached to the container. Any foam produced on shaking may be slow to subside because of the stabilizing effect of the small particles at the liquid/air interface. To ensure adequate wetting, the interfacial tension between the solid and the liquid must be reduced so that absorbed air is displaced from solid surface by the liquid. The particles will then disperse readily through the liquid, particularly if intense shearing is used during mixing.16
Thus, key ingredients of suspensions are surfactants, substances that alter the conditions prevailing at an interface, causing, for example, a marked decrease in the surface and interfacial tension of water. These substances are of importance in a wide variety of fields as emulsifying agents, detergents, solubilizing agents, wetting agents, foaming and antifoaming agents and flocculants and deflocculants, and in drug stability and drug absorption.
All surfactants are characterized by having two regions in their molecular structure: a lyophobic (or hydrophobic) group, such as a hydrocarbon chain that has no affinity for aqueous solvent; and a lyophilic (or hydrophilic) group that has affinity for water. To have such an affinity, the group must possess an appreciable polar character, for example, an ion or group with a large permanent dipole. A molecule or ion that possesses this type of structure is termed amphipathic.16
The hydrocarbon chains of a water-dispersible surfactant are adsorbed to the hydrophobic particle surfaces, while the polar groups project into the aqueous medium becoming hydrated. Thus, wetting of solid will occur due to a fall in both the interfacial tension between the solid and the liquid, and to a lesser extent, between the liquid and air.
Even when dispersion has been achieved, suspensions may be disadvantaged by numerous factors such as excessive foaming, rapid separation or caking, taste issues caused by solubilization and change in bioavailability.
Formulation development of riluzole 5 mg/mL oral suspension
To successfully develop a suspension formulation, it is critical that the drug substance must comprise finely divided particles of appropriate particle size in order to produce a homogenous dispersion. This is achieved by the use of micron-sized drug particles in the suspension product. Riluzole is a highly hydrophobic material, particularly when presented as a finely divided powder. Excipient selection then becomes key to ensuring physical and chemical stability of riluzole oral suspension. The qualitative composition is provided in Table 1.12,17
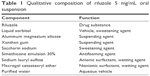  | Table 1 Qualitative composition of riluzole 5 mg/mL oral suspension |
Materials such as alcohol, glycerol and glycols that are water-miscible solvents will reduce the liquid/air interfacial tension. The riluzole suspension formulation contains sorbitol (D-glucitol), a hexahydric alcohol. Sorbitol will penetrate the loose agglomerates of drug particles displacing the air from the pores of the individual drug particles, thus enabling wetting to occur by the aqueous medium.
To further facilitate wetting of the drug substance and reduce the interfacial tension between the lipophilic solid and the hydrophilic vehicle, a combination of anionic and nonionic surfactants has been used.14,15
Sodium lauryl sulfate (anionic) and macrogol cetostearyl ether (nonionic) surfactants are used as wetting agents in appropriate concentrations in riluzole oral suspension. These surfactants are classified according to the nature of the ionic type of the hydrophilic group (Table 2).
As suspensions are thermodynamically unstable, dispersed particles tend to aggregate and/or sediment with time; in order to minimize settling and prevent caking of dispersed particles of riluzole, controlled flocculation has been implemented utilizing functional excipients to produce a stabilized suspension which is readily re-dispersed upon shaking. Flocculation is the process where suspended particles agglomerate to form loosely structured flocs, which are held together in a high-volume-network-like structure. Flocculated particles are therefore weakly bonded, and as such, they do not form a cake and are readily resuspended. The supernatant liquid is typically clear as colloidal particles are trapped within the flocs and sediment with them.
The hydrophilic colloid xanthan gum, a naturally occurring cellulose polymer, has been utilized in the patented formulation as a protective colloid, which coats the hydrophobic drug particles with a multimolecular layer. This layer imparts a hydrophilic character to the solid drug particles and promotes both wetting and suspension.
Controlled flocculation is dependent on a combination of particle size control, the use of electrolytes to control zeta potential and the addition of polymers to enable cross-linking between particles. Flocculation is typically achieved in part by neutralizing the charge of the suspended particles. Riluzole oral suspension thus remains in a controlled flocculated state, by the innovative combination of anionic and nonionic surfactants and hydrophilic polymers. Ionic surface-active agents cause flocculation by neutralization of the charge on each particle, whereas nonionic surfactants will have little effect on charge density, but because of their linear configurations, they may absorb onto more than one particle to promote a loose flocculated structure.
The suspending polymer xanthan gum also contributes to controlling the degree of flocculation. The polymer molecules promote a gel-like network within the system and adsorb onto the surface of the dispersed particles, thus holding them in a flocculated state. Although some settling is likely, the sedimentation volume remains large and can persist for a prolonged period.
An ideal pharmaceutical suspension will exhibit a high apparent viscosity at low rates of shear so that on storage, the particles either settle very slowly or remain permanently suspended. At higher rates of shear, such as those caused by moderate shaking, the apparent viscosity should fall sufficiently for the product to be readily and accurately dispensed.
A flocculated system, in part, meets these criteria, in that in such a system, pseudoplastic or plastic flow is exhibited as the structure progressively breaks down under shear. The product then shows time-dependent reversibility of the loss of structure, or thixotrophy. The inventors report that the chosen combination of excipients confers a high degree of flocculation.14,15
Suspending agents are used in riluzole oral suspension to impart viscosity to the vehicle and facilitate a stable dispersion by minimizing sedimentation and agglomeration of drug particles. Importantly, the viscosity of the suspension must be optimized to minimize sedimentation of drug particles under the static conditions of storage, while maintaining acceptable flow properties upon the application of shear in the form of gentle agitation. Such properties facilitate ease of resuspendability and dispensing of product by the patient and the absence of caking upon prolonged storage. However, controlled flocculation must be maintained, and the combination of suspending agents to enhance and preserve physical stability and flocculation is crucial.14,15 Riluzole oral suspension also contains sweetener (saccharin), antifoam and a further suspending agent aluminum magnesium silicate, which may also contribute advantages by associating with dissolved drug, thereby reducing the anesthetic effect.
Riluzole oral suspension is an innovative, physically and chemically stable aqueous liquid formulation, which has been developed utilizing expertise in pharmaceutical oral suspension technology and the design of optimized delivery systems for human administration, which is exemplified by a product shelf-life of 3 years, or 15 days after first opening.17
Clinical performance and bioequivalence of oral suspension
Riluzole oral suspension has been reported to exhibit a surprisingly favorable palatability profile compared with riluzole solution, indicating a significant advance especially in providing an effective treatment for ALS with potential for a high degree of patient acceptability and compliance.14,15
Although comprising dissolved drug and solid drug particles suspended in an aqueous vehicle, there results minimal or no anesthetic effect in the mouth upon oral administration.14,15 The unique and patented liquid suspension formulation of riluzole appears to minimize the local anesthetic effect of drug particles in the mouth by providing a protective barrier between the drug particles and the oral mucosa and tongue. Furthermore, this is in contrast to the presentation of crushed riluzole tablets that are noted to have a significant anesthetic effect on the tongue.6,10
Although the pathogenesis of ALS is not completely elucidated, it is suggested that glutamate (the primary excitatory neurotransmitter in the central nervous system) plays a role for cell death in the disease. Riluzole is proposed to act by inhibiting glutamate processes, although the mode of action is unclear. Riluzole oral suspension is indicated to extend life or the time to mechanical ventilation for patients with ALS. Clinical trials have demonstrated that riluzole extends survival for patients with ALS, with survival being defined as patients who were alive, not intubated for mechanical ventilation and tracheotomy-free.17
Riluzole tablets are rapidly absorbed after oral administration with maximal plasma concentrations occurring within 60–90 minutes (Cmax =173±72 [standard deviation] ng/mL). About 90% of the dose is absorbed, and the absolute bioavailability is 60%±18%. The rate and extent of absorption is reduced when riluzole is administered with high-fat meals (decrease in Cmax of 44%, decrease in AUC of 17%).17
In a bioequivalence study between novel riluzole oral suspension and Rilutek® (tablets), the total exposure of riluzole 50 mg tablets and riluzole 5 mg/mL oral suspension was equivalent. (ratio: 106.84%; 90% confidence interval [CI]: 96.98%–117.71%). Understandably, riluzole is more rapidly absorbed after the administration of oral suspension (Tmax approximately 30 minutes), with a Cmax approximately 20% higher than after the administration of riluzole tablets. (ratio: 122.32%; 90% CI: 103.28%–144.88%).17
The innovative technology employed in riluzole oral suspension clearly provides an opportunity for more accurate dosing and enhanced patient compliance compared with crushed tablets administered with food puree, yoghurt or via an enteral feeding tube. The suspension is readily administered by means of a graduated oral dosing syringe, and the concentration is consistent with a volume that is easy to measure and not burdensome. No product manipulation or premixing is required, and dilution with liquids is not necessary. Crucially, it is reported that there is minimal or no anesthetic effect in the mouth upon administration of the oral suspension.14,15
Conclusion
Riluzole is a disease-modifying treatment that has been shown to slow the course of ALS. Based on the recognized efficacy of riluzole, the demonstrated bioequivalence of riluzole oral suspension, reduced anesthesia in the mouth and the significant advantages of a liquid presentation exhibiting shear-thinning (pseudoplastic) behavior over tablets for ALS patients, riluzole 5 mg/mL oral suspension is the drug of choice. Teglutik® is a patented formulation that represents a significant therapeutic advancement for patients with ALS.
Disclosure
Ann Margaret Dyer is the sole director of PharmaSci Consulting Limited, and Alan Smith is an independent expert and a member of the Scientific Advisory Board of PharmaSci Consulting Limited. The authors are solely responsible for the article content. PharmaSci Consulting was invited by Martindale Pharma to conduct an independent evaluation of available riluzole formulations. The authors report no other conflicts of interest in this work.
References
Kühnlein P, Gdynia HJ, Sperfeld AD, et al. Diagnosis and treatment of bulbar symptoms in amyotrophic lateral sclerosis. Nat Clin Prac Neurol. 2008;4(7):366–374. | ||
Miller RG, Mitchell JD, Moore DH. Riluzole for amyotrophic lateral sclerosis (ALS)/motor neurone disease (MND). Cochrane Database Syst Rev. 2012;(3):CD001447. | ||
National Institute for Health and Care Excellence (NICE). Guidance on the use of riluzole (Rilutek) for the treatment of motor neurone disease. Technology appraisal guidance 20. 2001. Available from: https://www.nice.org.uk/guidance/ta20?unlid=4284488912016317234612. Accessed August 19, 2016. | ||
Keating GM. Riluzole oral suspension in amyotrophic lateral sclerosis: a guide to its use. Drugs Ther Perspect. 2016;32(7):282–286. | ||
Stegemann S, Gosch M, Breitkreutz J. Swallowing dysfunction and dysphagia is an unrecognized challenge for oral drug therapy. Int J Pharm. 2012;430(1–2):197–216. | ||
Royal Pharmaceutical Society of Great Britain. Pharmaceutical issues when crushing, opening or splitting oral dosage forms [updated June 2011]. Available from: https://www.rpharms.com/support-pdfs/pharmaceuticalissuesdosageformsjune-2011.pdf. Accessed July 29, 2016. | ||
Clifton M. NEEMMC guidelines for tablet crushing and administration via enteral feeding tubes. Colchester: National Health Service [updated April 2012]. Available from: http://www.stch.org.uk/wp-content/uploads/pct-version-neemmc-guidelines-for-tablet-crushing-april-2012.pdf. Accessed July 29, 2016. | ||
British Association for Parenteral and Enteral Nutrition (BAPEN). Administering drugs via enteral feeding tubes: a practical guide. Available from: http://www.bapen.org.uk/pdfs/d_and_e/de_pract_guide.pdf. Accessed August 19, 2016. | ||
Barnett N, Parmar P. Tailoring medication formulations for patients with dysphagia. Pharm J. 2016;297(7892):106–108. | ||
martindalepharma.co.uk/news [homepage on the Internet]. Martindale Pharma announces first oral liquid form of riluzole, TEGLUTIK®, launched in the UK. Romford: Martindale Pharma [cited December 18, 2015]. Available from: http://www.martindalepharma.co.uk/first-oral-liquid-form-of-riluzole-teglutik-launched-in-the-uk/. Accessed July 29, 2016. | ||
teglutik.co.uk [homepage on the Internet]. Romford: Martindale Pharma [updated May 2016]. Available from: http://www.teglutik.co.uk/. Accessed July 29, 2016. | ||
TEGLUTIK® (riluzole) 5 mg/mL oral suspension [patient information leaflet]. Romford: Martindale Pharma; 2015. | ||
BMJ Group and Royal Pharmaceutical Society. British National Formulary (BNF) 71. London: BMJ Group and Royal Pharmaceutical Society; 2016. | ||
Artico R, Adami M, Barbareschi D, Moscoso J, Oldoni T, Mascagni P, inventors; Italfarmaco SpA, assignee. Riluzole aqueous suspensions. European Patent EP 2405890 B1. Nov 28, 2012. | ||
Artico R, Adami M, Barbareschi D, Moscoso J, Oldoni T, Mascagni P, inventors; Italfarmaco SpA, assignee. Riluzole aqueous suspensions. United States patent US 8765150 B2. Jul 1, 2014. | ||
Aulton ME, Taylor KMG. Aulton’s Pharmaceutics: The Design and Manufacture of Medicines. 4th ed. New York: Churchill Livingstone; 2013. | ||
TEGLUTIK® (riluzole) 5 mg/mL oral suspension [summary of product characteristics]. Romford: Martindale Pharma Limited; 2015. |
 © 2016 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2016 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.

