Back to Journals » Drug Design, Development and Therapy » Volume 13
Renoprotective Effects Of Isoliquiritin Against Cationic Bovine Serum Albumin-Induced Membranous Glomerulonephritis In Experimental Rat Model Through Its Anti-Oxidative And Anti-Inflammatory Properties
Authors Liu Y, Xu X, Xu R, Zhang S
Received 23 April 2019
Accepted for publication 8 August 2019
Published 30 October 2019 Volume 2019:13 Pages 3735—3751
DOI https://doi.org/10.2147/DDDT.S213088
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 2
Editor who approved publication: Prof. Dr. Tin Wui Wong
Yingying Liu,1,* Xiaohua Xu,1,* Ruisi Xu,2 Siqi Zhang1
1Department of Nephrology, China-Japan Union Hospital of Jilin University, Changchun, Jilin 130033, People’s Republic of China; 2Endoscopy Center, China-Japan Union Hospital of Jilin University, Changchun, Jilin 130033, People’s Republic of China
*These authors contributed equally to this work
Correspondence: Siqi Zhang
Department of Nephrology, China-Japan Union Hospital of Jilin University, Changchun, Jilin 130033, People’s Republic of China
Tel +86 183 3112 5890
Email [email protected]
Ruisi Xu
Endoscopy Center, China-Japan Union Hospital of Jilin University, Changchun, Jilin 130033, People’s Republic of China
Tel +86 187 3220 0909
Email [email protected]
Background: Membranous glomerulonephritis (MGN) is a nephrotic syndrome which shows the symptoms of heavy proteinuria and immune complex deposition in glomerular sub-epithelial space and finally leads to chronic kidney disease. Isoliquiritin (ILQ) is a flavonoid with a wide range of pharmacological properties, including antioxidant and anti-inflammatory activity. The present study was undertaken to investigate the possible mechanisms by which ILQ ameliorates cationic bovine serum albumin (C-BSA) induced MGN in rat model.
Methods: The MGN condition was confirmed by the 24 hr proteinuria and ILQ (10 mg/kg/bw/day) or TPCA-1 (10 mg/kg/bw/day; IKKβ inhibitor) was administered to successfully induce rats for 4 weeks.
Results: The present study revealed that MGN rats treated with ILQ showed significantly ameliorated kidney dysfunction and histopathological changes in kidneys. ILQ treated MGN rats alleviated the oxidative stress and were presented with increased anti-oxidative status in kidneys. Furthermore, ILQ treatment to MGN rats showed anti-oxidative effects through the prominent stimulation of Nrf2 signaling pathway and inhibition of Keap1, which consequently increases the Nrf2 nuclear translocation and thereby induces expression of NQO1 and HO-1. In addition, ILQ-treated MGN rats demonstrated anti-inflammatory effects by inhibiting NF-κB signaling pathway through decreased mRNA and protein expressions of NF-κB p65, IKKβ, COX-2, iNOS, p38-MAPK, p-p38-MAPK, TNF-α, IL-1β, IL-8, ICAM-1, E-selectin and VCAM-1 and reduced the nuclear translocation of NF-κB p65.
Conclusion: The protective effect of ILQ on MGN can be explained by its anti-oxidative and anti-inflammatory activities, which in turn due to the activation of Nrf2 and downregulation of NF-κB pathway.
Keywords: isoliquiritin, membranous glomerulonephritis, oxidative stress, inflammation
Introduction
Membranous glomerulonephritis (MGN) is one of the major autoimmune-mediated nephrotic syndrome that affects mainly the adult population.1,2 MGN shows the symptoms of immune complex deposition in glomerular subepithelial space and heavy proteinuria.3,4 The major complications associated with MGN are chronic inflammation, oxidative stress, glomerular and tubulointerstitial lesions.5 Approximately, 30–40% of the MGN cases progress towards renal impairment and finally ends up with renal failure after 10–15 years.6 Previously, it has been reported that induced MGN animals presented with glomerular lesions,2 oxidative stress in terms of increased production of reactive oxygen species (ROS) and impaired production of anti-oxidant molecules7 and induction of inflammatory mediators.2 In addition, overproduction of ROS by inflammatory nephritis cells can cause tissue damage, intensify inflammation and aggravate nephritis progression.8 There is ample evidence that oxidative stress and its interrelationship with inflammation plays an important role in kidney injury.9 In recent years, the treatment of MGN with immunosuppressive drugs has been surfaced, but the treatment regimen is not that effective and also often associated with adverse effects.10–12 Hence, there is a quest for therapy which could provide an alternative for MGN. Nowadays, the usage of herbal originated bioactive compounds is pervasive due to its natural origin and less adverse effects.13
Isoliquiritin (ILQ) is one of the active constituents of traditional Chinese medicinal plant, Glycyrrhiza uralensis which has been used in herbal medicine for hundreds of years for the treatment of detoxification, swelling and injuries.14,15 ILQ is a flavonoid responsible for broad spectrum of pharmacological properties such as anti-neurotoxic,16 anti-depressant,17 anti-angiogenic,18 inhibition of p53-dependent pathway and proved crosstalk between Akt,19 phytoestrogenic,20 anti-genotoxic,21 anti-tumor,22 anti-fungal,23 anti-viral,24 anti-oxidant19,25 and anti-inflammatory16 activities. ILQ exerts its anti-oxidant property by an increase in the activities of superoxide dismutase and catalase and by decrease in the contents of ROS and malondialdehyde.16 Previous, Wang et al26 reported that ILQ suppresses the lipopolysaccharide (LPS) induced inflammatory responses in murine macrophages through the suppression of inflammatory mediators.
Considering the complications associated with MGN and pharmacological activities of ILQ, the present study is designed to evaluate the possible renoprotective effects of ILQ in C-BSA induced MGN using rat as an experimental model. To study the therapeutic effects of ILQ in induced-MGN rat model, several physiological, biochemical, molecular, immunological and histopathological parameters were studied with a special focus on the anti-oxidant and anti-inflammatory effects. The scope of the study is further broadened by studying the roles of Nrf-2 and NF-kB in mediating anti-oxidant and anti-inflammatory effects, respectively.
Materials And Methods
Chemicals
Isoliquiritin (ILQ) was purchased from Xi’an Xiaocao Botanical Development Co., Ltd. (purity >98%, Shanxi, China). The cationic bovine serum albumin (C-BSA) was bought from Chondrex, Inc. (Cat. No. 9058; Redmond, WA 98052, USA). Additionally, IKKβ inhibitor (2-[(aminocarbonyl)amino]-5-(4-fluorophenyl)-3-thiophenecarboxamide; TPCA-1) was purchased from Medchem Express (MCE Co. Ltd., Shanghai, China). All other chemicals and reagents used in the study were of analytical grade.
Animals
Adult male Sprague–Dawley (SD) rats, weighing between 225±20 g were purchased from Zhengzhou University, China. The animals were kept in polypropylene cages with controlled temperature (22 ± 2°C), light (12:12 hrs light:dark cycle) and relative humidity (55±10%). Animals were allowed ad libitum access to commercially available rodent feed and tap water. The experiments were conducted in accordance with the National Institutes of Health Guide for Laboratory Animals Care and Use and approved by the University Ethics Committee at Jilin University’s China-Japan Union Hospital, China.
Experimental Design
Initially, the animals were randomly divided into two groups, i.e. normal control group and experimental group. The experimental group of rats was daily injected intravenously with cationic C-BSA for 4 weeks. MGN was induced by the administration of a C-BSA regimen with a gradual dose increase from day 1 to day 7, i.e. 1 mg, 1 mg, 1 mg, 1.5 mg, 1.5 mg, 2 mg and 2 mg, and subsequently a constant dose of 2.5 mg was given for next 3 weeks. The MGN condition in rats was confirmed by the determination of 24 hrs proteinuria using Bradford assay kit (Sigma-Aldrich) and successfully induced rats were further taken for the main study. ILQ was dissolved in distilled water and TPCA-1 (IKKβ inhibitor) was dissolved in dimethyl sulfoxide (DMSO) and both drugs were administered to MGN rats orally with the help of oral gavage tube for 28 consecutive days. The rats were assigned to four groups of six animals in each as follows:
Group 1: Normal control rats administered with distilled water.
Group 2: MGN control rats administered with distilled water.
Group 3: MGN rats treated with ILQ at a dose of 10 mg/kg/day).17
Group 4: MGN rats treated with 10 mg/kg/day of TPCA-1 (IKKβ inhibitor).27
The dose was selected based on preliminary experiments using 10 and 20 mg/kg. The dose of 50 mg/kg had a significant renoprotective effect in induced-MGN rats as assessed by functional and histologic parameters and no further improvement was observed at 20 mg/kg. So, a dose of 50 mg/kg was taken for main study.
Sample Collection And Preparation
The day before the completion of experimental period, the rats were positioned in the metabolic cages in such a way to collect the urine for analysis. After experimental period, animals were sacrificed and blood was collected by cardiac puncture into separate tubes for serum separation. Immediately, kidneys were collected, weighed and stored in −80°C for further biochemical, molecular and histological analyses. Further, kidneys were fixed in 10% neutral formalin buffer for histological studies.
Determination Of Renal Functional Biomarkers
The commercially available colorimetric assay kits (Abnova Corporation, Taipei, Taiwan) were purchased to assess renal function, which includes proteinuria (Cat. No. 9040; Chondrex Inc., Redmond, WA 98052, USA), serum creatinine (Cat. No. KA0849), total cholesterol and triglycerides (Abnova Corporation). Blood urea nitrogen (BUN) was calculated by using the following formula:
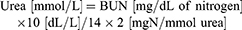
Determination Of Oxidative And Anti-Oxidative Status
The determination of lipid peroxidation products in kidney homogenate was done by using thiobarbituric acid reactive substances (TBARS) as described previously by Ohkawa et al.28 The oxidation products of nitric oxide (NO) were measured relative to the concentration of nitrite/nitrate according to the method given by Miranda et al.29 The activity levels of superoxide dismutase (SOD) were estimated as per previous method.30 Catalase (CAT) activity levels were estimated according to the protocol by the method described by Bonaventura et al.31 Moreover, glutathione peroxidase (GPx) activity levels were measured as per the method of Rotruck et al.32 The reduced glutathione (GSH) levels were determined spectrophotometrically following the method of Hissin and Hilf.33 The total protein levels were estimated by using Bradford protein assay kit (Sigma-Aldrich).
ELISA
The tumor necrosis factor-alpha (TNF-α; ab100785) and interleukin 1 beta (IL-1β; ab100768) levels were assessed in kidney homogenate by using ELISA kits (R&D Systems, Minneapolis, MN, USA) following manufacturer’s instructions. NF-κBp65 DNA binding activity was estimated by kit (Cat#40010, Active Motif, Carlsbad, CA, USA) in the nuclear extracts of kidney.
Quantitative Reverse Transcription Polymerase Chain Reaction (PCR) Analysis
Total RNA was extracted using a TRIzol® reagent, quantified using a Nanodrop 2000, and reverse transcription performed using cDNA synthesis kit (Applied Biosystems, Foster City, CA, USA).
The following sets of primers were used for RT-PCR. NAD(P)H:quinone oxidoreductase 1 (NQO-1): forward, 5′-GAGAAGAGCCCTGATTGTACTG-3′; reverse, 5′-ACCTCCCATCCTCTCTTCTT-3′; Heme oxygenase-1 (HO-1): forward, 5′-CTCCCTGTGTTTCCTTTCTCTC-3′; reverse, 5′-CTGCTGGTTTCAAAGTTCAG-3′; inhibitory kappa B kinase β (IKKβ): forward, 5′-CACCACAGAAGCACAACAATG-3′; reverse, 5′-CCCGCAGCAGTCCAAAGG-3′; interleukin-8 (IL-8): forward, 5′-TCAACGGGCAGAATCAAAGAG-3′; reverse 5′-CTCAGACAGCGAGGCACATC-3′; intercellular adhesion molecule 1 (ICAM-1): forward, 5′-CCTGTTTCCTGCCTCTGAA-3′; reverse, 5′-GTCTGCTGAGACCCCTCTTG-3′; vascular cell adhesion molecule 1 (VCAM-1): forward, 5′-TGACAAGTCCCCATCGTTGA-3′; reverse, 5′-ACCTCGCGACGGCATAATT-3′; E-selectin: forward, 5′- TGAACTGAAGGGATCAAGAAGACT-3′; reverse, 5′-GCCGAGGGACATCATCACAT-3′; monocyte chemoattractant protein-1 (MCP-1): forward, 5′-TCTCTTCCTCCACCACTATGCA-3′; reverse, 5′-GGCTGAGACAGCACGTGGAT-3′; β-actin: forward, 5′-GGTATCCTGACCCTGAAGTA-3′; reverse, 5′-CACACGCAGCTCATTGTAGA-3′. The RT-PCR was carried out in triplicates using SYBR GREEN PCR master mix (Invitrogen) on Applied Biosystems 7900 Fast Real-Time PCR System. The relative quantitation of mRNA expression was performed by 2−ΔΔCt method. Ct values for the housekeeping gene β-actin were first standardized within the sample before comparison across normal group samples.
Histopathological Analysis
The kidneys from normal and experimental group rats were fixed in 4% paraformaldehyde, processed, embedded in paraffin, sectioned to 5μm and then stained with hematoxylin and eosin (H&E). The sections were visualised under Leica DM 68 microscope and images were captured.
Immunohistochemistry
The kidney sections were deparaffinized, rehydrated and antigen retrieved with 10 mM citrate buffer (pH 6.0). Then, sections were blocked with bovine serum albumin (BSA) and allowed to incubate with primary antibody (Nrf2: ab62352; Keap1: ab218815; NQO1: ab28947; HO-1: ab13248; p38-MAPK: ab31828; p-p38-MAPK: ab4822; NF-κB p65: ab16502; IKKβ: ab124957; COX-2: ab15191; iNOS: ab15323; Abcam, Cambridge, UK) at a dilution of 1:100 in 5% bovine serum albumin (BSA). After 3 times washing with phosphate buffer saline (PBS), the sections were incubated with appropriate biotinylated secondary antibody for 1 hr and the immunoreaction was identified after incubation with 3,3′-diaminobenzidine (DAB). The sections were counterstained with hematoxylin to identify cell nuclei and the slides were mounted ones to observe and capture the images.
Protein Extraction And Western Blotting
Nuclear and cytoplasmic extracts were prepared as previously described by Jain et al.34 The protein samples of 30μg were then mixed with loading dye and boiled for 10 mins and subjected to electrophoresis in 12% sodium dodecyl sulfate-polyacrylamide gel (SDS-PAGE) and transferred onto polyvinylidene difluoride (PVDF) membrane at 120 V for 1.5 hrs. The membranes were blocked with 5% BSA for 1 hr and then probed overnight at 4°C with primary antibody at 1:10,000 in PBST. The membranes were washed 3 times with PBST and then incubated with secondary antibody at room temperature for 1 hr. The blots were washed and visualized by Colorimetric Western Blotting Kit (Sigma-Aldrich). The signals were washed 3 times with PBST and then visualized under ChemiDoc™ Imaging System (Bio-Rad). The results were normalized by β-actin content and were expressed as arbitrary units.
Data Analysis
Data are the mean of ± S.D. One-way ANOVA followed by Tukey post hoc test was used to compare differences between control and experimental groups. To denote statistical significance, the p value less than or equal to 0.05 was used.
Results
Effects Of ILQ On Body Weight And Kidney Indices In MGN Rats
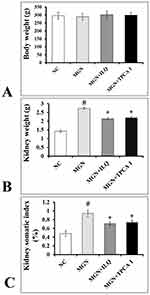
No significant difference (p > 0.05) was observed in the body weights of experimental rats when compared to control rats (Figure 1A). Conversely, a significant increase in kidney weights (p < 0.01; Figure 1B) and its somatic indices (p < 0.01; Figure 1C) were observed in MGN untreated rats when compared to control rats. Treatment with ILQ or IKKβ inhibitor (TPCA-1) to MGN rats significantly reduced the weights (p < 0.01) and somatic indices (p < 0.01) of kidney.
Effects Of ILQ On Biochemical Parameters In MGN Rats
The MGN untreated rats were presented with significantly increased levels of urinary protein (p < 0.001; Figure 2A), serum creatinine (p < 0.001; Figure 2B), BUN (p < 0.001; Figure 2C), adiponectin (p < 0.01; Figure 2D), total cholesterol (p < 0.001; Figure 2E), serum triglycerides (p < 0.01; Figure 2F) when compared to controls. While MGN rats treated with either ILQ or TPCA-1 significantly reduced the urinary protein (p < 0.01), serum creatinine (p < 0.01), BUN (p < 0.01), adiponectin (p < 0.01), total cholesterol (p < 0.01), serum triglycerides (p < 0.01) levels when compared to MGN untreated rats.
Effects Of ILQ On Histopathological In Kidney Of MGN Rats
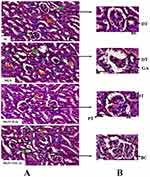
Histological analysis by hematoxylin and eosin (Figure 3A and B; H&E), pictures indicated control rats had normal renal tissue morphology with intact basement membranes of glomerular capillaries and tubular epithelium, clear Bowman’s capsule space with parietal epithelial cells and podocytes, normal size mesangium. While, MGN untreated rats were presented with thick glomerular basement membrane, glomerular atrophy. However, ILQ or TPCA-1 treatment to MGN rats clearly attenuated the severe pathological condition to mild pathological changes in the mesangium and interstitium.
Effects Of ILQ On Nrf2 Signaling Pathway In Kidney Of MGN Rats
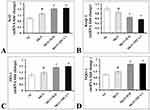
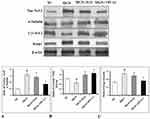
The gene expression data of Nrf2 signaling pathway are presented in Figure 4. The results have shown that the renal mRNA expression levels of Nrf2 (Figure 4A; p < 0.01), HO-1 (Figure 4C; p < 0.01), NQO1 (Figure 4D; p < 0.01) levels were slightly upregulated with downregulation in the mRNA expression levels of Keap1 (Figure 4B; p < 0.01) in MGN untreated group of rats when compared with control group of rats. In addition, a further conspicuous up-regulation in the renal Nrf2, HO-1, NQO1 mRNA levels with down-regulation of Keap1 mRNA levels was observed in MGN rats treated with ILQ or TPCA-1 when compared to MGN untreated rats. The results were mirrored by immunohistochemistry studies, which revealed that significantly increased protein distribution of Nrf2 (Figure 5A and E; p < 0.01), HO-1 (Figure 5C and G; p < 0.01) and NQO1 (Figure 5D and H; p < 0.01) proteins and decreased distribution of Keap1 (Figure 5B and F; p < 0.01) protein in glomerular and tubular regions of kidney in MGN untreated rats. While treatment with ILQ or TPCA-1 to MGN rats showed prominent distribution of Nrf2, HO-1 and NQO1 proteins with lesser Keap1 distribution in glomerular and tubular region of kidney as compared with that of MGN untreated rats.
The present study results confirmed that a significantly increased nuclear Nrf2 (Figure 6A; p < 0.01) and Keap1 (Figure 6C; p < 0.01) protein expression with decreased cytosolic protein expressions of Nrf2 (Figure 6B; p < 0.01) protein expression in kidney of MGN untreated rats. However, ILQ and TPCA-1 treatment results in a further downregulation in the nuclear Nrf2 and Keap1 (Figure 6A; p < 0.001 and Figure 6C; p < 0.001) and upregulation in the cytosolic Nrf2 (Figure 6B; p < 0.001) protein expression.
Effects Of ILQ On Renal Oxidant And Renal Anti-Oxidant Levels In MGN Rats
The renal lipid peroxidation products MDA (Figure 7A; p < 0.001) and NO (Figure 7B; p < 0.001) levels were significantly increased in MGN untreated rats compared to normal control rats. On the other hand, activity levels of renal SOD (Figure 7C; p < 0.001), CAT (Figure 7D; p < 0.001), GPx (Figure 7E; p < 0.001) and levels of GSH (Figure 7F; p < 0.001) were significantly decreased in MGN untreated rats. While ILQ as well as TPCA-1 treated MGN rats showed significantly reduced levels of MDA (p < 0.01) and NO (p < 0.01) associated with significantly increased activity levels of SOD (p < 0.05), CAT (p < 0.05), GPx (p < 0.01) and levels of GSH (p < 0.01) when compared to MGN untreated rats.
Effects Of ILQ On Inflammatory Markers In Kidney Of MGN Rats
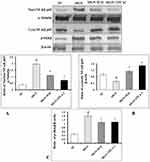
The mRNA expression levels of renal NF-κB p65 (Figure 8A; p < 0.001), IKKβ (Figure 8B; p < 0.001), TNF-α (Figure 8C; p < 0.001), IL-1β (Figure 8D; p < 0.001), IL-8 (Figure 8E; p < 0.001), ICAM-1 (Figure 8F; p < 0.001), VCAM-1 (Figure 8G; p < 0.001), MCP-1 (Figure 8H; p < 0.001) and E-selectin (Figure 8I; p < 0.001) were significantly upregulated in MGN untreated rats in comparison with control rats. The MGN rats treated with either ILQ or TPCA-1 exhibited significant downregulation in the mRNA expression levels of renal NF-κB p65 (p < 0.01), IKKβ (p < 0.05), TNF-α (p < 0.01), IL-1β (p < 0.05), IL-8 (p < 0.01), ICAM-1 (p < 0.01), VCAM-1 (p < 0.01), MCP-1 (p < 0.001) and E-selectin (p < 0.01) when compared to MGN untreated rats.
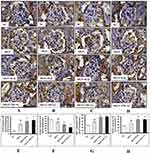
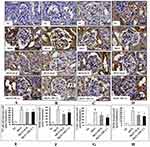
The immunohistochemistry results confirmed that the higher expression levels of NF-κB p65 (Figure 9A and E), IKKβ (Figure 9B and F), COX-2 (Figure 9C and G) and iNOS (Figure 9D and H) in glomerular and tubular region of kidney in MGN untreated rats when compared to control rats. The Western blot analysis results also confirmed that decreased cytosolic (Figure 10B) and increased nuclear (Figure 10A) NF-κB p65 protein levels along with increased p-IKKβ (Figure 10C) protein expression level in kidney of MGN untreated rats when compared with control rats. However, MGN rats treated with either ILQ or TPCA-1 displayed significantly lower NF-κB p65, IKKβ, COX-2 and iNOS protein levels and lesser distribution levels in glomerular region of kidney when compared to MGN untreated rats. Also, increased cytosolic and decreased nuclear NF-κB p65 protein levels with decreased IKKβ protein expression levels were observed in kidney of MGN rats treated with ILQ or TPCA-1 when compared with MGN untreated rats.
Effects Of ILQ On P38 MAPK And P-P38 MAPK Expression Levels In Kidney Of MGN Rats
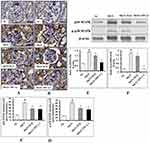
Immunohistochemistry analysis revealed that p38-MAPK (Figure 11A and C) and p-p38-MAPK (Figure 11B and D) proteins distribution level were highly (p < 0.001) expressed in glomerular region of kidney in MGN untreated rats. Whereas treatment with ILQ or TPCA-1 to MGN rats showed lower distribution levels of proteins p38-MAPK (p < 0.05) and p-p38-MAPK (p < 0.01) in glomerular and tubular region of kidney when compared with MGN untreated rats. Western blotting results to further confirm that p38-MAPK (Figure 11E; p < 0.01) and p-p38-MAPK (Figure 11F; p < 0.01) protein levels were significantly higher in MGN untreated rats when compared with control rats. On the other hand, ILQ or TPCA-1 treated MGN rats showed lower expression levels of p38-MAPK (p < 0.001) and p-p38-MAPK (p < 0.001) when compared with MGN untreated rats.
Discussion
The present work has been undertaken to investigate the potential benefits of isoliquiritin (ILQ), an active constituent isolated from the Glycyrrhiza uralensis plant in membranous glomerulonephritis (MGN). Previously, it has been demonstrated the anti-oxidant, free radical scavenging and anti-inflammatory properties of ILQ. In the current study, C-BSA induced MGN rat model was used to demonstrate the therapeutic efficacy of ILQ. To the best of our knowledge, this is the first study to demonstrate that ILQ significantly ameliorates the proteinuria and pathological changes of MGN rat. Furthermore, the results of this study indicated that ILQ’s renoprotective effects on experimental MGN are at least in part due to the activation of Nrf2 antioxidant pathway and inhibition of NF-κB inflammatory responses.
In the present study, body weights of the animals are not significantly altered in any of the groups indicating no systemic toxicity. Whereas the weights of kidney and somatic indices were significantly increased in MGN rats when compared to control rats. The increase might be due to the inflammatory effects observed in the study. Similar results were observed in rats induced with membranous nephropathy.35 On the other hand, treatment with ILQ or TPCA-1 significantly reduced the kidney weights and its somatic indices when compared to MGN rats.
The aetiology for any pathologic condition in nephrotic syndrome is the increased glomerular permeability to proteins. Hence, the proteinuria is commonly considered to be as a sensitive indicator for MGN.36 Further, creatinine and BUN give valuable information regarding the glomerular filtration rate as a measure of kidney function. In the present study, the MGN rats were presented with significantly increased levels of urinary protein, serum creatinine and BUN when compared to control rats. Treatment of ILQ or TPCA-1 markedly reduced proteinuria, serum creatinine and BUN when compared to MGN rats. Generally, proteinuria is commonly associated with dyslipidemia as loss of even small levels of urinary protein can alter the lipid levels.37 The results of the present study also revealed a significant elevation in the levels of serum cholesterol and triglycerides in MGN rats when compared to controls. The results are in consonance with the earlier reports.35 Treatment with ILQ or TPCA-1 significantly improved the MGN induced hypercholesterolemia and hypertriglyceridemia in rats. The improvement of dyslipidemia after renal injury in MGN rats might be due to the amelioration of proteinuria in ILQ or TPCA-1 treated rats.
Previously, it was reported that hyperlipidemia can aggravate the progression of kidney damage through oxidative stress.38 The increased ROS generation plays a major role in MGN pathogenesis.35 In the current study, a significant increase in MDA and NO levels associated with a significant decrease in SOD, CAT, GPx and GSH activity was observed in the MGN rats kidney compared to control rats. The overproduction of ROS and reactive nitrogen species (RNS) by inflammatory cells in nephritis can intensity inflammation thereby causes tissue damage and ultimately progress towards nephritis.39 The treatment of MGN rats with ILQ or TPCA-1 significantly reduced the renal levels of MDA and NO and significantly increased the activities of SOD, CAT, GPx and levels of GSH when compared to MGN rats.
However, the mechanisms by which ILQ shows its anti-oxidant activity against MGN are still remained to be elucidated. There is a large body of evidence that Nrf2, an essential transcription factor involved in the regulation of antioxidant responsive element (ARE) DNA sequences of Phase II anti-oxidant genes that includes a battery of genes such as NAD(P)H dehydrogenases (NQOs) and HO-1.40,41 Under normal conditions, Nrf2 is negatively regulated by Keap1 in the cytoplasm. Upon activation, Nrf2 dissociates from Keap1 and subsequently translocates to the nucleus,42 dimerizes with Maf protein and binds with cis-acting ARE and thereby regulates genes such as NQOs and HO-1.41 In the present study, MGN rats showed slightly increased mRNA and protein expression levels of Nrf2 and its targeted genes NQO1 and HO-1 along with decreased mRNA and protein expression levels of Keap1 when compared to controls in kidney. The results are in agreement with the observations made by Sutariya, Saraf43 Additionally, treatment of either ILQ or TCPA-1 to MGN rats significantly upregulated the renal mRNA and protein expression levels of Nrf2 and its targeted genes NQO1 and HO-1 with downregulation in the mRNA and protein expression levels of Keap1 compared with that of MGN control rats. These findings are supported by the recent study which shows ILQ treatment induces the expression of NQO1 and HO-1 through the Keap1–Nrf2 pathway.26 So, when Nrf2 is activated, it can translocate to the nucleus and regulates the battery of genes such as NQO1 and HO-1. The results of the present study also revealed an upregulation in the nuclear protein expression of Nrf2 than cytosolic Nrf2 expression with a decreased protein expression of Keap-1 in the cytosol. Thus, the antioxidant activity of ILQ may be associated with activation of Nrf2 signaling pathway through the downregulation of Keap-1, nuclear translocation of Nrf2 and an elevated expression of Nrf2 regulated genes such as NQO1 and HO-1.
The two important pathophysiological processes involved in the regulation of oxidative stress and inflammation are Nrf2 and NF-κB, respectively. Earlier it was reported that Nrf2 signaling pathway could modulate the overproduction of pro-inflammatory cytokines and chemokines and thereby inhibits the activation of NF-κB.44 In the resting state, NF-κB resides in the cytoplasm by attaching to inhibitor of alpha (IκBα) protein, but on stimulation of specific stimulants, such as ILQ, IκBα gets phosphorylated by IKKβ and undergoes proteosomal degradation and then NF-κB translocates to the nucleus to induce the expression of various inflammatory cytokines, chemokines, iNOS and COX-2.26 To test this hypothesis, IKKβ inhibitor, i.e. TPCA-1 was also included in the study. In MGN-treated rats, an upregulated renal mRNA expression levels of NF-κB p65, IKKβ, TNF-α, IL-1β and IL-8 and protein expressions of NF-κB p65, IKKβ, COX-2 and iNOS were observed in comparison with that of control rats. Conversely, treatment of MGN rats with either ILQ or TPCA-1 significantly reduced the mRNA expression levels of NF-κB, IKKβ, TNF-α, IL-1β and IL-8 and protein expression levels of NF-κB p65, IKKβ, COX-2 and iNOS when compared to only C-BSA induced MGN rats. Also, Western blot analysis revealed an upregulated expression of nuclear NF-κB and cytosolic IKKβ protein expressions with downregulated protein expression of cytosolic NF-κB in MGN rats. However, ILQ- or TPCA-1-treated MGN rats showed downregulation in the protein expressions of nuclear NF-κB and cytosolic IKKβ protein expressions in association with an upregulated protein expression of cytosolic NF-κB compared to MGN rats. From the results, it is evident that the anti-inflammatory responses of ILQ or TPCA-1 are mediated through the downregulation of IKKβ and reduced nuclear translocation of NF-κB to limit the expression of different inflammatory mediators.
The MAPKs have been implicated in the regulation of cellular growth and differentiation and plays a critical role in the control of the plethora of cellular response to cytokines and stresses. Accumulating evidence also suggests that MAPKs are involved in the activation of NF-κB pathway, which results in the overexpression of inflammatory mediators.45 Interestingly, various animal models with inhibition in the p38, mitigated the pro-inflammatory cytokines suggesting P38 a suitable candidate for targeted inhibition of inflammation.46 The results of the present study indicated that expression levels of p38 MAPK and p-p38 MAPK were upregulated in MGN rats when compared to that of control rats. Moreover, ILQ or TPCA-1 treatment to MGN rats significantly reduced the renal mRNA and protein expression levels of p38 MAPK and p-p38 MAPK when compared with MGN rats. In this study, the observed downregulation in the expression levels of iNOS, COX-2, TNF-α, IL-1β and IL-8 after treatment with either ILQ or TPCA-1 was linked to the inhibition of IKKβ/NF-κB and MAPKs pathways.
Previous reports suggested that various intercellular adhesion molecules were increasingly expressed upon activation of inflammatory signalling pathways.47 In the current study, an upregulated mRNA expression levels of ICAM-1, VCAM-1, E-selectin and MCP-1 were observed in MGN rats when compared to control rats. On the other hand, ILQ or TPCA-1 treatment downregulated the mRNA expression levels of ICAM-1, VCAM-1, E-selectin and MCP-1 indicating the inhibition of MAPKs activation and attenuation of inflammation.
In this study, the kidney histopathological evaluation revealed diffuse thickening of glomerular basement membrane with tubular atrophy in MGN rats. Whereas treatment of ILQ to MGN rats improved the pathological changes from severe to mild pathological changes in mesangium and interstitium. The improvement in the pathological changes might be due to the antioxidant and anti-inflammatory activities.
To conclude, the present study showed that the therapeutic effect of ILQ in rats on C-BSA induced MGN. The therapeutic effects are associated with its ability to upregulate the Nrf2 signalling pathway against oxidative damage and to inhibit the inflammation by downregulating the NF-κB signalling pathway. The present study demonstrates that ILQ has mitigated kidney impairment in C-BSA induced MGN rats through its anti-oxidant and anti-inflammatory effects through orchestrated Nrf2 and NF-κB signalling cascades. These findings strongly suggest that ILQ is a promising candidate against damage to the kidney during MGN condition. A treatment regimen involving ILQ could be considered as a potential new therapeutic intervention for MGN in the future. However, this non-clinical research further warrants clinical studies to apply these results in humans.
Disclosure
The authors report no conflicts of interest in this work.
References
1. Lai WL, Yeh TH, Chen PM, et al. Membranous nephropathy: a review on the pathogenesis, diagnosis, and treatment. J Formos Med Assoc. 2015;114(2):102–111. doi:10.1016/j.jfma.2014.11.002
2. Wu J, Liu B, Liang C, et al. Zhen-wu-tang attenuates cationic bovine serum albumin-induced inflammatory response in membranous glomerulonephritis rat through inhibiting AGEs/RAGE/NF-kappaB pathway activation. Int Immunopharmacol. 2016;33:33–41. doi:10.1016/j.intimp.2016.01.008
3. Huang YM, Zhou HR, Zhang L, Yang KK, Luo JX, Zhao HL. Spontaneous remission of membranous glomerulonephritis with successful fetal outcome: a case report and literature review. Medicine. 2016;95(26):e4022. doi:10.1097/MD.0000000000004864
4. Steyaert S, Van Dorpe J, Hoorens A, Van Biesen W, Van Laecke S. Intravenous immunoglobulins modify relapsing membranous glomerulonephritis after kidney transplantation: a case report. Acta Clin Belg. 2018;73(3):229–232. doi:10.1080/17843286.2017.1361622
5. Song J, Wang Y, Liu C, et al. Cordyceps militaris fruit body extract ameliorates membranous glomerulonephritis by attenuating oxidative stress and renal inflammation via the NF-kappaB pathway. Food Funct. 2016;7(4):2006–2015. doi:10.1039/C5FO01017A
6. Hladunewich MA, Troyanov S, Calafati J, Cattran DC. The natural history of the non-nephrotic membranous nephropathy patient. Clin J Am Soc Nephrol. 2009;4(9):1417–1422. doi:10.2215/CJN.01330209
7. Li Y, Yan M, Yang J, et al. Glutathione S-transferase Mu 2-transduced mesenchymal stem cells ameliorated anti-glomerular basement membrane antibody-induced glomerulonephritis by inhibiting oxidation and inflammation. Stem Cell Res Ther. 2014;5(1):19. doi:10.1186/scrt408
8. Shah SV, Baliga R, Rajapurkar M, Fonseca VA. Oxidants in chronic kidney disease. J Am Soc Nephrol. 2007;18(1):16–28. doi:10.1681/ASN.2006050500
9. Rojas-Rivera J, Ortiz A, Egido J. Antioxidants in kidney diseases: the impact of bardoxolone methyl. Int J Nephrol. 2012;2012:321714. doi:10.1155/2012/321714
10. Cattran D. Management of Membranous Nephropathy: When and What for Treatment. 2005.
11. Glassock RJ. The treatment of idiopathic membranous nephropathy: a dilemma or a conundrum? Am J Kidney Dis. 2004;44(3):562–566.
12. Du Buf-Vereijken PW, Branten AJ, Wetzels JF. Idiopathic membranous nephropathy: outline and rationale of a treatment strategy. Am J Kidney Dis. 2005;46(6):1012–1029. doi:10.1053/j.ajkd.2005.08.020
13. Lavecchia T, Rea G, Antonacci A, Giardi MT. Healthy and adverse effects of plant-derived functional metabolites: the need of revealing their content and bioactivity in a complex food matrix. Crit Rev Food Sci Nutr. 2013;53(2):198–213. doi:10.1080/10408398.2010.520829
14. Jeong SJ, Lim HS, Seo CS, et al. Traditional herbal formula Jakyakgamcho-tang (Paeonia lactiflora and Glycyrrhiza uralensis) impairs inflammatory chemokine production by inhibiting activation of STAT1 and NF-kappaB in HaCaT cells. Phytomedicine. 2015;22(2):326–332. doi:10.1016/j.phymed.2014.12.002
15. Yin L, Guan E, Zhang Y, et al. Chemical profile and anti-inflammatory activity of total flavonoids from glycyrrhiza uralensis fisch. Iran J Pharm Res. 2018;17(2):726–734.
16. Zhou YZ, Li X, Gong WX, et al. Protective effect of isoliquiritin against corticosterone-induced neurotoxicity in PC12 cells. Food Funct. 2017;8(3):1235–1244. doi:10.1039/C6FO01503D
17. Wang W, Hu X, Zhao Z, et al. Antidepressant-like effects of liquiritin and isoliquiritin from Glycyrrhiza uralensis in the forced swimming test and tail suspension test in mice. Prog Neuropsychopharmacol Biol Psychiatry. 2008;32(5):1179–1184. doi:10.1016/j.pnpbp.2007.12.021
18. Kobayashi S, Miyamoto T, Kimura I, Kimura M. Inhibitory effect of isoliquiritin, a compound in licorice root, on angiogenesis in vivo and tube formation in vitro. Biol Pharm Bull. 1995;18(10):1382–1386. doi:10.1248/bpb.18.1382
19. Zhou Y, Ho WS. Combination of liquiritin, isoliquiritin and isoliquirigenin induce apoptotic cell death through upregulating p53 and p21 in the A549 non-small cell lung cancer cells. Oncol Rep. 2014;31(1):298–304. doi:10.3892/or.2013.2849
20. Kang K, Ra J, Jo E. Phytoestrogenic composition comprising an extract of chinese licorice root, liquiritin or isoliquiritin. Google Patents. 2005.
21. Kaur P, Kaur S, Kumar N, Singh B, Kumar S. Evaluation of antigenotoxic activity of isoliquiritin apioside from Glycyrrhiza glabra L. Toxicol In Vitro. 2009;23(4):680–686. doi:10.1016/j.tiv.2009.01.019
22. Yang R, Wang L-Q, Liu Y. Antitumor activities of widely-used Chinese Herb – licorice. Chin Herbal Med. 2014;6(4):274–281. doi:10.1016/S1674-6384(14)60042-3
23. Luo J, Li Z, Wang J, Weng Q, Chen S, Hu M. Antifungal activity of isoliquiritin and its inhibitory effect against peronophythora litchi Chen through a membrane damage mechanism. Molecules. 2016;21(2):237. doi:10.3390/molecules21020237
24. Fukuchi K, Okudaira N, Adachi K, et al. Antiviral and antitumor activity of licorice root extracts. In Vivo. 2016;30(6):777–785.
25. Sun YX, Tang Y, Wu AL, et al. Neuroprotective effect of liquiritin against focal cerebral ischemia/reperfusion in mice via its antioxidant and antiapoptosis properties. J Asian Nat Prod Res. 2010;12(12):1051–1060. doi:10.1080/10286020.2010.535520
26. Wang R, Zhang CY, Bai LP, et al. Flavonoids derived from liquorice suppress murine macrophage activation by up-regulating heme oxygenase-1 independent of Nrf2 activation. Int Immunopharmacol. 2015;28(2):917–924. doi:10.1016/j.intimp.2015.03.040
27. Schopf L, Savinainen A, Anderson K, et al. IKKbeta inhibition protects against bone and cartilage destruction in a rat model of rheumatoid arthritis. Arthritis Rheum. 2006;54(10):3163–3173. doi:10.1002/art.22081
28. Ohkawa H, Ohishi N, Yagi K. Assay for lipid peroxides in animal tissues by thiobarbituric acid reaction. 1979;95(2):351–358. doi:10.1016/0003-2697(79)90738-3
29. Miranda KM, Espey MG, Wink DA. A rapid, simple spectrophotometric method for simultaneous detection of nitrate and nitrite. Nitric Oxide. 2001;5(1):62–71. doi:10.1006/niox.2000.0319
30. Misra HP, Fridovich I. The role of superoxide anion in the autoxidation of epinephrine and a simple assay for superoxide dismutase. J Biol Chem. 1972;247(10):3170–3175.
31. Bonaventura J, Schroeder W, Fang SJ. Human erythrocyte catalase: an improved method of isolation and a reevaluation of reported properties. Arch Biochem Biophys. 1972;150(2):606–617. doi:10.1016/0003-9861(72)90080-x
32. Rotruck JT, Pope AL, Ganther HE, Swanson A, Hafeman DG, Hoekstra WJS. Selenium: biochemical role as a component of glutathione peroxidase. Science. 1973;179(4073):588–590. doi:10.1126/science.179.4073.588
33. Hissin PJ, Hilf R. A fluorometric method for determination of oxidized and reduced glutathione in tissues. Anal Biochem. 1976;74(1):214–226. doi:10.1016/0003-2697(76)90326-2
34. Jain AK, Bloom DA, Jaiswal AK. Nuclear import and export signals in control of Nrf2. J Biol Chem. 2017;292(5):2052. doi:10.1074/jbc.A117.502083
35. Sutariya B, Taneja N, Saraf M. Betulinic acid, isolated from the leaves of Syzygium cumini (L.) Skeels, ameliorates the proteinuria in experimental membranous nephropathy through regulating Nrf2/NF-kappaB pathways. Chem Biol Interact. 2017;274:124–137. doi:10.1016/j.cbi.2017.07.011
36. Taal MW, Brenner BM. Renal risk scores: progress and prospects. Kidney Int. 2008;73(11):1216–1219. doi:10.1038/ki.2008.36
37. Levy J. Proteinuria, renal impairment, and death. BMJ. 2006;332(7555):1402–1403. doi:10.1136/bmj.332.7555.1402
38. Trevisan R, Dodesini AR, Lepore GJ. Lipids and renal disease. J Am Soc Nephrol. 2006;17(4 suppl 2):S145–S147. doi:10.1681/ASN.2005121320
39. Shah SV, Baliga R, Rajapurkar M, Fonseca VAJ. Oxidants in chronic kidney disease. J Am Soc Nephrol. 2007;18(1):16–28. doi:10.1681/ASN.2006050500
40. Rada P, Rojo AI, Chowdhry S, McMahon M, Hayes JD, Cuadrado A. SCF/{beta}-TrCP promotes glycogen synthase kinase 3-dependent degradation of the Nrf2 transcription factor in a Keap1-independent manner. Mol Cell Biol. 2011;31(6):1121–1133. doi:10.1128/MCB.01204-10
41. Hirotsu Y, Katsuoka F, Funayama R, et al. Nrf2-MafG heterodimers contribute globally to antioxidant and metabolic networks. Nucleic Acids Res. 2012;40(20):10228–10239. doi:10.1093/nar/gks827
42. Kansanen E, Kuosmanen SM, Leinonen H, Levonen AL. The Keap1-Nrf2 pathway: mechanisms of activation and dysregulation in cancer. Redox Biol. 2013;1:45–49. doi:10.1016/j.redox.2012.10.001
43. Bloomfield MG, Page M, McLachlan AG, Studd RC, Blackmore TK. Routine ertapenem prophylaxis for transrectal ultrasound guided prostate biopsy does not select for carbapenem resistant organisms: a prospective cohort study. J Urol. 2017;198(2):362–368. doi:10.1016/j.juro.2017.03.077
44. Ahmed SMU, Luo L, Namani A, Wang XJ, Tang X. Nrf2 signaling pathway: pivotal roles in inflammation. Biochimica Et Biophysica Acta Mol Basis Dis. 2017;1863(2):585–597. doi:10.1016/j.bbadis.2016.11.005
45. Jiang S-Y, Zou Y-Y, Wang J-T. p38 mitogen-activated protein kinase-induced nuclear factor kappa-light-chain-enhancer of activated B cell activity is required for neuroprotection in retinal ischemia/reperfusion injury. 2012;18(2096).
46. Ten Hove T, van Den Blink B, Pronk I, Drillenburg P, Peppelenbosch M, van Deventer SJ. Dichotomal role of inhibition of p38 MAPK with SB 203580 in experimental colitis. Gut. 2002;50(4):507–512. doi:10.1136/gut.50.4.507
47. Sans E, Delachanal E, Duperray A. Analysis of the roles of ICAM-1 in neutrophil transmigration using a reconstituted mammalian cell expression model: implication of ICAM-1 cytoplasmic domain and Rho-dependent signaling pathway. J Immunol. 2001;166(1):544–551. doi:10.4049/jimmunol.166.1.544
 © 2019 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2019 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.