Back to Journals » Drug Design, Development and Therapy » Volume 9
Regulation of bone formation by baicalein via the mTORC1 pathway
Authors Li S, Tang J, Chen J, Zhang P, Wang T, Chen T, Yan B, Huang B, Wang L, Huang M, Zhang Z, Jin D, Luo H
Received 25 January 2015
Accepted for publication 31 March 2015
Published 9 September 2015 Volume 2015:9 Pages 5169—5183
DOI https://doi.org/10.2147/DDDT.S81578
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 3
Editor who approved publication: Professor Shu-Feng Zhou
Sheng-fa Li,1,2,* Jia-jun Tang,1,2,* Jian Chen,1–3,* Pei Zhang,4,* Ting Wang,5 Tian-yu Chen,1,2 Bo Yan,1,2 Bin Huang,1,2 Liang Wang,1,2 Min-jun Huang,1,2 Zhong-min Zhang,1,2 Da-di Jin1,2
1Academy of Orthopedics of Guangdong Province, Guangzhou, People’s Republic of China; 2Department of Orthopedics, The Third Affiliated Hospital, Southern Medical University, Guangzhou, Guangdong, People’s Republic of China; 3Three Gorges Central Hospital of Chongqing, Chongqing, People’s Republic of China; 4School of Traditional Chinese Medicine, Southern Medical University, Guangzhou, Guangdong, People’s Republic of China; 5Department of Cell Biology, School of Basic Medical Science, Southern Medical University, Guangzhou, Guangdong, People’s Republic of China
*These authors contributed equally to this work
Abstract: Osteoporosis is a systemic skeletal disease that is characterized by low bone density and microarchitectural deterioration of bone tissue. The increasing prevalence of osteoporosis has attracted much attention. In this study, MC3T3-E1 pre-osteoblasts were treated with the natural compound, baicalein (0.1 µmol/L, 1 µmol/L, 10 µmol/L), to stimulate differentiation over a 14-day period. In addition, a canonical ovariectomized (OVX) mouse model was used to investigate the effect of 3-month baicalein treatment (10 mg/kg per day) in preventing postmenopausal osteoporosis. In vitro, we found that baicalein induced activation of alkaline phosphatase, stimulated the mammalian target of rapamycin complex 1 (mTORC1) signaling pathway, and induced expression of osteoblast differentiation markers, ie, osteocalcin, osterix, collagen Iα1, and runt-related transcription factor 2 (RUNX2), in osteoblasts. In vivo, several bone parameters, including trabecular thickness, trabecular bone mineral density, and trabecular number, in the distal femoral metaphysis were significantly increased in OVX mice treated intragastrically with baicalein for 3 months compared with OVX mice that were not treated with baicalein. We also found that expression of osteocalcin and RUNX2 was decreased in primary ossified tissue from the OVX group, and baicalein increased the levels of osteocalcin and RUNX2 in OVX mice. These data suggest that baicalein can stimulate MC3T3-E1 cells to differentiate into osteoblasts via activation of the mTORC1 signaling pathway, which includes protein kinases and transcription factors such as P-4E/BP1 and P-S6K1.
Keywords: osteoblast, osteoporosis, menopause, mTOR
Introduction
During bone remodeling, a balance, is elaborately maintained by two phases, ie, bone formation by osteoblasts and bone resorption by osteoclasts.1 Generally speaking, an imbalance in these phases directly leads to osteoporosis. One cannot ignore the fact that, in this cycle, the removal of old bone from the skeleton is performed by osteoclasts, which are derived from hematopoietic stem cells, and the addition of new bone occurs through differentiation/mineralization by osteoblasts, which are derived from mesenchymal stem cells.2,3 If the balance between bone formation and bone resorption is disturbed, and less bone formation than bone resorption takes place, adult skeletal diseases could result, such as osteoporosis. Osteoporosis contributes to many adverse outcomes, including substantial skeletal deformity, pain, functional limitation, increased mortality, and severe economic burden.4
Currently, the clinical treatments for osteoporosis are antiresorptive drugs. These include bisphosphates, estrogen receptor analogs, estrogen, and calcitonin. These compounds maintain bone mass by inhibiting osteoclast function. Due to the potential complications associated with antiresorptive drugs, ie, breast cancer, uterine bleeding, and cardiovascular events, their use is restricted for treating osteoporosis. Therefore, it is desirable to identify better and safe anabolic agents that have lower toxicity. Since new bone formation is primarily mediated by osteoblasts, agents that act by either increasing osteoblast proliferation or inducing osteoblast differentiation can enhance bone formation.5,6
Baicalein (BN) is a coumarin-like derivative extracted from Chinese herbs. It has been used to treat bone diseases for thousands of years. Scutellaria baicalensis Georgi root (Huangqin) is one of the herbs commonly used in products prescribed for the treatment of fractures and bone and joint diseases. BN is the main active ingredient extracted from the root of S. baicalensis Georgi. It has been reported that BN stimulates osteoblast differentiation by a coordinated activation of mitogen-activated protein kinases and transcription factors,7 inhibition of osteoclast differentiation, and induction of apoptosis in mature osteoclasts.8 However, the effects of BN on bone formation in vivo and the underlying molecular mechanisms remain poorly understood. In this study, we clarified the detailed molecular mechanisms of BN in MC3T3-E1 cells. Moreover, we used a combination of in vitro and in vivo approaches to test the hypothesis that BN promotes differentiation of osteoblasts. The results demonstrate that BN induces pre-osteoblasts to differentiate into osteoblasts by activation of mammalian target of rapamycin complex 1 (mTORC1) signaling.
Materials and methods
Materials and reagents
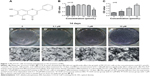
BN (Figure 1) was obtained from Sigma-Aldrich (St Louis, MO, USA), and had a molecular weight of 270.24 Da and purity of more than 97%. The molecular sequences have been deposited in a publicly accessible database in PubChem with the research number CID 5281605. Stock solutions of BN were prepared in dimethyl sulfoxide (Sigma-Aldrich) and stored at −20°C.
Animals and drug treatment
Eight-week-old female C57/BL6 mice (n=30) weighing 18–20 g were purchased from Southern Medical University (Guangzhou, People’s Republic of China). The mice were randomly divided into sham, ovariectomized (OVX), and OVX + BN groups. Mice in the OVX + BN group (n=10) were treated intragastrically with BN at a dose of 10 mg/kg per day for 5 days before ovariectomy and BN intragastric administrations were maintained for 3 months after ovariectomy. Mice in the OVX group (n=10) were treated with vehicle only after ovariectomy. Mice in the sham group (n=10) had some fat tissue around the ovaries removed. Ethical approval to conduct this study was given by the medical ethics committee of Southern Medical University (2014-091). The mice were kept following the animal care guidelines of Southern Medical University laboratory animal welfare and ethics committee charter.
Cell culture and osteoblast differentiation
MC3T3-E1 cells (subclone 14; American Tissue Culture Collection, Manassas, VA, USA) were cultured in α-minimal essential medium (Gibco, Grand Island, NY, USA) containing 10% fetal bovine serum (Gibco) and 1% penicillin–streptomycin reagent (Gibco). The cells were grown at 37°C in humidified air containing 5% CO2 and fed with fresh medium every 3 days. MC3T3-E1 cells were seeded into six-well plates at a density of 1×105 cells/well and grown to 95% confluence. MC3T3-E1 pre-osteoblasts were then induced by 10% fetal bovine serum osteogenic medium (α-minimal essential medium supplemented with 50 μmol/L ascorbic acid, 0.1 μmol/L dexamethasone, and 10 mmol/L β-glycerol phosphate). At the same time, we added BN (0.1 μM–10 μM) to the differentiation medium. The medium was changed every 3 days and the cells were maintained in differentiation medium for 14 days.
Cell proliferation assay
The Cell Counting Kit-8 (CCK-8; Keygen Biotech, Nanjing, People’s Republic of China) colorimetric assay was used to assess cell proliferation and viability from triplicate cultures, according to the manufacturer’s protocol. The MC3T3-E1 pre-osteoblasts were seeded into 96-well plates at a density of 5×104 cells/well. After incubation with BN (0.1 μmol/L, 1 μmol/L, 10 μmol/L, 100 μmol/L, 1,000 μmol/L) for 14 days, the CCK-8 reagent was added to each well and the cells were incubated at 37°C for 4 hours. The absorbance (optical density) at 450 nm was measured and used as an index of cell proliferation and viability.
ALP activity/staining assay
Cells were plated into six-well plates at a density of 2×105 cells/well. Following treatment with BN (0.1 μM–10 μM) and osteoblast differentiation for 2 weeks, the cells were washed twice with phosphate-buffered saline (PBS), scraped into 500 μL of 10 mM Tris-HCl buffer (pH 7.6) containing 0.1% Triton X-100, placed on ice, and sonicated to lyse the cells. Protein concentrations in the lysates were determined using the Bradford protein assay. Alkaline phosphatase (ALP) activity in the cellular fraction was measured using a fluorometric detection kit (Nanjing Jiancheng Biotechnology Co Ltd, Nanjing, People’s Republic of China). A standard curve was created using p-nitrophenol as the standard, and the ALP activity of each sample was calculated from optical density (450 nm) values. Parallel wells were stained for ALP. Cells were fixed in 4% paraformaldehyde for 20 minutes at room temperature, washed, incubated with ALP staining buffer (NBT-BCIP, Sigma-Aldrich) at 37°C for 30 minutes, and washed with PBS to remove excess dye.
Quantitative PCR
Total RNA was isolated from cultures with TRIzol reagent (Life Technologies, Grand Island, NY, USA) according to the manufacturer’s protocol. After MC3T3-E1 cells were incubated in differentiation medium for 14 days, reverse transcription was performed using 1 μg of total RNA with a complementary DNA synthesis kit (Takara Biotechnology Co Ltd, Dalian, China). The polymerase chain reaction (PCR) cycle parameters were as follows: 30°C for 10 minutes; 42°C for 50 minutes; and 95°C for 5 minutes. Real-time PCRs were performed in duplicate using the SYBR Premix Ex Taq II kit (Takara Biotechnology Co Ltd) and a Rotor-Gene Q thermal cycler (Qiagen, Hilden, Germany). The primer sequences are shown in Table 1. The thermal cycling conditions were as follows: initial denaturation at 95°C for 30 seconds, followed by 40 cycles of 95°C for 5 seconds, 60°C for 20 seconds, and 72°C for 15 seconds. Expression levels were normalized to those of endogenous glyceraldehyde-3-phosphate dehydrogenase, and the data were analyzed using the ΔΔ-Ct method.9
Western blot assay
After treatment with BN for 14 days in osteoblast differentiation medium, the MC3T3-E1 cells were lysed immediately in Laemmli buffer (62.5 mM Tris-HCl, pH 6.8, 2% sodium dodecyl sulfate, 10% glycerol, 50 mM dithiothreitol, 0.01% bromophenol blue) for 5 minutes at 95°C. Cell lysates were analyzed by sodium dodecyl sulfate polyacrylamide gel electrophoresis and transferred by electrophoresis to a nitrocellulose membrane (Millipore, Billerica, MA, USA). Membranes were blocked for 1 hour at room temperature using 5% (w/v) skim milk-TBST solution. The membranes were then incubated with primary antibodies, ie, RUNX2 (product code sc-390351, diluted 1:2,000, Santa Cruz Biotechnology, Santa Cruz, CA, USA), osteocalcin (product code sc-376835, diluted 1:3,000, Santa Cruz Biotechnology), β-actin (product code 4967, diluted 1:4,000, Cell Signaling Technology Inc, Danvers, MA, USA), phospho-4E (eIF4E)-binding protein 1 (P-4E/BP1, product code 9456, diluted 1:5,000, Cell Signaling Technology), 4E (eIF4E)-binding protein 1 (4E/BP1, product code 9452, diluted 1:5,000, Cell Signaling Technology), phospho-p70 ribosomal S6 kinase 1 (P-S6K1, product code 4858, diluted 1:6,000, Cell Signaling Technology), p70 ribosomal S6 kinase 1 (S6K1, product code 2708, diluted 1:6,000, Cell Signaling Technology) at 4°C overnight. Blots were then incubated with anti-rabbit immunoglobulin G peroxidase conjugate (product code 7071, all diluted 1:4,500, Cell Signaling Technology) for 1 hour at room temperature. Signals were revealed using an enhanced chemiluminescence kit (Cell Signaling Technology).
Immunocytofluorescence assays
MC3T3-E1 cells were seeded into six-well plates containing glass cover slides at a density of 2×105 cells/well and grown to 95% confluence. The cells received osteoblast differentiation medium containing BN for 2 weeks. Following fixation in 4% paraformaldehyde at room temperature for 30 minutes, the cells were washed three times with PBST (0.1% Triton X-100 in PBS) and blocked in PBS-bovine serum albumin (1% bovine serum albumin in PBS) for 1 hour at room temperature. Cells were incubated with antibodies against collagen Iα1 (product code ab21286, diluted 1:100, Abcam, Cambridge, MA, USA) for 1 hour at room temperature. The samples were then washed three times in PBS for 10 minutes each, and incubated with anti-rabbit immunoglobulin G (H+L), F (ab′)2 Fragment Alexa Fluor 594 conjugated secondary antibodies (product code 8889, diluted 1:100, Cell Signaling Technology) for 1 hour at room temperature. For nuclear staining, cells were counterstained with 4′,6-diamidino-2-phenylindole (DAPI; Cell Signaling Technology) for 5 minutes. Cells were examined using a laser confocal microscope (FV1000, Olympus Optical Co Ltd, Tokyo, Japan). Positive cells were evaluated using Image-Pro Plus software (Media Cybernetics Inc, Rockville, MD, USA) to measure cellular fluorescence intensity. Cells with fluorescence intensity ≥150% of background were considered positive.
Immunocytochemistry assay
Cell were seeded into six well plates at a density of 2×105 cells and grown to 95% confluence. The cells then received osteoblast differentiation medium containing BN for 2 weeks. Following fixation and blocking, the cells were incubated with antibodies against osterix (product code ab22552, diluted 1:100, Abcam) for 2 hours at room temperature and goat-anti-rabbit horseradish peroxidase-conjugated secondary antibodies (product code 7071, diluted 1:100, Cell Signaling Technology) for 1 hour at room temperature. The immunostained sections were then incubated with 3,3′-diaminobenzidine (DAB, ZSGB-Bio, Beijing, People’s Republic of China). Finally, the sections were counterstained with hematoxylin, mounted with coverslips, and examined using a light microscope (BX43, Olympus).
Immunofluorescence assay
After ovariectomy and treatment with BN, the mice were euthanized with pelltobarbitalum natricum (1%, 10 mg/kg, Southern Medical University, Guangdong, People’s Republic of China). Femurs were fixed in 4% paraformaldehyde in 0.1 M PBS (pH 7.4). Fixed bones were decalcified by immersion in 10% ethylenediaminetetraacetic acid (pH 7.0) for 14 days and embedded in paraffin. The paraffin-embedded longitudinal bone sections with 5 μm slice thickness were then blocked by 5% goat serum for 1 hour at room temperature, and incubated overnight with the mouse monoclonal osteocalcin antibodies (product code sc-376835, diluted 1:50, Santa Cruz Biotechnology). Sections were then incubated with anti-rabbit immunoglobulin G (H+L), F (ab′)2 Fragment Alexa Fluor 594 conjugated (product code 8889, diluted 1:100, Cell Signaling Technology) for 1 hour at room temperature. Controls included substitution of the primary antibody with rabbit immunoglobulin G Femur histological sections were imaged using a FV1000 confocal microscope and positive cells were evaluated using the Image-Pro Plus software program.
Micro-CT assay
Long bones were collected from the mice, dissected free of soft tissue, fixed overnight in 10% formalin, and analyzed by high-resolution micro-computed tomography (μCT 80, Scanco Medical, Brüttisellen, Zurich, Switzerland). The micro-CT scanner was set at a voltage of 89 kVp and a current of 112 μA to collect images at a resolution of 8.66596 μm per pixel. Serial cross-sectional images of the distal femur were collected to perform three-dimensional histomorphometric analysis of the trabecular bone. The sample area selected for scanning was a 1.5 mm length of the metaphyseal secondary spongiosa that originated 1.0 mm below the epiphyseal growth plate and extended caudally. Our analyses included various bone parameters, ie, trabecular thickness, trabecular number, and trabecular bone mineral density (BMD).
Histochemical and histological assays
All bones were fixed in buffered aqueous formalin, decalcified, embedded in paraffin, sectioned, and stained for ALP (product code 86R-1KT, Sigma-Aldrich) and hematoxylin and eosin, and dyeing steps were followed with the product manual.
Immunohistochemical assay
For immunohistochemistry, long bones were obtained after the mice had been treated with BN for 3 months. The paraffin-embedded bones were then sectioned using a paraffin microtome (RM2125 RTS, Leica, Wetzlar, Germany) and 5 μm sections were stored at −20°C. For immunostaining, the sections were deparaffinized and briefly washed with TBS (100 mmol/L Tris, pH 7.5; 150 mmol/L NaCl). This step was followed by incubation for 30 minutes in 3% H2O2 to quench endogenous peroxidase activity. Appropriate primary antibodies (anti-mouse-RUNX2, product code sc-390351, diluted 1:100, Santa Cruz Biotechnology) were then applied overnight at 4°C. After incubation with the primary antibody, sections were washed three times with TBS and incubated with the goat-anti-mouse horseradish peroxidase-conjugated secondary antibodies (product code 7072, diluted 1:100, Cell Signaling Technology) for 1 hour at room temperature. Immunostained sections were then incubated with DAB. Finally, the sections were dehydrated, mounted with coverslips, and examined using the Olympus light microscope.
Quantitative method
Western blot data were evaluated as follows: the gray value of the Western blot bandings was examined by Image-Pro Plus in the control and experimental groups; the gray value of interest protein of each group was divided by the gray value of internal reference protein themselves; the result for the experimental group from the preceding step were divided by the result for the control group from the preceding step; and the final results are shown as “fold change”.
Quantification of immunocytofluorescence, immunocytochemistry, immunofluorescence, histochemistry, and immunohistochemistry was very similar. The total integral optical density (IOD SUM) was examined by Image-Pro Plus in the fluorescence image. The IOD SUM was then divided by the total measurement area (area SUM). Finally, the results were shown as “mean optical density”. The computational formula was given by: mean optical density = (IOD SUM)/area SUM.
Statistical analysis
The data are representative of at least three independent experiments. Each experiment was done in triplicate. The data were graphed using GraphPad Prism software version 3.0 (GraphPad Software Inc, La Jolla, CA, USA). One-way analysis of variance followed by the Student’s t-test was used to determine statistical differences. Error bars represent the standard error of the mean in the cell experiment and the standard deviation in the animal experiment.
Results
Effect of BN on cell proliferation
As shown in Figure 1, we measured the effect of BN on MC3T3-E1 cell proliferation using the CCK-8 assay. We found that BN at concentrations ranging from 0.1 μM to 100 μM did not significantly affect cell growth after treatment for 14 days.
Effect of BN on osteogenesis of MC3T3-E1 cells in vitro
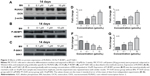
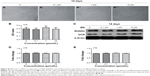
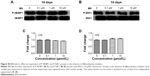
Our first experiments did not show an effect of BN alone on MC3T3-E1 cells (Figure S1). In the absence of differentiation medium, treatment with BN for 2 weeks did not significantly promote changes in osteogenic proteins, ie, ALP (Figure S1), osteocalcin (Figure S1), and RUNX2 (Figure S1). However, to further investigate the consequences of adding BN in vitro, MC3T3-E1 cells were cultured for 14 days in osteoblast differentiation medium containing BN. First, we found BN could promote protein expression of RUNX2 (Figure S2) and osteocalcin (Figure S2) and mRNA expression of ALP (Figure S2), RUNX2 (Figure S2), osteocalcin (Figure S2), and collagen Iα1 (Figure S2) in differentiation medium compared with the group that only received differentiation medium (Figure S2). Under these conditions, BN increased the expression of ALP (Figure 1) by at least 30% in cultured MC3T3-E1 cells. RUNX2 expression detected by quantitative real-time PCR (Figure 2) and Western blot (Figure 3) was also upregulated. In addition, BN increased the expression of osteocalcin mRNA and protein levels measured by quantitative real-time PCR (Figure 2) and Western blot (Figure 3), respectively. As markers of osteoblasts at different stages of differentiation, the mRNA of early-stage markers, such as ALP (Figure 2), and late-stage markers, such as collagen Iα1 (Figure 2), were both upregulated in the presence of BN. Moreover, immunostaining for osterix (Figure 4) and collagen Iα1 (Figure 4) showed that BN could increase the expression of osterix (Figure 4) and collagen Iα1 (Figure 4), and this response was dose-dependent and peaked at 10 μM. These data suggest that BN promotes differentiation of osteoblasts in vitro.
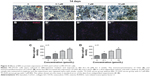
Effect of BN on microstructural parameters and histologic appearance of femoral bone
To confirm the effect of ovariectomy, body weight (Figure S3) and mass of the uterus (Figure S3) were recorded. Body weight increased in the OVX and OVX + BN groups. The mass of the uterus in the sham group was significantly greater than in the OVX or OVX + BN mice. These data confirm that excision of the ovaries was successful. To evaluate the effect of BN on bone formation in mice, we performed micro-CT analyses on the sham, OVX, and OVX + BN mice. Micro-CT results for the long bones of the OVX + BN (Figure 5) mice demonstrate a clear trend in which trabecular thickness (Figure 5) was increased relative to the OVX group (Figure 4). Moreover, trabecular BMD (Figure 5) and trabecular number (Figure 5) in bones from the OVX + BN group were significantly higher than in bones from the OVX group. However, these parameters were not significantly different between the OVX and sham groups (Figure 5). Hematoxylin and eosin staining of femoral bone sections (Figure 5) indicated that the number of trabeculae in OVX mice (Figure 5) was significantly decreased when compared with the OVX + BN group (Figure 5).
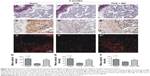
Effect of BN on expression of ALP, RUNX2, and osteocalcin in vivo
To examine the molecular and cellular changes associated with BN-mediated protection against bone loss in vivo, we examined the epiphyseal growth plate, where bone formation occurs on a cartilaginous template.10,11 Expression of ALP (Figure 6), RUNX2 (Figure 6), and osteocalcin (Figure 6) was markedly reduced in OVX mice (Figure 6) when compared with OVX + BN mice (Figure 6). However, there were no statistically significant differences between the sham group (Figure 6) and the OVX + BN group (Figure 6). Our in vitro and in vivo data indicate that BN-induced osteogenesis positively regulates bone formation.
Effect of BN on mTORC1 signaling
BN had no effect on protein expression of P-4E/BP1 and P-S6K1 in the absence of differentiation medium, as shown in Figure S4. BN did not alter protein expression of P-4E/BP1 (Figure S4) and P-S6K1 (Figure S4) with BN (1–10 μM) cultured for 14 days in the complete medium. To gain further insight into the function of BN in differentiation of osteoblasts in cultured MC3T3-E1 cells, we examined the expression of P-4E/BP1 (Figure 3) and P-S6K1 (Figure 3) in vitro. Western blot results show that expression of P-S6K1 was enhanced (Figure 3) and expression of P-4E/BP1 was decreased (Figure 3). Collectively, these data indicate that mTORC1 signaling is upregulated in MC3T3-E1 cells in the presence of BN and suggest that BN cooperates with mTORC1 signaling to regulate ossification.
Discussion
BN is a tricyclic aromatic compound present in the root of S. baicalensis Georgi. Previous studies have shown that BN has many pharmacological properties. These include anti-inflammatory,12,13 antiallergic,14 antioxidative,15 antiviral,16 and antigenotoxic17 effects, and the potential to modulate activation of ERK.18,19 The effects of BN on differentiation of osteoclasts8 and osteoblasts7 have also been studied. However, the mechanism of action of BN in vivo is poorly understood. In our study, we found that BN upregulated the mTORC1 pathway and bone formation activity in MC3E3-E1 cells in a dose-dependent manner in vitro. Furthermore, in the absence of BN, bone loss caused by ovariectomy was accelerated, and expression of bone markers, ie, ALP, RUNX2, and osteocalcin, were reduced in growth plate osteoblasts.
The mammalian target of rapamycin (mTOR) pathway, when genetically downregulated, increases the life span and homeostasis of stem cells in evolutionarily diverse organisms, including mammals.20 It plays an important role in postnatal bone formation and bone remodeling.21 Therefore, suppression of peroxisome proliferator-activated receptor gamma activity in osteoblasts significantly increases osteoblast differentiation and trabecular number. Furthermore, endogenous peroxisome proliferator-activated receptor gamma in osteoblasts strongly inhibits Akt/mTOR/p70S6k activity and leads to decreased osteoblast differentiation.22 Osteoblast insulin-like growth factor 1 released from the bone matrix during remodeling stimulates differentiation of recruited mesenchymal stem cells into mature osteoblasts by activation of mTOR. These steps maintain the appropriate bone microarchitecture and mass.23 In addition, suppressing the mTOR signaling pathway by using rapamycin, which is a specific inhibitor of mTOR, could lead pre-osteoblasts to express low levels of cyclin A and D1 protein.24 In differentiating osteoblasts, rapamycin significantly reduced osteoblast-specific osteocalcin, bone sialoprotein, and osterix mRNA expression, ALP activity, and mineralization capacity.24 In our study, expression of P-4E/BP1 and P-S6K1 (key downstream proteins in the mTORC1 pathway) was decreased and increased, respectively, with administration of BN in vitro.
Growing evidence suggests that maintenance of bone mass during early adult life is a key contributor to bone strength during aging. This is despite the fact that most efforts to prevent fracture in osteoporosis have been directed at inhibiting age-related bone loss.25–28 Previous studies have found that RUNX229–32 is a critical regulator of differentiation of osteoblasts and chondrocytes and essential for skeletal development. Osteocalcin and collagen Iα1, which are essential bone proteins, are secretory glycoproteins produced almost exclusively by osteoblasts.33–35 Previous studies have found that osteocalcin levels are significantly lower in postmenopausal women.33,36 ALP and osterix are markers of osteoblast differentiation. Some researchers have also found that ALP is an ubiquitous enzyme with an important role in osteoid formation and bone mineralization.37 Since ALP is a biochemical marker of bone metabolism, ALP levels are monitored in postmenopausal women.37 In addition, several previous studies have found that RUNX2 may be a useful genetic marker for bone metabolism in menopausal women and may play an important role in their BMD.38–41 In our study, we used female OVX mice to mimic the osteoporosis that occurs in postmenopausal women. We found that BN decreased the rate of bone metastasis after 3 months of treatment and promoted osteogenesis. These BN-dependent changes in bone turnover were associated with increased levels of ALP, RUNX2, and osteocalcin measured in vitro and in vivo, and of osteoblast markers, osterix and collagen Iα1, measured in vitro. In summary, our study indicates that BN stimulates osteoblast differentiation by activation of mTORC1 signaling as shown in Figure 7. BN may have potential application in the treatment of osteoporosis and nonunion fractures.
Acknowledgments
The authors thank Wu Xiu-hua (Guangzhou Zhongke Medical Technology Co, Guangdong, People’s Republic of China) for excellent technical support with micro-CT. This work was supported by the State Key Development Program for Basic Research of China (2013CB945203).
Author contributions
All authors made substantial contributions to conception and design, acquisition of data and interpretation of data; took part in either drafting the article or revising it critically for important intellectual content; gave final approval of the version to be published; and agree to be accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved.
Disclosure
The authors report no conflicts of interest in this work.
References
Parfitt AM. Bone remodeling and bone loss: understanding the pathophysiology of osteoporosis. Clin Obstet Gynecol. 1987;30: 789–811. | ||
Harada S, Rodan GA. Control of osteoblast function and regulation of bone mass. Nature. 2003;423:349–355. | ||
Boyle WJ, Simonet WS, Lacey DL. Osteoclast differentiation and activation. Nature. 2003;423:337–342. | ||
NIH Consensus Development Panel on Osteoporosis Prevention D, and Therapy. Osteoporosis Prevention, Diagnosis, and Therapy. JAMA. 2001;285:785–795. | ||
Ducy P, Amling M, Takeda S, et al. Leptin inhibits bone formation through a hypothalamic relay: a central control of bone mass. Cell Biochem Biophys. 2000;100:197–207. | ||
Lane NE, Kelman A. A review of anabolic therapies for osteoporosis. Arthritis Res Ther. 2003;5:214–222. | ||
Kim JM, Lee SU, Kim YS, Min YK, Kim SH. Baicalein stimulates osteoblast differentiation via coordinating activation of MAP kinases and transcription factors. J Cell Biochem. 2008;104:1906–1917. | ||
Kim MH, Ryu SY, Bae MA, Choi JS, Min YK, Kim SH. Baicalein inhibits osteoclast differentiation and induces mature osteoclast apoptosis. Food Chem Toxicol. 2008;46:3375–3382. | ||
Livak KJ, Schmittgen TD. Analysis of relative gene expression data using real-time quantitative PCR and the 2(-Delta Delta C(T)) method. Methods. 2001;25:402–408. | ||
Karsenty G. The complexities of skeletal biology. Nature. 2003;423: 316–318. | ||
Kronenberg HM. Developmental regulation of the growth plate. Nature. 2003;423:332–336. | ||
Chen CJ, Raung SL, Liao SL, Chen SY. Inhibition of inducible nitric oxide synthase expression by baicalein in endotoxin/cytokine-stimulated microglia. Biochem Pharmacol. 2004;67:957–965. | ||
Hong T, Jin GB, Cho S, Cyong JC. Evaluation of the anti-inflammatory effect of baicalein on dextran sulfate sodium-induced colitis in mice. Planta Med. 2002;68:268–271. | ||
Kimata M, Shichijo M, Miura T, Serizawa I, Inagaki N, Nagai H. Effect of luteolin, quercetin and baicalein on immunoglobulin E-mediated mediator release from human cultured mast cells. Clin Exp Allergy. 2000;30:501–508. | ||
Hwang JM, Tseng TH, Tsai YY, et al. Protective effects of baicalein on tert-butyl hydroperoxide-induced hepatic toxicity in rat hepatocytes. J Biomed Sci. 2005;12:389–397. | ||
Ono K, Nakane H. Mechanisms of inhibition of various cellular DNA and RNA polymerases by several flavonoids. J Biochem. 1990; 108:609–613. | ||
Lee BH, Lee SJ, Kang TH, et al. Baicalein: an in vitro antigenotoxic compound from Scutellaria baicalensis. Planta Med. 2000;66:70–71. | ||
Chen YC, Chow JM, Lin CW, Wu CY, Shen SC. Baicalein inhibition of oxidative-stress-induced apoptosis via modulation of ERKs activation and induction of HO-1 gene expression in rat glioma cells C6. Toxicol Appl Pharmacol. 2006;216:263–273. | ||
Nakahata N, Tsuchiya C, Nakatani K, Ohizumi Y, Ohkubo S. Baicalein inhibits Raf-1-mediated phosphorylation of MEK-1 in C6 rat glioma cells. Eur J Pharmacol. 2003;461:1–7. | ||
Russell RC, Fang C, Guan KL. An emerging role for TOR signaling in mammalian tissue and stem cell physiology. Development. 2011; 138:3343–3356. | ||
Chen J, Long F. mTORC1 signaling controls mammalian skeletal growth through stimulation of protein synthesis. Development. 2014; 141:2848–2854. | ||
Sun H, Kim JK, Mortensen R, Mutyaba LP, Hankenson KD, Krebsbach PH. Osteoblast-targeted suppression of PPARgamma increases osteogenesis through activation of mTOR signaling. Stem Cells. 2013;31:2183–2192. | ||
Xian L, Wu X, Pang L, et al. Matrix IGF-1 maintains bone mass by activation of mTOR in mesenchymal stem cells. Nat Med. 2012;18: 1095–1101. | ||
Singha UK, Jiang Y, Yu S, et al. Rapamycin inhibits osteoblast proliferation and differentiation in MC3T3-E1 cells and primary mouse bone marrow stromal cells. J Cell Biochem. 2008;103:434–446. | ||
Bonjour JP, Chevalley T, Ferrari S, Rizzoli R. [The importance and relevance of peak bone mass in the prevalence of osteoporosis]. Salud Publica Mex. 2009;51 Suppl 1:S5–S17. Spanish. | ||
Bluher M, Kahn BB, Kahn CR. Extended longevity in mice lacking the insulin receptor in adipose tissue. Science. 2003;299:572–574. | ||
Liu JM, Zhao HY, Ning G, et al. IGF-1 as an early marker for low bone mass or osteoporosis in premenopausal and postmenopausal women. J Bone Miner Metab. 2008;26:159–164. | ||
Ohlsson C, Mellstrom D, Carlzon D, et al. Older men with low serum IGF-1 have an increased risk of incident fractures: the MrOS Sweden study. J Bone Miner Metab. 2011;26:865–872. | ||
Ducy P, Zhang R, Geoffroy V, Ridall AL, Karsenty G. Osf2/Cbfa1: a transcriptional activator of osteoblast differentiation. Cell. 1997;89: 747–754. | ||
Otto F, Thornell AP, Crompton T, et al. Cbfa1, a candidate gene for cleidocranial dysplasia syndrome, is essential for osteoblast differentiation and bone development. Cell. 1997;89:765–771. | ||
Komori T, Yagi H, Nomura S, et al. Targeted disruption of Cbfa1 results in a complete lack of bone formation owing to maturational arrest of osteoblasts. Cell. 1997;89:755–764. | ||
Mundlos S, Otto F, Mundlos C, et al. Mutations involving the transcription factor CBFA1 cause cleidocranial dysplasia. Cell. 1997;89: 773–779. | ||
Shinkov AD, Borissova AM, Kovatcheva RD, Atanassova IB, Vlahov JD, Dakovska LN. Age and menopausal status affect osteoprotegerin and osteocalcin levels in women differently, irrespective of thyroid function. Clin Med Insights Endocrinol Diabetes. 2014;7:19–24. | ||
Hauschka PV, Lian JB, Cole DE, Gundberg CM. Osteocalcin and matrix Gla protein: vitamin K-dependent proteins in bone. Physiol Rev. 1989;69:990–1047. | ||
Gossl M, Modder UI, Atkinson EJ, Lerman A, Khosla S. Osteocalcin expression by circulating endothelial progenitor cells in patients with coronary atherosclerosis. J Am Coll Cardiol. 2008;52:1314–1325. | ||
Undale A, Srinivasan B, Drake M, et al. Circulating osteogenic cells: characterization and relationship to rates of bone loss in postmenopausal women. Bone. 2010;47:83–92. | ||
Bhattarai T, Bhattacharya K, Chaudhuri P, Sengupta P. Correlation of common biochemical markers for bone turnover, serum calcium, and alkaline phosphatase in post-menopausal women. Malays J Med Sci. 2014;21:58–61. | ||
Lee HJ, Koh JM, Hwang JY, et al. Association of a RUNX2 promoter polymorphism with bone mineral density in postmenopausal Korean women. Calcif Tissue Int. 2009;84:439–445. | ||
Vaughan T, Pasco JA, Kotowicz MA, Nicholson GC, Morrison NA. Alleles of RUNX2/CBFA1 gene are associated with differences in bone mineral density and risk of fracture. J Bone Miner Res. 2002;17: 1527–1534. | ||
Doecke JD, Day CJ, Stephens AS, et al. Association of functionally different RUNX2 P2 promoter alleles with BMD. J Bone Miner Res. 2006;21:265–273. | ||
Vaughan T, Reid DM, Morrison NA, Ralston SH. RUNX2 alleles associated with BMD in Scottish women; interaction of RUNX2 alleles with menopausal status and body mass index. Bone. 2004;34:1029–1036. |
Supplementary materials
 © 2015 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2015 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.