Back to Journals » Drug Design, Development and Therapy » Volume 14
Radix Polygoni Multiflori and Its Main Component Emodin Attenuate Non-Alcoholic Fatty Liver Disease in Zebrafish by Regulation of AMPK Signaling Pathway
Authors Yu L, Gong L, Wang C, Hu N, Tang Y, Zheng L, Dai X, Li Y
Received 28 December 2019
Accepted for publication 5 March 2020
Published 15 April 2020 Volume 2020:14 Pages 1493—1506
DOI https://doi.org/10.2147/DDDT.S243893
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 3
Editor who approved publication: Dr Jianbo Sun
Linyuan Yu, Lihong Gong, Cheng Wang, Naihua Hu, Yunqiu Tang, Li Zheng, Xuyang Dai, Yunxia Li
School of Pharmacy, Chengdu University of Traditional Chinese Medicine; Key Laboratory of Standardization for Chinese Herbal Medicine, Ministry of Education; National Key Laboratory Breeding Base of Systematic Research, Development and Utilization of Chinese Medicine Resources, Chengdu 611137, People’s Republic of China
Correspondence: Yunxia Li
School of Pharmacy, Chengdu University of Traditional Chinese Medicine; Key Laboratory of Standardization for Chinese Herbal Medicine, Ministry of Education; National Key Laboratory Breeding Base of Systematic Research, Development and Utilization of Chinese Medicine Resources, No. 1166, Liu Tai Avenue, Wenjiang District, Chengdu, Sichuan, People’s Republic of China
Tel +86-13699021135
Email [email protected]
Purpose: Nonalcoholic fatty liver disease (NAFLD) has become a predictor of death in many diseases. This study was carried out to investigate the therapeutic effect of Radix Polygoni Multiflori Preparata (RPMP) and its main component emodin on egg yolk powder-induced NAFLD in zebrafish. Further investigation was performed to explore whether emodin was the main component of RPMP for the treatment of NAFLD as well as the underlying therapeutic mechanism of RPMP and emodin.
Methods: Zebrafish were divided into control group, egg yolk powder group, RPMP group and emodin group. The obesity of zebrafish was evaluated by body weight, body length and BMI. The content of lipid was detected by triglyceride (TG), total cholesterol (TC) reagent kit and the fatty acid was detected by nonesterified free fatty acids (NEFA) reagent kit. HE staining was used to detect the histological structure of liver. Whole-mount Oil red O staining and Frozen oil red O staining were carried out to investigate the lipid accumulation in liver. KEGG and STRING databases were performed to analyze the potential role of AMPK between insulin resistance (IR) and fatty acid oxidation. Western blot and RT-qPCR were carried out for mechanism research.
Results: RPMP and emodin significantly reduced zebrafish weight, body length and BMI. Both RPMP and emodin treatment could reduce the lipid deposition in zebrafish liver. RPMP significantly reduced the content of TG. However, emodin significantly reduced the contents of TG, TC and NEFA in zebrafish with NAFLD. The protein interaction network indicated that AMPK participated in both IR and fatty acid oxidation. Further investigation indicated that RPMP and emodin reduced hepatic lipogenesis via up-regulating the expressions of phosphatidylinositol 3-kinase (PI3K), protein kinase B (AKT2), amp-activated protein kinase alpha (AMPKα), proliferator-activated receptor alpha (PPARα), carnitine palmitoyl transferase 1a (CPT-1a) and acyl-coenzyme A oxidase 1 (ACOX1).
Conclusion: These findings suggest that emodin is the main component of RPMP for the treatment of NAFLD, which is closely related to the regulation of AMPK signaling pathway which increases IR and fatty acid oxidation.
Keywords: RPMP, emodin, zebrafish, AMPK, nonalcoholic fatty liver disease
Introduction
Non-alcoholic fatty liver disease (NAFLD) is a chronic liver disease referring to the presence of hepatic steatosis without significant intake of alcohol,1 which encompasses a wide spectrum of liver pathologies ranging from simple hepatic steatosis to inflammatory nonalcoholic steatohepatitis (NASH), and subsequently progresses to liver fibrosis, cirrhosis, and ultimately hepatocellular carcinoma in about 10% of patients.2,3 NAFLD is widespread in all countries, with the highest incidence in the United States 31% and the Middle East 32%, respectively, followed by Asia and Europe with 27% and 23%, respectively.4 The exact mechanisms underlying the pathogenesis of NAFLD still remain unclear, which limits the development of effective treatments. Presently, drugs specifically designed for the treatment of NAFLD have not been discovered yet.
The pathogenesis of NAFLD is very complex. Understanding of the etiology and different dietary factors that induce the “two hits” is essential in the prevention and treatment of NAFLD.5 The first hit includes lipid accumulation and insulin resistance (IR). The second hit is caused by various factors such as mitochondrial beta-oxidation, oxidative stress, lipid peroxidation, which lead to inflammatory necrosis and fibrosis of liver cells. NAFLD is common among overweight and obese individuals.6 As an important risk factor leading to metabolic syndrome, obesity generally has lipid metabolism disorder which increases free fatty acids (FFA) in blood circulation and liver triglyceride (TG) synthesis.7 In addition, it has been reported that obesity can inhibit liver glucose output and promote muscle glucose uptake, subsequently causing IR and promoting the occurrence of NAFLD.8 AMPK (AMP-activated protein kinase) is considered to be a cellular energy sensor that contributes to regulate energy balance and caloric intake,9 which is closely related to IR and is involved in fatty acid oxidation. In obese humans, a reduction of AMPK activity in adipose tissue is correlated with whole body IR, suggesting that adipose tissue AMPK may be important for obesity and NAFLD.10 From the perspective of traditional Chinese medicine, the main pathogenesis of fatty liver is related to both Liver and Kidney deficiency and Blood and Qi deficiency.11
Radix Polygoni Multiflori is the dried root tuber of Fallopia multiflora (Thunb.) Harald which has been applied to treat fatty liver for more than 2000 years. In the ancient herbal book Compendium of Materia Medica, Radix Polygoni Multiflori Preparata (RPMP) was recorded to tonify liver and kidney, nourish the blood and strengthen the bones and muscles. In modern pharmacology, RPMP possesses an effect of reducing blood lipid and is widely used in the treatment of blood deficiency, lumbar debility, hyperlipidemia, etc,12 as recorded In Chinese Pharmacopoeia (2015 edition). Kong Xianglian collected 40 cases in which 94 drugs were used for the treatment of fatty liver and Radix Polygonum multiflorum ranked 7th in terms of usage frequency.13 The commonly used Chinese patent medicine Kezhi capsule and Jiangzhining tablet containing Radix Polygonum multiflorum have been approved by the Chinese Food and Drug Administration for the treatment of fatty liver and Hyperlipidemia due to the effect of invigorating liver and kidney. Studies claimed that Radix Polygonum multiflorum can reduce lipids by improving IR and promoting fatty acid oxidation.14 The main active components of Radix Polygonum multiflorum are anthraquinones, stilbenes, phospholipids, flavonoids and phenols, among which stilbene glycosides, emodin and physcion are considered to be the main components with lipid-lowering effect.15
The results of our previous experiments on the lipid-lowering effect of Radix Polygonum multiflorum provided the following findings: (a) 50% alcohol extract of RPMP possessed predominant therapeutic effect than aqueous extract of RPMP; (b) The best effective concentration of 50% RPMP alcohol extract to treat NAFLD was 1–2 mg/mL; (c) Most components of 50% alcohol extract and aqueous extract were similar. However, significant difference was found in the emodin contents between aqueous extract and 50% alcohol extract of RPMP. Thus, we hypothesized that emodin might be the main component of RPMP accounting for the lowering lipid effect.
Over the past decade, emodin has received increasing attention as many studies have demonstrated its anti-inflammatory, anti-tumor, immunoregulation and lipid-lowering effects.16 The chemical structure of emodin is shown in Figure 1A. Modern research finds that emodin is an AMPK activator, which can attenuate lipid accumulation by decreasing lipogenesis and increasing mitochondrial fatty acid β-oxidation mediated via activation of the AMPK signaling pathway, and emodin also activates AMPK by an indirect mechanism similar to berberine.5,17 However, most of the current researches on emodin treatment of NAFLD have focused on the anti-inflammatory mechanism which has no relationship with the treatment of NAFLD by RPMP.18
In the present study, we tried to find the intrinsic relationship between RPMP and emodin in the treatment of NAFLD via AMPK signaling pathway. And found that both RPMP and emodin could activate AMPK and participate in the regulation of IR and fatty acid oxidation.
Materials and Methods
Materials
Larval-AP100 was obtained from Zeigler Bros. Inc (PA, USA). Egg yolk powder was obtained from Yuanye Bio-Technology Co., Ltd (Shanghai, China). Triglyceride (TG) and total cholesterol (TC) reagent kits were purchased from Nanjing Jiancheng Institute of Biotechnology (Nanjing, China). Nonesterified fatty acids (NEFA) reagent kit was purchased from elabscience Biotechnology Co., Ltd (Wuhan, China). Oil red O dyeing solution was collected from Shanghai solarbio Bioscience and Technology Co., Ltd (Shanghai, China). HE dye solution was collected from Servicebio (Wuhan, China). SuperLumia ECL HRP Substrate Kit was obtained from Abbkine Scientific Co., Ltd (CA, USA). 5×All-In-one MasterMix and EvaGreen 2×qRT-PCR were provided by Applied Biological Materials Inc (VAN, Canada). Gene-specific primer sequences for RT-qPCR were synthesized by TSINGKE Biological Technology (Chengdu, China). Formic acid, Acetonitrile and methanol were provided by Thermo Fisher Scientific Co., Ltd (MA, USA). Fluorescence microscope (Leica, Germany), microplate reader (BioTek, USA), high-performance liquid chromatography (PerkinElmer, USA). The materials are listed in Table 1.
 |
Table 1 The Item Number and Manufacturer of Reagents |
Extraction of Alcohol Extract from RPMP
0.25 g RPMP was accurately weighed, placed in a round-bottom flask and mixed with 20 times of 50% ethanol. The mixture was soaked for 30 min and heated to reflux for 60 min followed by filtrating. The filtrate was evaporated using a rotary evaporator at 45°C until discarding the totality of ethanol.
Analysis of the Major Components in 50% Alcohol Extract and Aqueous Extract of RPMP
The major components of RPMP were analyzed by high-performance liquid chromatography (HPLC) referred to the previous methods established by our lab.19 The determination was carried out in ZORBAX C18 analytical column (4.6 mm × 250 mm, 5 μm) with the pre-column ZORBAX C18 (4.6 mm × 12.5 mm, 5 μm). Gradient elution was performed with mobile phase composed of (a) 0.1% formic acid and (b) acetonitrile at the flow rate of 1 mL/min. Percentage of acetonitrile was 5–10% (0–5 min), 10–22% (5–30 min), 22–25% (30–38 min), 25–32% (38–48 min), 32–45% (48–55 min), 45–85% (55–65 min), 85–95% (65–70 min), 95% (70–72 min). The detection wavelength was set at 275 nm, and the column temperature was 30°C. The peaks of different components were identified through comparing their retention time values and UV spectra with standard solution (catechin, gallic acid ester, stilbene glycoside, emodin glycoside, emodin and physcion).
Animals and Methods
Zebrafish (Danio rerio) of wild-type AB strain from Pharmacy College of Chengdu University of Traditional Chinese Medicine were maintained and raised under standard conditions as previously described.20 The embryos were cultured at 28.5°C from natural mating. The zebrafish experiments were performed under the approval of the Institutional Animal Care and Use Committee of Chengdu University of Traditional Chinese Medicine. Egg yolk powder was used as the induction factor of zebrafish obesity due to its excellent water solubility and high-fat content. AP100, a standard diet for zebrafish juveniles, was chosen as a control diet. As shown in Figure 1B, 5-day post-fertilization (5 dpf) zebrafish were randomly divided into six groups and maintained for 72 h as follows: (1) the control group of zebrafish exposed to AP100; (2) the model group of zebrafish exposed to 2 mg/mL egg yolk powder; (3) RPMP treatment groups of zebrafish exposed to cotreatment of 2 mg/mL egg yolk powder and 1 mg/mL, 0.5 mg/mL 50% alcohol extraction of RPMP, respectively; (4) emodin treatment groups of zebrafish exposed to cotreatment of 2 mg/mL egg yolk powder and 0.5 µg/mL, 0.25 µg/mL emodin, respectively. Zebrafish were fasted for 1 day before sample collection for further analysis of histologist, TG, TC, NEFA, WB and RT-qPCR.
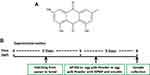 |
Figure 1 (A) Chemical structure of emodin. (B) Experimental outline of the feeding protocol. |
Measurement of Body Index
After 72 hrs RPMP and emodin administration, the body weight and length of zebrafish were measured. Before measurements, zebrafish were anesthetized with tricaine at 1:20 dilution for 15 s. The body length of zebrafish is the distance from the most front of the head of zebrafish to the most end of zebrafish tail. Once the measurement was finished, zebrafish were put back into fresh water. Body mass index (BMI) was calculated as weight/length2.
Whole-Mount Oil Red O Staining
After fasting for 1 day, zebrafish larvae were fixed overnight with 4% PFA at 4°C and washed 3 times with PBS. Subsequently, zebrafish larval were orderly immersed in 25%, 50%, 75% and 100% 1,2-propanediol at room temperature for 20 min, respectively. Then, fresh 0.5% Oil Red O solution was added and zebrafish larval were dyed at room temperature for 12 h. Then, zebrafish larval samples were washed with 100%, 75%, 50%, 25% 1.2-propanediol, respectively. Finally, the lipid droplets in liver tissue were observed and photographed on fluorescence microscope. The oil red O positive staining of zebrafish liver was measured by Image J software.
Hematoxylin and Eosin (HE) Staining
Zebrafish larvae in each group were fixed with 4% PFA for 24 h, dehydrated and embedded in paraffin at 65°C. Paraffin sections were cut by paraffin slicer and counterstained to visualize the nuclei. All other HE staining processes were performed in accordance with standard. Then, all the staining was observed and photographed under microscope.
Frozen Oil Red O Staining (ORO)
Zebrafish larvae were fixed with 4% PFA for 24 h at 4°C and washed 3 times with PBS. Subsequently, the zebrafish samples were placed at 4°C and dehydrated and precipitated with 15% sucrose solution and 30% sucrose solution. The surface water was sucked slightly with filter paper. Then, the zebrafish samples were embedded by optimum cutting temperature (OCT) compound and sliced after OCT compound turned white and hard.
The slices were baked at 60°C for 30 min, then infiltrated with 85% and 100% 1.2-propanediol for 5 min. Next, the slices were dyed with freshly prepared 0.5% Oil Red O dye solution at room temperature for 2 h. The dye solution was lightly rinsed off with PBS. The background color was removed with 100% 1.2-propanediol for 1 min. The slices were re-dyed in hematoxylin dye solution for 10 s, rinsed with clear water and sealed with gelatin. Finally, the lipid droplets (dyed red) were observed under the microscope and photos were taken at the same time.
Triglyceride (TG) and Total Cholesterol (TC) Test
After 25 larvae were cleaned by precooled PBS for three times, 300 µL absolute ethanol were added and homogenized. After being centrifuged at 4°C, 10,000 ×g for 10 min, the supernatants were aspirated according to the instructions of TG and TC reagent kit at a wavelength of 510 nm in the microplate reader. The contents of TG and TC were detected according to the kit instruction.
Non-Esterified Fatty Acid (NEFA) Test
Sixty larvae were cleaned by precooled PBS for three times and 2 mL extraction liquid was added to mechanically homogenize the larvae samples under temperature of the mixture of ice and water. The supernatants were then collected after being centrifuged at 4°C for 10 min (10,000 ×g) and divided into control tube and measuring tube with addition of 0.5 mL contrast liquid and reaction liquid, respectively. The mixture was placed on ice for measurement. The standard tubes were prepared according to the instruction. The control tube, measuring tube and standard tube were vortexed for 3 min followed by 3 min treatment at room temperature. Then, 0.3 mL of the upper layer liquid was collected for microplate reader analysis at a wavelength of 715 nm and the content of NEFA was detected according to the kit instruction.
Construction of Protein Interaction Networks
For protein interaction network construction, keywords of “insulin resistance” and “fatty acid oxidation” were imported into Kyoto Encyclopedia of Genes and Genomes (KEGG) database. The protein information concerning “insulin resistance” and “fatty acid oxidation” were collected and screened. Pathway analysis was carried out according to KEGG database. And, protein–protein interactions (PPI) were analyzed by SRING database.
Protein Isolation and Western Blot
Thirty-five zebrafish larvae were lysed on ice and the protein was isolated by using RIPA buffer supplemented with protease inhibitors (RIPA lysate: Phenyl methyl sulfonyl fluoride: Protein phosphatase inhibitor = 100:1:1). Centrifuging for 15 min and collecting the supernatant. The protein concentration was detected with the BCA kit, and then the protein concentration was adjusted to be consistent with the Lysis Buffer. Protein loading buffer was added (total protein: loading buffer=4:1) and heated for 5 min at 100°C, then cooled to room temperature. Equal amounts of sample protein were loaded and separated on 10% SDS-PAGE gels and electrotransferred onto polyvinylidene fluoride membranes. The polyvinylidene fluoride membranes were blocked with 5% milk for 2.5 h at room temperature and immunoblotted with primary antibodies AMPKα (1:1000), PAMPKα (1:1000), GAPDH (1:1000) overnight at 4°C. After incubating with the corresponding secondary antibodies (1:5000), the signals were detected with an enhanced chemiluminescence kit and exposed in gel imager. The net optical density was quantificationally analyzed with the gel imager analysis software.
Real-Time Quantitative PCR (qRT-PCR) Analysis
Total RNA was extracted from 38 juvenile fish using Trizol reagent, and then dissolved in 50 μL of RNase-free water. The final RNA purity was detected by measuring the OD260/280 value using a nucleic acid/protein analyzer and RNA integrity was verified by Gold View I type on 1% agarose gel. The reaction conditions were as follows: 95°C 10 min, 95°C 15 s, 60°C 30 s (40 cycles). Ct values were obtained, and the relative expression of target gene mRNA was calculated using the 2−ΔΔCt method. Melting curve analysis was performed at the end of each PCR run to ensure amplification of a single product. Gene-specific primer sequences for qRT-PCR are listed in Table 2.
 |
Table 2 Primers Used for qRT-PCR |
Results
The Content Comparison of 50% Alcohol Extracts and Aqueous Extracts
From HPLC chromatogram (Figure 2), 6 peaks have been assigned using reference compounds both in 50% alcohol-extracted and aqueous-extracted of RPMP (catechin, gallic acid ester, stilbene glycoside, emodin glycoside, emodin and physcion). The content of emodin in 50% alcohol-extract was 12.99 times that in aqueous extract, as shown in Figure 2A and B. However, the content of catechin, gallic acid ester, stilbene glycoside, emodin glycoside and physcion in the alcohol extract were 1.12 times, 1.09 times, 1.38 times, 1.97 times and 0.88 times that in the aqueous extract, respectively.
 |
Figure 2 (A) HPLC chromatogram of the 50% ethanol extract of RPMP. (B) HPLC chromatogram of the aqueous extract of RPMP. |
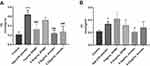
Length, Weight and BMI
After 72 hrs of the feeding experiment, the body weight and length of the zebrafish larval were detected, as shown in Figure 3. Compared with the control group, the body length (Figure 3A), body weight (Figure 3B) and BMI (Figure 3C) of zebrafish larval in the model group were increased. After 72 hrs of administration, compared with the model group, the body weight and body length were decreased in RPMP group and emodin group. In addition, both 1 mg/mL RPMP group and 0.5 μg/mL emodin and 0.25 μg/mL emodin groups could reduce the BMI of zebrafish compared to the model group, which indicated that RPMP and emodin could reduce zebrafish obesity.
Whole-Mount Oil Red O Staining
As shown in Figure 4, the liver of control group was not stained after the Whole-mount Oil Red O staining, but the liver of model group was stained. The staining of zebrafish liver and blood vessel staining was correspondingly lighter after administration (Figure 4A). The statistical results of image J software also showed that the gray value of the liver part in the model group was significantly higher than that in control group, RPMP treatment group and emodin treatment group (Figure 4B). Among which, 1mg/mL RPMP, 0.5 μg/mL, 0.25 μg/mL emodin exhibited an obvious effect on alleviating the liver staining in zebrafish. These results demonstrated that RPMP and emodin could reduce liver lipid accumulation.
HE Staining
To further evaluate the liver histological changes, the liver sections were stained by H&E staining. The whole fish was embedded, sectioned and photographed. As shown in Figure 5, the morphology of zebrafish liver tissues in control group was normal, and hepatocytes were arranged closely. However, a large number of vacuoles and irregular arrangement of liver cells were observed in pathological sections of model group due to the lipid droplets deposited in zebrafish liver tissue. But the lipid droplets and the vacuoles in hepatocyte space of liver were reduced after treating with RPMP and emodin, and the arrangement was tended to be regular. The liver tissues of zebrafish in 0.5μg/mL emodin treatment group were regularly arranged and were similar to that of the control group, which showed an optimal therapeutic effect.
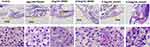 |
Figure 5 Effect of RPMP and emodin on liver tissue morphology of zebrafish (yellow arrow refers to liver). |
Frozen Oil Red O Staining
As shown in Figure 6, compared with the control group, the model group showed an increase of lipid droplets and diffuse red stained lipid droplets in liver cells. Compared with the model group, red lipid droplets in liver were decreased after administrating with RPMP and emodin. According to the diffuse of red-stained lipid in liver, 1mg/mL RPMP, 0.5μg/mL and 0.25μg/mL emodin showed an optimal elimination on the lipid deposition in zebrafish liver. These results suggested that RPMP and emodin could protect against liver steatosis induced by egg yolk powder. And the effect of emodin on reducing liver lipid deposition was more obvious.
 |
Figure 6 Effect of RPMP and emodin on lipid deposition in zebrafish liver. |
Analysis of TG and TC Content
As shown in Figure 7, the contents of TG and TC were significantly increased in the model group compared with control group. Although 1mg/mL RPMP, 0.5μg/mL and 0.25μg/mL emodin could decrease the TG content compared with the model group. The inhibition of TC expression was only found in 0.5 µg/mL emodin treatment group, but not RPMP treatment group.
Analysis of Non-Esterified Fatty Acid (NEFA) Content
As shown in Figure 8, the content of NEFA was significantly increased in the model group compared with control group. Emodin treatment group significantly decreased the content of NEFA, while there was no statistical significance between RPMP treatment group and model group.
Protein Interaction Networks Construction
As shown in Figure 9, the hub value of proteins was carried out using SRING database (Figure 9). It was found that AMPK participated in the regulation of IR and fatty acid oxidation, which indicated that AMPK might be a crucial protein during the development of NAFLD.
 |
Figure 9 PPI plot of IR and fatty acid oxidation. |
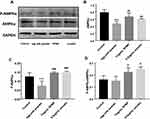
Western Blot
The expressions of proteins were shown in Figure 10A. We found decreased expression of AMPKα and P-AMPKα in the model group, and RPMP significantly increased the expression of AMPKα (Thr172) (Figure 10B) and P-AMPKα (Figure 10C) compared with model group. According to the value of P-AMPKα/AMPKα, both RPMP and emodin significantly increased phosphorylation of AMPKα (Figure 10D). It suggested that RPMP treatment of NAFLD might be related to the AMPK pathway.
RT-qPCR
The mRNA expression level is shown in Figure 11A–FG. Compared with the model group, the expression level of target gene PI3K, AKT2, AMPKα, PPARα, ACOX1, CPT-1a was significantly increased after treatment with RPMP and emodin. The expression level of adipoR2 was not significantly increased in RPMP group. However, the expression level of adipoR2 was significantly enhanced in emodin group, as shown in Figure 11G. These results indicated that emodin might be the main component of RPMP in the treatment of NAFLD via promoting IR and fatty acid oxidation.
 |
Figure 11 Continued. |
Discussion
As a traditional medicinal herb in East Asia for more than three thousand years, RPMP has been proved to have the effect of lowering blood lipid and is used as a therapeutic drug for NAFLD in clinic. However, the effective substances of RPMP have not been fully confirmed and the exact mechanism of its protective effect has not been fully understood.
Accumulating evidence suggested that emodin can improve key features of NAFLD, including hepatic steatosis, obesity and hyperlipidemia.21,22 In previous study carried out in our lab, it was preliminarily found that emodin may be the potential main effective component for RPMP’s treatment for NAFLD. In the present study, the therapeutic effects of RPMP and emodin on NAFLD were explored by activating AMPK to clarify that emodin may be the main active component of RPMP.
Studies have shown that the main lipid-lowering ingredients in RPMP include TSG, emodin and physcion. TSG showed the best cholesterol-reducing effect, especially LDL. Emodin showed the best TG-reducing effect and physcion showed the best VLDL-reducing effect.15 In preliminary experiment, the better effect of 50% alcohol extract than water extract of RPMP suggested the different efficacy might result from the great concentration difference of emodin which was 12.99 times in 50% alcohol extract than water extract of RPMP. Our findings also suggested that egg yolk powder induced NAFLD model significantly increased TG expression. RPMP and emodin reduced TG more significantly than TC, which suggested that emodin was the main component in the treatment of NAFLD in RPMP.
A vicious cycle is formed due to excessive production of FFA in body and leads to the increase of liver TG.23 The accumulation of FFA in muscle and liver induced by increased lipolysis in turn interferes insulin’s utilization, glucose metabolism and causing IR.24 The peripheral tissue glucose uptake and utilization capacity are decreased by IR,25 and excess glucose reaches the liver through blood circulation and deposits in the form of TG. The decomposition of TG into FFA has been proved to be a determining factor for changes in insulin sensitivity.26 In addition, lipid synthesis of adipose tissue is weakened while lipolysis is strengthened when IR occurs.27 Therefore, TG and FFA play an important role in IR in NAFLD patients. The PI3K-AKT signaling pathway is one of the major pathway of IR. It is an effective strategy to prevent IR and type 2 diabetes by regulating AKT and AMPK.28–30 For example, AMPK can regulate the activity of PI3K and AKT by combining IRS-1 (insulin receptor substrate-1).31 The AMPK is a serine/threonine protein kinase that is highly conserved in higher eukaryotes.32 Threonine 172 is involved in AMPK activation and its phosphorylation plays an important role in the regulation of AMPK activity.33,34 After AMPK is activated, the expression of target genes that promote fatty acid oxidation are increased.35 Emodin can attenuate lipid accumulation by decreasing lipogenesis and increasing mitochondrial fatty acid β-oxidation mediated by activation of the AMPK signaling pathway.17
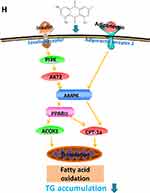
The dynamic balance of liver TG content cannot be maintained without mitochondrial fatty acid beta-oxidation. AdipoR2 mainly exists in liver and related to mitochondrial fatty acid beta-oxidation. The accumulation of hepatic triglyceride can be increased by adiponectin-lowering level. Adiponectin binds to AdipoR2 and the affinity is related to adiponectin sensitivity,36 which regulates substrate metabolism by activating several key participants in cell energy management, including AMPK, PPARα and adiponectin,37 that leads to enhanced fatty acid oxidation, thus preventing hepatic steatosis and enhancing insulin sensitivity.38 The pathway of CPT-1 transport across the mitochondrial outer membrane is catalyzed by the oligoyl-CoA synthetase on the outer membrane of mitochondria, which determines the rate of mitochondrial β-oxidation.39 ACOX1, the rate-limiting enzyme for β-oxidation of peroxisome fatty acids, mainly regulates the β-oxidation process of fatty acids in mitochondria and peroxisome,40 which determines the oxidation rate of peroxisome. PPARα is an important regulatory factor in fatty acid oxidation, which regulates the expression of its downstream target proteins CPT1, ACOX1,41 and regulates fatty acid oxidation. AMPK can indirectly regulate PPARα, but the mechanism of correlation is not clear. Studies have shown that pretreatment with AMPK inhibitor Compound C attenuates the effects of lipid accumulation and expression of PPARa proteins,42 which suggest that PPARα is a downstream gene of AMPK. Inhibition of AMPK activity leads to reduced expression of PPARα. AMPK activation enhances PPARα activity may be via ERK1/2 MAPK signaling pathway.43 The AMPK signaling pathway related to emodin and RPMP treatment of NAFLD is shown in Figure 11H. In this study, both 50% alcohol-extracted of RPMP and emodin can improve IR through PI3K/AKT2/AMPKα pathway and promote the expression of PPARα, CPT-1a and ACOX1 to enhance the oxidation of fatty acids. Emodin exhibited a preferable effect on lipid-lowering relative to RPMP for it can reduce both the content of TG and TC. In addition, RPMP had no significant effect on lowering FFA and increasing expression of adipoR2. Some studies show that adiponectin will play an import role in lipid-lowering only after binding to its receptor,44 which consequently promotes FFA oxidation.45 Therefore, we suspected that adipoR2 was one of the main targets to promote fatty acid oxidation. Our results indicated that emodin was a main effective component of RPMP in the treatment of NAFLD. But there may be other components in RPMP that cooperate with emodin for the treatment of NAFLD, which provided an interesting consideration for lipid-lowering research of other components of RPMP.
Conclusions
Our research indicates that emodin is the main component of RPMP in the treatment of NAFLD, which is closely related to the regulation of AMPK signaling pathway, and the increase of IR and fatty acid oxidation.
Author Contributions
All authors contributed to data analysis, drafting or revising the article, gave final approval of the version to be published, and agree to be accountable for all aspects of the work.
Funding
The study was supported by the National Natural Science Foundation of China (No: 81373943, 81573583), Sichuan Provincial Science and Technology Department of Youth Science and Technology Innovation Research Team Program (2017TD0001), National Natural Science Foundation of China (U19A2010).
Disclosure
The authors declare no conflict of interest in this work.
References
1. Nseir W, Mahamid M. Statins in nonalcoholic fatty liver disease and steatohepatitis: updated review. Curr Atheroscler Rep. 2013;3:305. doi:10.1007/s11883-012-0305-5
2. Yari Z, Hekmatdoost A. Dietary interventions in fatty liver. In: Dietary Interventions in Gastrointestinal Diseases. 2019;245–257.
3. Gaemers IC, Groen AK. New insights in the pathogenesis of non-alcoholic fatty liver disease. Curr Opin Lipidol. 2006;3:268–273. doi:10.1097/01.mol.0000226118.43178.98
4. Younossi Z, Anstee QM, Marietti M, et al. Global burden of NAFLD and NASH: trends, predictions, risk factors and prevention. Nat Rev Gastroenterol Hepatol. 2018;1:11–20. doi:10.1038/nrgastro.2017.109
5. Chen ZF, Zhang L, Yi JY, et al. Promotion of adiponectin multimerization by emodin: a novel AMPK activator with PPARγ-agonist activity. J Cell Biochem. 2012;11:3547–3558. doi:10.1002/jcb.24232
6. Chang Y, Jung HS, Cho J, et al. Metabolically healthy obesity and the development of nonalcoholic fatty liver disease. Am J Gastroenterol. 2016;8:1133–1140. doi:10.1038/ajg.2016.178
7. Boden G. Role of fatty acids in the pathogenesis of insulin resistance and NIDDM. Diabetes. 1997;1:3–10. doi:10.2337/diab.46.1.3
8. Saltiel AR, Kahn CR. Insulin signalling and the regulation of glucose and lipid metabolism. Nature. 2001;6865:799–806. doi:10.1038/414799a
9. Dzamko N, van Denderen BJ, Hevener AL, et al. Ampk β1 deletion reduces appetite, preventing obesity and hepatic insulin resistance. J Biol Chem. 2010;1:115–122. doi:10.1074/jbc.M109.056762
10. Gauthier MS, O’Brien EL, Bigornia S, et al. Decreased AMP-activated protein kinase activity is associated with increased inflammation in visceral adipose tissue and with whole-body insulin resistance in morbidly obese humans. Biochem Biophys Res Commun. 2011;1:382–387. doi:10.1016/j.bbrc.2010.11.127
11. Gong LB, Pang HL, Li ZL. Common Use of Chinese Patent Medicines. Beijing, China: Military Science Publishing House; 2009:1–2.
12. Chinese Pharmacopoeia Commission. Pharmacopeia of the People’s Republic of China. Beijing, China: The medicine science and technology press of China; 2015.
13. Kong XL. Chinese Medicine Treatment of Fatty Liver. Beijing, China: Press of Traditional Chinese Medicine; 2005.
14. Choi RY, Lee HI, Ham JR, et al. Heshouwu, (polygonum multiflorum, thunb.) ethanol extract suppresses pre-adipocytes differentiation in 3T3-L1 cells and adiposity in obese mice. Biomed Pharmacother. 2018;106:355–362. doi:10.1016/j.biopha.2018.06.140
15. Wang W, He Y, Lin P, et al. In vitro effects of active components of polygonum multiflorum radix on enzymes involved in the lipid metabolism. J Ethnopharmacol. 2014;3:763–770. doi:10.1016/j.jep.2014.03.042
16. Li J, Ding L, Song B, et al. Emodin improves lipid and glucose metabolism in high fat diet-induced obese mice through regulating srebp pathway. Eur J Pharmacol. 2016;770:99–109. doi:10.1016/j.ejphar.2015.11.045
17. Tzeng TF, Lu HJ, Liou SS, et al. Emodin, a naturally occurring anthraquinone derivative, ameliorates dyslipidemia by activating AMP-activated protein kinase in high-fat-diet-fed rats. Evid Based Complement Alternat Med. 2012;2012:781812. doi:10.1155/2012/781812
18. Choi Y, Abdelmegeed MA, Song BJ. Diet high in fructose promotes liver steatosis and hepatocyte apoptosis in C57BL/6J female mice: role of disturbed lipid homeostasis and increased oxidative stress. Food Chem Toxicol. 2017;103:111–121. doi:10.1016/j.fct.2017.02.039
19. Zhou YM, Zhao MJ, Gong XH. The influence of different processing time on composition and content of polygoni multiflori. Nat Prod Res Dev. 2017;10:129–135.
20. Westerfield M. The Zebrafish Book. A Guide for the Laboratory Use of Zebrafish (Danio Rerio). America, oregon: Inst of Neuro Science; 2000.
21. Tzeng TF, Lu HJ, Liou SS, et al. Emodin protects against high-fat diet-induced obesity via regulation of AMP-activated protein kinase pathways in white adipose tissue. Planta Med. 2012;10:943–950.
22. Wang S, Li X, Guo H, et al. Emodin alleviates hepatic steatosis by inhibiting SREBP1 activity via the CaMKK-AMPK-mTOR-p70S6K signaling pathway. Hepatol Res. 2016;7:683–701.
23. Ma M, Duan R, Zhong H, et al. The crosstalk between fat homeostasis and liver regional immunity in NAFLD. J Immunol Res. 2019;2019:1–10. doi:10.1155/2019/3954890
24. Dirks ML, Wall BT, van de Valk B, et al. One week of bed rest leads to substantial muscle atrophy and induces whole-body insulin resistance in the absence of skeletal muscle lipid accumulation. Diabetes. 2016;65(10):2862–2875. doi:10.2337/db15-1661
25. Lu J, Xie G, Jia W, et al. Insulin resistance and the metabolism of branched-chain amino acids. Front Med. 2013;1:53–59. doi:10.1007/s11684-013-0255-5
26. Girousse A, Tavernier G, Valle C, et al. Partial inhibition of adipose tissue lipolysis improves glucose metabolism and insulin sensitivity without alteration of fat mass. PLoS Biol. 2013;2:e1001485. doi:10.1371/journal.pbio.1001485
27. Perry RJ, Shulman GI. Treating fatty liver and insulin resistance. Aging. 2013;11:791–792. doi:10.18632/aging.100617
28. Mazibuko-Mbeje S, Dludla P, Roux C, et al. Aspalathin-enriched green rooibos extract reduces hepatic insulin resistance by modulating PI3K/AKT and AMPK pathways. Int J Mol Sci. 2019;3:633. doi:10.3390/ijms20030633
29. Mackenzie RW, Elliott BT. Akt/PKB activation and insulin signaling: a novel insulin signaling pathway in the treatment of type 2 diabetes. Diabetes Metab Syndr Obes. 2014;7:55–64. doi:10.2147/DMSO
30. Jung JY, Lee CW, Park SM, et al. Activation of AMPK by Buddleja officinalis Maxim. Flower extract contributes to protecting hepatocytes from oxidative stress. Evid Based Compl Alt. 2017;1:1–15.
31. Tao R, Gong J, Luo X, et al. AMPK exerts dual regulatory effects on the PI3K pathway. J Mol Signal. 2010;1:1–9. doi:10.1186/1750-2187-5-1
32. Shaw RJ. LKB1 and AMP-activated protein kinase control of mTOR signalling and growth. Acta Physiol. 2009;1:65–80. doi:10.1111/j.1748-1716.2009.01972.x
33. Woods A, Vertommen D, Neumann D, et al. Identification of phosphorylation sites in AMP-activated Protein Kinase (AMPK) for upstream AMPK kinases and study of their roles by site-directed mutagenesis. J Biol Chem. 2003;31:28434–28442. doi:10.1074/jbc.M303946200
34. Chen PC, Kryukova YN, Shyng SL. Leptin regulates KATP channel trafficking in pancreatic β-cells by a signaling mechanism involving AMP-activated Protein Kinase (AMPK) and cAMP-dependent Protein Kinase (PKA). J Biol Chem. 2013;47:34098–34109. doi:10.1074/jbc.M113.516880
35. Ali F, Ismail A, Esa NM, et al. Cocoa polyphenols treatment ameliorates visceral obesity by reduction lipogenesis and promoting fatty acid oxidation genes in obese rats through interfering with AMPK pathway. Eur J Lipid Sci Tech. 2016;4:564–575. doi:10.1002/ejlt.201400581
36. Kawano J, Arora R. The role of adiponectin in obesity, diabetes, and cardiovascular disease. J Cardiometab Syndr. 2009;1:44–49. doi:10.1111/j.1559-4572.2008.00030.x
37. Dasarathy J, Periyalwar P, Allampati S, et al. Hypovitaminosis D is associated with increased whole body fat mass and greater severity of non-alcoholic fatty liver disease. Liver Int. 2014;6:e118–e127. doi:10.1111/liv.12312
38. Polyzos SA, Toulis KA, Goulis DG, et al. Serum total adiponectin in nonalcoholic fatty liver disease: a systematic review and meta-analysis. Metabolism. 2011;3:313–326. doi:10.1016/j.metabol.2010.09.003
39. Ji S, You Y, Kerner J, et al. Homozygous carnitine palmitoyltransferase 1b (muscle isoform) deficiency is lethal in the mouse. Mol Genet Metab. 2008;3:314–322. doi:10.1016/j.ymgme.2007.10.006
40. Yang L, Zhang Y, Wang S, et al. Decreased liver peroxisomal β-oxidation accompanied by changes in brain fatty acid composition in aged rats. Neurol Sci. 2014;2:289–293. doi:10.1007/s10072-013-1509-3
41. Zhou M, Zeng D, Ni X, et al. Effects of bacillus licheniformis on the growth performance and expression of lipid metabolism-related genes in broiler chickens challenged with clostridium perfringens-induced necrotic enteritis. Lipids Health Dis. 2016;1:48–58. doi:10.1186/s12944-016-0219-2
42. Lee MS, Kim D, Jo K, et al. Nordihydroguaiaretic acid protects against high-fat diet-induced fatty liver by activating AMP-activated protein kinase in obese mice. Biochem Biophys Res Commun. 2010;1:92–97. doi:10.1016/j.bbrc.2010.09.016
43. Meng R, Pei Z, Zhang A, et al. AMPK activation enhances PPARα activity to inhibit cardiac hypertrophy via ERK1/2MAPK signaling pathway. Arch Biochem Biophys. 2011;1–2:1–7. doi:10.1016/j.abb.2011.04.010
44. Punyadeera C, Zorenc AHG, Koopman R, et al. The effects of exercise and adipose tissue lipolysis on plasma adiponectin concentration and adiponectin receptor expression in human skeletal muscle. Eur J Endocrinol. 2005;3:427–436. doi:10.1530/eje.1.01872
45. Yamauchi T, Kamon J, Ito Y, et al. Cloning of adiponectin receptors that mediate antidiabetic metabolic effects. Nature. 2003;6941:762–769. doi:10.1038/nature01705
 © 2020 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2020 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.