Back to Journals » Drug Design, Development and Therapy » Volume 10
Protective effects of vitamin C and vitamin E against hysterosalpingography-induced epithelial degeneration and proliferation in rat endometrium
Authors Pala Ş, Atilgan R , Kuloğlu T, Kara M, Başpınar M , Can B, Artaş G
Received 14 July 2016
Accepted for publication 17 November 2016
Published 15 December 2016 Volume 2016:10 Pages 4079—4089
DOI https://doi.org/10.2147/DDDT.S117207
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 3
Editor who approved publication: Dr Qiongyu Guo
Şehmus Pala,1 Remzi Atilgan,1 Tuncay Kuloğlu,2 Murat Kara,3 Melike Başpinar,1 Behzat Can,1 Gökhan Artaş4
1Department of Obstetrics and Gynecology, 2Department of Histology and Embriology, Firat University School of Medicine, Elazig, 3Department of Medical Biology and Genetics, Muğla Sıtkı Koçman University School of Medicine, Muğla, 4Department of Pathology, Firat University School of Medicine, Elazig, Turkey
Aim: The aim of this study was to examine the protective effects of vitamin C (VC) and vitamin E (VE) against hysterosalpingography (HSG)-induced epithelial degeneration and proliferation in rat endometrium.
Materials and methods: A total of 28 female Wistar albino rats were randomized into four groups: G1 (n=7; abdomen was opened and closed), G2 (n=7; 0.1 mL Lipiodol [ethiodized oil] was administered to each uterine horn in conjunction with X-ray irradiation), G3 (n=7; 50 mg/kg of intraperitoneal (ip) VC was administered, followed by the administration of 0.1 mL of ethiodized oil into the uterine horns after 15 minutes), and G4 (n=7; 50 mg/kg of ip VE was administered, followed by the administration of 0.1 mL of ethiodized oil into the uterine horns after 15 minutes). After abdominal closure, rats in G2, G3 and G4 groups were exposed to whole-body X-irradiation three times with 2-minute intervals at a total dose of 15–20 mrad. Three hours after exposure, abdominal cavities of all the rats were reopened and uterine horns were removed. The right uterine horns were embedded into paraffin blocks after fixing in 10% formaldehyde for histopathological and immunohistochemical examination. Uterine horns on the other side were rapidly excised and stored at -80°C for the examination of expression of microRNAs (miRNAs) and oxidant, antioxidant, apoptotic and antiapoptotic gene expression using real-time polymerase chain reaction (RT-PCR) method.
Results: No differences were observed in terms of expression of miRNAs and oxidant, antioxidant, apoptotic and anti-apoptotic gene expression between the study groups. Congestion, epithelial degeneration and malondialdehyde immunoreactivity were significantly lower in G3 and G4 groups than in G2 group; no differences were observed between G1, G3 and G4 groups. Ki-67 immunoreactivity score was significantly higher in G2 group when compared with G1, G3 and G4 groups. Caspase-3 immunoreactivity was not statistically different between the groups.
Conclusion: VC and VE may confer cellular protection against radiation injury induced by HSG in endometrial epithelium.
Keywords: vitamin C, vitamin E, radiation, endometrium, rat, miRNA
Introduction
Hysterosalpingography (HSG) is a screening and diagnostic tool used in the radiographic examination of ovarian tubes and uterine cavity with injection of a contrast medium. However, HSG is also associated with certain disadvantages since it can be painful and bothersome for the patient and also involves exposure to ionizing radiation, although in low doses.1,2 Ionizing radiation can be defined as an electromagnetic wave or particle that can trigger certain chemical alterations through freeing ions on surfaces with which it interacts. Radiation damage is caused by either a direct interaction with target molecules or indirectly through the effect of chemically or pharmacologically active elements derived mainly from water molecules. After being absorbed, radiation affects the electrons of atoms and molecules in tissue. This process leads to the formation of positive ions. However, from a biological viewpoint, the most significant alteration at the cellular level is the generation of molecules that carry an unpaired electron in one of the orbitals known as free radicals. Free radicals are compounds that exist only for several seconds. Radioprotective mechanisms involve the inactivation of free radicals, hydrogen atom binding to target molecules, formation of mixed sulfide compounds and delay in cellular division and induction of hypoxia in tissues.3
Although the toxicity of exogenous materials has been extensively examined in numerous previous studies,4,5 studies on these chemicals are relatively scarce in number. Therefore, we designed the present study on the basis of the assumption that antioxidant defense mechanisms, apoptotic and anti-apoptotic genes and certain microRNAs (miRNAs) could be activated in response to immune system activity and that the free radicals are released into the organism through the effect of radiation. Thus, from this viewpoint, determination of the expression of antioxidant genes bears significance, which include glutathione reductase (GSR); glutathione peroxidase 3 (GPX3); superoxide dismutase 1 (SOD1); nitric oxide synthase 2 (RAI-NOS); the apoptotic–anti-apoptotic genes heat shock 70 kDa protein4 (HSP7), Bcl2-associated X protein (BAX), B-cell lymphoma gene-2 (Bcl-2), caspase-3 (CASP3) and malate dehydrogenase 1 (MDH1) and miR-15b, miR-21, miR-34 and miR-98 in the endometrial tissues. Antioxidant response in mammalian cells occurs through the enzymatic and nonenzymatic pathways.4 Although various enzymes play key roles in the enzymatic pathways, the primary function of these enzymes is the maintenance of the redox balance.5
Antioxidant nutrients such as vitamin C (VC) and vitamin E (VE) have also been reported to exhibit protective effects against ionizing radiation damage. In experimental animal studies, VC and VE, when present in biological systems during exposure to radiation, have been shown to decrease the frequency of chromosomal aberrations through binding free radicals.6–9
Materials and methods
The present study was conducted at the Laboratory Center for Experimental Studies, Firat University (Firat University Experimental Investigations Department). Twenty-eight albino female Wistar albino rats with regular cycles weighing 190–220 g and 12–14 weeks old were kept under a 12-hour artificial light–dark cycle (8 am to 22 pm) at a temperature of 21°C–23°C in groups of five per cage to maintain the regularity of their biological rhythms. Standard pellets and tap water were used.10 To determine the estrous cycles of the rats, vaginal smear method used by Mallenby et al11 was employed. Twenty-eight rats were randomized prospectively into four groups, with seven in each group. Rats at the estrous stage of the cycle as documented by vaginal cytology were anesthetized by intramuscular 70 mg/kg ketamine (Ketalar, Eczacıbaşı, Turkey) and 10 mg/kg xylazine (Rompun, Bayer, Turkey) and then were placed in the supine position on the operation table and an incision was made in the middle of the abdomen.
- G1: n=7; abdomen was opened and closed, without any further intervention.
- G2: n=7; abdomen was opened and 0.1 mL Lipiodol (ethiodized oil) ampul (Villepinte, Guerbet, France) was administered to each uterine horn in conjunction with X-ray irradiation.
- G3: n=7; abdomen was opened and 50 mg/kg of intraperitoneal (ip) VC (500 mg/5 mL; Redox-C ampul; Bayer AG, Leverkusen, Germany) was administered, followed by the administration of 0.1 mL of ethiodized oil into the uterine horns after 15 minutes.
- G4: n=7; abdomen was opened and 50 mg/kg of ip VE (300 mg/2 mL DL-alpha-tocopherol acetate; Evigen ampul; Aksu Farma, Istanbul, Turkey) was administered, followed by the administration of 0.1 mL of ethiodized oil into the uterine horns after 15 minutes.
After abdominal closure, rats in G2, G3 and G4 groups were exposed to whole-body X-irradiation three times with 2-minute intervals (total dose: 15–20 mrad). Following the exposure, abdominal cavity and the skin were closed with 3–0 silk sutures. After 3 hours, abdominal cavities of all the rats were reopened and uterine horns were removed. The right uterine horns were embedded into paraffin blocks after fixing in 10% formaldehyde. Uterine horns on the other side were rapidly excised and stored at −80°C.
Histological examination
Paraffin blocks were prepared for histological assays. The 5–6 μm thick cross-sections obtained from the paraffin blocks were stained with Masson’s trichrome staining. Epithelial degeneration was evaluated as no (0), light (1), middle (2) and severe (3). Scoring was done for each rat, and mean values were detected for each group.
Immunohistochemical examination
The 5–6 μm thick cross-sections prepared from paraffin blocks were placed on polylysine-coated microscopic slides. Routine immunohistochemical staining was performed according to the method of Kuloğlu and Aydin.12 Primary antibodies (rabbit polyclonal anti-malondialdehyde antibody, ab6463; Abcam, Cambridge, UK, and CASP3 rabbit polyclonal immunoglobulin G, ab2302; Abcam) immunoreactivity were studied with avidin–biotin–peroxidase methodology. Ki-67 (monoclonal mouse, anti-human, clone MIB-1 code 7240, Dako Denmark A/S, Glostrup, Denmark) antibody was performed with automatic painting device (SN:712299; Ventana Medical Systems, Inc., Tucson, AZ, USA). Light microscopy and photography were done using an Olympus BX50 light microscopy (Olympus Corporation, Tokyo, Japan). A histoscore was derived from the distribution (0.1: <25%, 0.4: 26%–50%, 0.6: 51%–75%, 0.9: 76%–100%) and intensity (0: no staining, +0.5: very little staining, +1: little staining, +2: medium, +3: very strong) of staining immunoreactivity (histoscore = distribution × intensity).13
TUNEL staining
Cross-sections of 5–6 μm thickness from the paraffin blocks were placed onto microscopic slides. Cells with apoptotic activity were determined using ApopTag Plus Peroxidase In Situ Apoptosis Detection Kit (catalog number: S7101; Chemicon International, Temecula, CA, USA) in accordance with the relevant information provided by the manufacturer. Nuclei staining blue with Harris hematoxylin were considered normal, while those staining brown were considered apoptotic. The extent of the staining was used as the primary assessment criteria in the evaluation of terminal deoxynucleotidyl transferase dUTP nick end labeling (TUNEL) staining. Preparations were evaluated through examination using microscope (Olympus BX50) and photographed. At least 500 cells, normal and apoptotic, were detected in the randomly selected regions of the sections at ×10 magnification. Apoptotic index (AI) was calculated from the proportion of apoptotic cells to total (normal + apoptotic) cells.
Isolation of total RNA from tissues and real-time polymerase chain reaction (RT-PCR) quantifications
Total RNA was isolated from the uterine horn by using a QIA amp RNA isolation kit (Qiagen, Hilden, Germany). RNA samples were preserved at −80°C until quantifications. Gene expression levels were evaluated by the RT-PCR method. For the gene expression studies, SYBR Green Master Mix from Thermo Fisher Scientific (Waltham, MA, USA) was used. Glyceraldehyde-3-phosphate dehydrogenase (GAPDH) gene was used as internal control. All expression analyses were evaluated using Rotor Gene 6000 RT-PCR Machine. (Rotor-Gene Q, Qiagen, Hilden, Germany).
Statistical analysis
The data obtained were presented as mean ± standard deviation. For statistical analysis, SPSS version 22.0 (IBM Corporation, Armonk, NY, USA) software package was used. For the statistical analysis of continuous and ordinal data, one-way analysis of variance and post-hoc Tukey’s HSD test were used. Differences between the groups were evaluated by independent-samples t-test. The level of significance was set at P<0.05.
Ethical standards
This study was approved by the local animal ethics committee of Firat University (date: 16.02.2012, session no: 2012/02, decision no: 27) and does not contain any studies with human participants performed by any of the authors. All experimental manipulations were conducted in accordance with the National Institutes of Health Guide for the Care and Use of Laboratory Animals.
Results
Histopathological findings
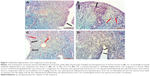
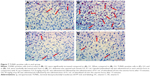
The examination of tissues stained with Masson’s trichrome under light microscope revealed normal appearance of uterine tissues in the G1 group (Figure 1A). A markedly increased epithelial degeneration and congestion was observed in the G2 group compared to the G1 group (P<0.05; Figure 1B). In the G3 (Figure 1C) and G4 groups (Figure 1D), a marked decrease in epithelial degeneration and congestion – close to those of controls (P>0.05) – was observed compared to the G2 group (P<0.05). No difference was observed between G3 and G4 groups (P>0.05; Figure 2).
Immunohistochemical findings
Malondialdehyde (MDA) immunoreactivity
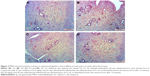
MDA immunoreactivity was observed in endometrial glands in uterus and in stromal cells. MDA immunoreactivity was significantly increased in G2 group (Figure 3B) compared to the G1 group (P<0.05; Figure 3A). Compared to G2 group, MDA immunoreactivity was significantly lower in the G3 (Figure 3C) and G4 (Figure 3D) groups (P<0.05; Figure 4). There were no significant differences between G1, G3 and G4 groups (P>0.05).
Ki-67 immunoreactivity
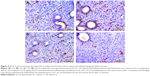
Immunoreactivity of Ki-67 in endometrial stromal cells and in endometrial glands was significantly increased in G2 group compared to G1 group (P<0.05). There was no difference between G1, G3 and G4 groups (P>0.05; Figures 4 and 5).
CASP3 immunoreactivity
There was no difference between groups when CASP3 immunoreactivity was evaluated (P>0.05; Figures 4 and 6).
TUNEL staining
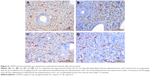
TUNEL-positive cells in G2 group (Figure 7B) were significantly increased compared with G1 group (P<0.05; Figure 7A). When compared with G2 group, TUNEL-positive cells in G3 (Figure 7C) and G4 groups (Figure 7D) were markedly decreased (P<0.05) and almost equal to that in G1 group (P>0.05; Figure 8). There was no difference between G3 and G4 groups (P>0.05).
No statistically significant differences were observed between the groups with respect to antioxidant, apoptotic and anti-apoptotic gene expressions (P>0.05; Table 1).
There were no significant differences in miR-15b, miR-21, miR-34 and miR-98 expressions between the groups (P>0.05; Table 2).
Discussion
In our study, we showed that HSG process significantly increases MDA and Ki-67 immunoreactivity and epithelial degeneration in endometrium. We also showed that VC and VE significantly decreased MDA and Ki-67 scores and epithelial degeneration in endometrium caused by radiation injury. In this aspect, our study is the first in this area.
The total dose of radiation exposure of the patient during an HSG shooting is calculated as 713 cGy·cm2 (range: 247–1,623 cGy·cm2).14 In another study, the average radiation dose exposure of the ovaries during an HSG procedure was found to be 500–1,000 mrad.15 According to these values, we chose the dose used in the HSG process in this study as follows: a human being has 17,000 cm2 average surface area and a rat that weighs 200 g has 325 cm2 average surface area. If we evaluate that a human being is exposed to an average of 700–1,000 cGy radiation during an HSG process, then a rat should take ~14–19 mrad radiation dose. When we searched PubMed, we did not find any study about the dose that should be given to HSG-applied rats. For this reason, in our study, we applied 15–20 mrad radiation during the HSG process.
Lee et al16 in their study, after exposing the mice to gamma radiation, examined primordial and primary follicular damage and found that the most significant damage appeared after 3 hours, with a subsequent decrease at 6 and 12 hours post exposure. Therefore, we examined uterus tissues 3 hours after radiation exposure, following decapitation of rats in all groups.
Reactive oxygen species (ROS) also alter the balance in the endogenous protective systems such as glutathione and enzymatic antioxidant defense systems.17,18 Endogenous antioxidant defense systems fail to eliminate free radicals induced by radiation. Addition of the appropriate antioxidants may inhibit or reduce the toxicity induced by free radicals and thus can provide a radioprotective effect, since exogenous compounds may potentially contribute to the antioxidant capacity of the system. Dietary supplementation of antioxidants such as vitamins, carotenes, carnitine, alpha lipoic acid and polyphenols can be effective in decreasing the sensitivity of the organism to the oxidative damage.18,19 VC, also known as ascorbic acid, is a water-soluble and essential vitamin with strong antioxidant properties. Even small amounts of VC can confer protection against the injury caused by free radicals and reactive oxygen derivatives in essential molecules of the body such as proteins, lipids, carbohydrates and nucleic acids (DNA and RNA). Radioprotective effect of ascorbic acid has been suggested to result from its interaction with free radicals induced by radiation.20 Similarly, pretreatment with ascorbic acid was found to inhibit lipid peroxidation induced by radiation.21 VC has also been shown to have protective effects against diseases in mice exposed to whole-body gamma radiation as well as decreases mortality and improves wound healing.22
VE, chemically belonging to tocopherols, is an important fat-soluble antioxidant that has significant antioxidant activity, particularly in cell membranes and lipoprotein structures. Protective effect of VE against oxidative damage caused by radiation has been demonstrated in vitro, and increased lipid peroxidation was observed in low doses compared to high doses of radiation. Ip administration of VE increased cellular and humoral immunity, indicative of its radioprotective effect. Accordingly, we opted for ip route of administration in our study. In vitro, VE also inhibits transformation due to radiation.23 In our experiment, we examined the radioprotective effects of antioxidant vitamins, such as VC and VE, in radiation damage.
Plasma MDA levels increase in the phase of increasing oxidative stress.24 MDA also increases during ischemia–reperfusion damage.25 Addition of VC and VE has been demonstrated to decrease MDA levels in breast cancer and fibroadenoma patients who had high MDA concentrations in plasma and erythrocytes.26 Likewise, in our experiment, immunohistochemical examination showed significantly lower MDA scores in G3 and G4 groups as compared to G2 group. This finding suggests that the endometrial epithelial degeneration observed in our experiment might be due to the increased ROS generation.27 Radiation could also have led to increased levels of MDA due to ischemia–reperfusion damage in endometrial tissues.
Said et al28 observed degeneration of mucosal epithelial cell layers of uterus in rats exposed to irradiation. One such alteration, membrane blebbing, is a feature of apoptosis and occurs during this process. In our experiment, we observed significantly higher congestion and epithelial degeneration of the endometrial glands in G2 group as compared to G1, G3 and G4 groups.
miRNAs have a number of important functions in cells. miRNA-15b, miRNA-21, miRNA-34 and miRNA-98 are expressed in mammalian cells that carry out many essential cellular functions. miRNAs regulate expression of the protein containing miR-98 and induce these cytokines.29 miRNA-15 is associated with many biological events such as cell division, cell metabolism, stress and angiogenesis. miRNA-21 is believed to be involved in various metabolic events.30 miRNA-34 is assumed to regulate the expressions of various genes (eg, p53, SIRT), which are associated with apoptosis. Free radicals are reactive molecules that are released as a response to cellular stress triggered by exogenous insults, and an increase in their concentration affects living cells adversely.31 Generation of free radicals in cells has strong effects on various primary metabolites and plays a role in the pathogenesis of diseases such as cancer. When administered exogenously, these substances are detected by the organism, which, in turn, develops defense mechanisms against their effects. Subsequently, antioxidant enzymes of the organism are activated for scavenging these molecules without harming the surrounding macromolecules. In case of irreversible damage in the cells, programmed cell death may be induced and apoptotic process begins.32 In our study, mRNA and miRNA genes associated with apoptotic pathway were evaluated in a radiation exposure model. Antioxidant, apoptotic and anti-apoptotic genes were used for the analysis of mRNA expression. Although we could not demonstrate significant differences between the groups in miRNA expression (except miRNA-34), which is assumed to be associated with the apoptotic pathway, an upregulation of miRNA expression was observed in G2 group as compared to G1 group. In comparison to G2 group, G3 group showed downregulation and G4 group showed upregulation. However, apoptosis scores for G1, G3 and G4 groups were similar and significantly increased for G2 group, as shown by TUNEL staining. In contrast to TUNEL staining, we found that there was no difference between groups with respect to CASP3 immunoreactivity. However, we observed similar results between CASP3 staining scores and biochemical evaluation in all groups. This shows us that although the TUNEL method is widely used to determine apoptosis in situ, using the TUNEL method alone is not very reliable, which supports the idea that this method should be used with other methods that determine routine morphology or apoptosis.33,34
It is reported that generally tumor growth is related to increased proliferation rate and low apoptosis rates that do not balance cell growth. In one study, it was shown that in case of high Ki-67 levels, there are low apoptotic scores. But after therapy, apoptotic scores increased and proliferation scores decreased.35 Nevertheless, there are studies that report high apoptotic scores with high Ki-67 scores.36–39 In our study, we showed that there was a significant increase in Ki-67 immunoreactivity in G2 group compared to G1, G3 and G4 groups. Also, the apoptotic scores evaluated with TUNEL staining were correlated with Ki-67 and there was a significant increase in G2 group than in the other groups. Our finding is compatible with the findings of McMenamin et al36 and Stoll et al.39 But we could not show any correlation between increasing Ki-67 and CASP3 immunoreactivity. Our findings show that the HSG process can initiate proliferative changes in the endometrial tissue. The Ki-67 immunoreactivity, which was significantly lower in G3 and G4 groups compared with G2 group, can indicate the protective effect of VC and VE against the proliferative effect of radiation.
In our study, we failed to demonstrate significant consistency between histochemical and biochemical findings. Factors that may account for this discrepancy include lower doses of radiation (total dose =15–20 mrad; 1 rad =0.01 Gy), short duration of exposure, early termination of the experiment (at 3 hours), possible effects of radiation on oxidative and antioxidant defenses and possible interference of other endogenous defense mechanisms.40 Further studies could provide clues on the possible causes of the discrepancy observed in our study between biochemical and histochemical findings. On the other hand, our histochemical observations confirmed that cellular microscopic alterations occur at a very early stage in this setting, suggesting that even a very low dose of radiation could have detrimental effects at the cellular level.
Conclusion
VC and VE could have cell protective effects against the injury induced by HSG in endometrial epithelium.
Acknowledgment
The study was funded by Firat University, Scientific Research Grant (project number: 29.08.2012 – TF.12.68).
Disclosure
The authors report no conflicts of interest in this work.
References
Simpson WL, Beitia LG, Mester J. Hysterosalpingography: a reemerging study. Radiographics. 2006;26(2):419–431. | ||
Baramki TA. Hysterosalpingography. Fertil Steril. 2005;83(6):1595–1606. | ||
Varanda EA, Tavares DC. Radioprotection: mechanisms and radioprotective agents including honeybee venom. J Venom Anim Toxins. 1998;4:5–21. | ||
Bartel DP. MicroRNAs: target recognition and regulatory functions. Cell. 2009;136(2):215–233. | ||
Du C, Liu C, Kang J, et al. MicroRNA miR-326 regulates TH-17 differentiation and is associated with the pathogenesis of multiple sclerosis. Nat Immunol. 2009;10(12):1252–1259. | ||
el-Nahas SM, Mattar FE, Mohamed AA. Radioprotective effects of vitamins C and E. Mutat Res. 1993;301(2):143–147. | ||
Felemovicius I, Bonsack ME, Baptista ML, Delaney JP. Intestinal radioprotection by vitamin E (alpha-tocopherol). Ann Surg. 1995;222(4):504–508. | ||
Prasad KN. Multiple dietary antioxidants enhance the efficacy of standard and experimental cancer therapies and decrease their toxicity. Integr Cancer Ther. 2004;3(4):310–322. | ||
Prasad KN. Rationale for using high. Dose multiple dietary antioxidants as an adjunct to radiation therapy and chemotherapy. J Nutr. 2004;134(11):3182–3183. | ||
National Institutes of Health. Principles of Laboratory Animal Care. Bethesda, MD: National Institutes of Health. NIH Publication no: 86–23, Revised 1985. | ||
Mallenby J, Dunyer J, Hawkins C, Hitchen C. Effects of experimental limbic on the estrus cycle and reproductive success in rats. Epilepsia. 1991;34(2):220–227. | ||
Kuloğlu T, Aydin S. Immunohistochemical expressions of adropin and inducible nitricoxide synthase in renal tissues of rats with streptozotocin-induced experimental diabetes. Biotech Histochem. 2014;89(2):104–110. | ||
Inan S, Vatansever S, Celik-Ozenci C, Sanci M, Dicle N, Dmir R. Immunolocalizations of VEGF, its receptors flt-1. KDR and TGF-beta’s in epithelial ovarian tumors. Histol Histopatol. 2006;21(10):1055–1064. | ||
Fernández JM, Vañó E, Guibelalde E. Patient doses in hysterosalpingography. Br J Radiol. 1996;69(824):751–754. | ||
Shirley LH. Ovarian radiation dosage during histerosalpingogram. Fertil Steril. 1971;22(2):83–85. | ||
Lee CJ, Park HH, Do BR, Yoon Y, Kim JK. Natural and radiation induced degeneration of primordial and primary follicles in mouse ovary. Anim Reprod Sci. 2000;59(1–2):109–117. | ||
Beutler E, Gelbart T, Pegelow C. Erythrocyte glutathione synthetase deficiency leads not only to glutathione but also to glutathione-S-transferase deficiency. J Clin Invest. 1986;77(1):38–41. | ||
Koracevic D, Koracevic G, Djordjevic V, Andrejevic S, Cosic V. Method for measurement of antioxidant activity in human fluids. J Clin Path. 2001;54(5):356–361. | ||
Urso ML, Clarkson PM. Oxidative stress, exercise, and antioxidant supplementation. Toxicology. 2003;189(1–2):41–54. | ||
Duchesne J, Gilles R, Mosora F. Effect of antioxidant substances on the level of free organic radicals naturally present in the rat diaphragm. C R Acad Sci Hebd Seances Acad Sci D. 1975;281(13):945–947. | ||
Jagetia GC. Ascorbic acid treatment reduces the radiation induced delay in the skin excision wound of Swiss albino mice. Indian J Radiat Res. 2004;1:7. | ||
Jagetia GC, Rajanikant GK, Mallikarjun Rao KV. Ascorbic acid increases healing of excision wounds of mice whole body exposed to different doses of gamma-radiation. Burns. 2007;33(4):484–494. | ||
Weiss JF, Landauer MR. Protection against ionizing radiation by antioxidant nutrients and phytochemicals. Toxicology. 2003;189(1–2):1–20. | ||
Frei B, Lynch SM. Effects of combined supplementation with antioxidants on low-density lipoprotein oxidation. Circulation. 1994;90(6):3119–3121. | ||
Sener G, Sener E, Sehirli O, et al. Ginkgo biloba extract ameliorates ischemia-reperfusion-induced injury in rats. Pharmacol Res. 2005;52(3):216–222. | ||
Kumaraguruparan R, Subapriya R, Viswanathan P, Nagini S. Tissue lipid peroxidation and antioxidant status in patients with adenocarcinoma of the breast. Clin Chim Acta. 2002;325(1–2):165–170. | ||
Beltran-Garcia MJ, Espinosa A, Herrera N, Perez-Zapata AJ, Beltran-Garcia C, Ogura T. Formation of copper oxychloride and reactive oxygen species as causes of uterine injury during copper oxidation of Cu-IUD. Contraception. 2000;61(2):99–103. | ||
Said RS, Nada AS, El-Demerdash E. Sodium selenite improves folliculogenesis in radiation-induced ovarian failure: a mechanistic approach. PLoS One. 2012;7(12):e50928. | ||
Hu G, Zhou R, Liu J, et al. MicroRNA-98 and let-7 confer cholangiocyte expression of cytokineinducible Src homology 2-containing protein in response to microbial challenge. J Immunol. 2009;183(3):1617–1624. | ||
Yan-nan B, Zhao-yan Y, Li-xi L, Jiang L, Qing-jie X, Yong Z. MicroRNA-21 accelerates hepatocyte proliferation in vitro via PI3K/Akt signaling by targeting PTEN. Biochem Biophys Res Commun. 2014;443(3):802–807. | ||
Sun Y. Free radicals, antioxidant enzymes, and carcinogenesis. Free Radic Biol Med. 1990;8(6):583–599. | ||
Mraz M, Malinova K, Kotaskova J, et al. miR-34a, miR-29c and miR-17-5p are downregulated in CLL patients with TP53 abnormalities. Leukemia. 2006;23(6):1159–1163. | ||
Thatte U, Dahanukar S. Apoptosis: clinical relevance and pharmacological manipulation. Drugs. 1997;54(4):511–532. | ||
Stuanton MJ, Gaffney EF. Apoptosis: basic concepts and potential significance in human cancer. Arch Pathol Lab Med. 1998;122(4):310–319. | ||
Matsushima H, Goto T, Hosaka Y, Kitamura T, Kawabe K. Correlation between proliferation, apoptosis, and angiogenesis in prostate carcinoma and their relation to androgen ablation. Cancer. 1999;85(8): 1822–1827. | ||
McMenamin ME, O’Neill AJ, Gaffney EF. Extent of apoptosis in ovarian serous carcinoma: relation to mitotic and proliferative indices, p53 expression, and survival. Mol Pathol. 1997;50(5):242–246. | ||
Tannapfel A, Hahn HA, Katalinic A, Fietkau RJ, Kühn R, Wittekind CW. Incidence of apoptosis, cell proliferation and P53 expression in renal cell carcinomas. Anticancer Res. 1997;17(2A):1155–1162. | ||
Rödel C, Grabenbauer GG, Rödel F, et al. Apoptosis, p53, bcl-2, and Ki-67 in invasive bladder carcinoma: possible predictors for response to radiochemotherapy and successful bladder preservation. Int J Radiat Oncol Biol Phys. 2000;46(5):1213–1221. | ||
Stoll C, Baretton G, Ahrens C, Löhrs U. Prognostic significance of apoptosis and associated factors in oral squamous cell carcinoma. Virchows Arch. 2000;436(2):102–108. | ||
Halliwell B. Reactive oxygen species in living system: source, biochemistry and role in human disease. Am J Med. 1991;91(3C):14–21. |
 © 2016 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2016 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.