Back to Journals » Drug Design, Development and Therapy » Volume 10
Protective effects of honokiol on ischemia/reperfusion injury of rat ovary: an experimental study
Authors Yaman Tunc S, Agacayak E , Yaman Goruk N, Icen MS, Turgut A, Alabalık U, Togrul C, Ekinci C, Ekinci A, Gul T
Received 5 August 2015
Accepted for publication 18 January 2016
Published 8 March 2016 Volume 2016:10 Pages 1077—1083
DOI https://doi.org/10.2147/DDDT.S93768
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 2
Editor who approved publication: Prof. Dr. Wei Duan
Senem Yaman Tunc,1 Elif Agacayak,1 Neval Yaman Goruk,2 Mehmet Sait Icen,1 Abdulkadir Turgut,1 Ulas Alabalik,3 Cihan Togrul,4 Cenap Ekinci,5 Aysun Ekinci,6 Talip Gul1
1Department of Obstetrics and Gynecology, School of Medicine, Dicle University, 2Department of Obstetrics and Gynecology, Diyarbakir Memorial Hospital, 3Department of Pathology, School of Medicine, Dicle University, Diyarbakir, 4Department of Obstetrics and Gynecology, School of Medicine, Hitit University, Corum, 5Department of Histology and Embryology, 6Department of Medical Biochemistry, School of Medicine, Dicle University, Diyarbakir, Turkey
Aim: The purpose of this study was to investigate the protective effect of honokiol on experimental ischemia/reperfusion injury of rat ovary.
Materials and methods: A total of 40 female Wistar albino rats were used in this study. The rats were divided into five groups as follows: sham (Group I), torsion (Group II), torsion + detorsion (Group III), torsion + detorsion + saline (Group IV), and torsion + detorsion + honokiol (Group V). Bilateral adnexa in all the rats except for those in the sham group were exposed to torsion for 3 hours. The rats in Group IV were administered saline, whereas the rats in Group V were administered honokiol by intraperitoneal route 30 minutes before detorsion. Tissue and plasma concentrations of malondialdehyde and nitric oxide were determined. Ovarian tissue was histologically evaluated. Data analyses were performed by means of Kruskal–Wallis test and Mann–Whitney U-test (Bonferroni correction) in SPSS 15.0 (Statistical Package for Social Sciences; SPSS Inc., Chicago, IL, USA).
Results: The torsion and detorsion groups had higher scores in vascular congestion, hemorrhage, and inflammatory cell infiltration compared with the sham group (P<0.005). In addition, total histopathological scores were significantly higher in the torsion and detorsion groups compared with the sham group (P<0.005). A significant reduction was observed in hemorrhage, inflammatory cell infiltration, and cellular degeneration scores, of all histopathological scores, in the honokiol group (P<0.005). Ovarian tissue concentrations of malondialdehyde were significantly higher in the torsion and detorsion groups compared with the sham and honokiol groups (P<0.005). Ovarian tissue concentrations of nitric oxide, on the other hand, were significantly higher in the torsion group compared with the sham, saline, and honokiol groups (P<0.005).
Conclusion: Honokiol has a beneficial effect on ovarian torsion-related ischemia/reperfusion injury.
Keywords: ovary, ischemia/reperfusion injury, honokiol, malondialdehyde, nitric oxide
Introduction
Ovarian torsion is a rare but important gynecologic surgical emergency which most commonly occurs in the first three decades of life.1 It might well occur in normal ovaries; however, it is usually associated with the (known) already existent tubal/ovarian pathologies.2 Detorsion might be considered as soon as the diagnosis of ovarian torsion is established in order to restore the ovarian blood supply with a surgical intervention. This way, potential necrosis can be avoided.3,4 However, the recovery of ovarian circulation after detorsion might cause a pathologic process called ischemia/reperfusion (I/R) injury characterized by oxidative stress, leading to deteriorated tissue damage.5 The causes of I/R injury of the ovary include elevated concentrations of nitric oxide (NO), apoptosis, neutrophil and thrombocyte activation, and free radical and cytokine release.6–9
In the post-I/R period, reactive oxygen species (ROS) are produced in large amounts, and antioxidant defense mechanisms of the body are undermined.10 Increased production of ROS impairs the DNA chain, causes denaturation of proteins containing enzymes and ion channels, and does cellular damage through lipid membrane peroxidation.11 Malondialdehyde (MDA) is the basic product of polyunsaturated fatty acid peroxidation and is quite a toxic molecule. Therefore, it is used to determine in vivo and in vitro oxidative stress levels.12 NO, which is produced by normal endothelium, is the main determinant of normal endothelial and vascular functions. In case of inflammation, overproduction of NO, together with ROS, causes oxidative stress.13 Honokiol is a natural biphenolic compound that is isolated from magnolia bark and has been used in the traditional Chinese and Japanese medicine as an antibacterial, antiemetic, antidepressant, antithrombotic, and anxiolytic agent. Studies have shown therapeutic effects including antioxidative and anti-inflammatory activities for honokiol.14–19 The effect of honokiol on ovarian torsion-related I/R injury has not been investigated earlier. The purpose of this study was to investigate the protective effect of honokiol on I/R injury in an experimental rat adnexal torsion model.
Materials and methods
The protocol of this study was approved by the Dicle University Ethics Committee of Animal Rights (DÜHADEK). All animal-handling procedures were performed according to the Guide for the Care and Use of Laboratory Animals of the National Institutes of Health and followed the guidelines of the Animal Welfare Act. A total of 40 female Wistar albino rats weighing between 220 g and 250 g were used in this study. The rats, which were given sufficient amount of water and food, were maintained at proper humidity and temperature in 12-hour light–dark cycle for at least 7 days. They were randomized into five groups, each consisting of eight rats as follows:
- Group I: sham (n=8)
- Group II: torsion (n=8)
- Group III: torsion + detorsion (n=8)
- Group IV: torsion + detorsion + saline (n=8)
- Group V: torsion + detorsion + honokiol (n=8)
Numbers of rats and groups were noted by one of the researchers. The researchers who performed biochemical and histopathological analyses were not provided information about randomization until the end of the study.
Each rat was weighed and anesthetized with intramuscular ketamine hydrochloride (50 mg/kg Ketalar; Eczacibasi, Istanbul, Turkey) and xylazine hydrochloride (10 mg/kg Rompun; Bayer AG, Leverkusen, Germany). Then, laparotomy was performed with a 2.5 cm midline abdominal incision after sufficient sterile conditions were ensured. In the sham group (Group I), uterine horns and adnexa were exposed to nearly 1 minute and abdominal wall was closed with 3/0 silk sutures (sham operation). Three hours later, relaparotomy was performed in this group, and both ovaries were surgically removed. Adnexal torsion was performed as follows: adnexa containing tuba and ovarian tissues were twisted 360° clockwise and fixed to abdominal wall.20 In the torsion group (Group II), relaparotomy was performed and both ovaries were surgically removed after 3 hours of ischemia. In the torsion + detorsion group (Group III), bilateral adnexal torsion (3 hours of ischemia) and bilateral detorsion (3 hours of reperfusion) were performed. Both ovaries were surgically removed after a total of 6 hours. In the torsion + detorsion + saline group (Group IV), 2 mL of saline was injected by intraperitoneal route 30 minutes before detorsion. Then, relaparotomy was performed for the purpose of detorsion in bilateral adnexa, and the incision was sutured. Both ovaries were surgically removed after 3 hours of reperfusion (Figures 1 and 2).
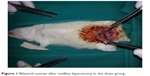  | Figure 1 Bilateral ovaries after midline laparotomy in the sham group. |
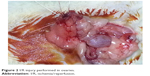  | Figure 2 I/R injury performed in ovaries. |
In the torsion + detorsion + honokiol group (Group V), honokiol (25 mg, Honokiol; Sigma-Aldrich Co., St Louis, MO, USA; honokiol dose: 2 mg of honokiol was dissolved in 2 mL of dimethylsulfoxide) was injected by intraperitoneal route 30 minutes before detorsion. Then, relaparotomy was performed for the purpose of detorsion in bilateral adnexa, and the incision was sutured. Both ovaries were surgically removed after 3 hours of detorsion (Figure 3). Intracardiac blood was taken from all the groups after completion of surgical procedures, and the rats were sacrificed. One of the ovaries that was removed was stored in a deep freezer at -80°C for biochemical analysis. The other ovary, on the other hand, was transferred to the laboratory in enumerated vessels containing 10% formaldehyde for histopathological examination. Blood specimens were sent to the biochemistry laboratory under proper conditions for biochemical analysis.
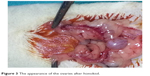  | Figure 3 The appearance of the ovaries after honokiol. |
Histologic evaluation
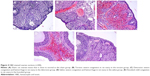
Ovarian tissues were fixed in 10% neutral buffered formalin solution for 48 hours and were dehydrated, cleared, and embedded in paraffin, as usual. Serial tissue sections, which were 4 μm thick, were cut using a microtome (Leica RM2125RTS) and stained with hematoxylin and eosin for general morphological observation. All the sections were examined with a light microscope (Olympus BX43), and the pieces were photographed. At least five microscopic regions were examined to score the specimens semiquantitatively. Criteria for ovarian injury were as follows: follicular cell degeneration (granulosa cells), vascular congestion, hemorrhage, and inflammation (neutrophil infiltration). Each specimen was scored for each criterion using a scale ranging from 0 to 3 (0, none; 1, mild; 2, moderate; 3, severe).21 Ovary sections were analyzed in a blinded fashion by the same pathologist.
Biochemical analyses
Blood specimens were centrifuged at 3,000 rpm for nearly 10 minutes at +4°C to obtain plasma. Plasma specimens that were obtained were analyzed for MDA and NO. Ovarian tissue homogenates were prepared at +4°C to measure MDA and NO concentrations. Tissues were weighed and sliced into small pieces. Then, they were homogenized with a homogenizer (IKA®Ultra-Turrax® T8 dispersing homogenizator; IKA-Werke GmbH, Staufen, Germany) using an ice cold solution of 10× phosphate-buffered saline (50 mM/L, pH 7.0). Homogenates were centrifuged at 15,000 rpm for nearly 10 minutes at +4°C to obtain supernatant. Supernatant specimens were used to measure MDA and NO concentrations.
MDA measurements
MDA concentrations in the specimens were determined by means of a predefined method22 as follows: 0.5 mL specimen (plasma/homogenate) was taken by pipette to a 10 mL centrifuge tube containing a solution of 1.0 mL of thiobarbituric acid (0.6%) and 2.5 mL of trichloroacetic acid (20%). Tubes were heated in boiling water bath for 30 minutes. Then, the reaction mix was cooled down in ten ice baths following the addition of 4.0 mL of n-butanol. Tubes were vortex mixed and centrifuged at 3,000 rpm for 10 minutes. Absorbance of the organic layer was measured at 535 nm.
NO measurements
The production of NO was determined by indirectly measuring nitrite concentrations based on the Griess reaction.23 At the beginning, specimens were deproteinized with 75 mmol/L ZnSO4. Following cleanup, equal volumes of specimens were treated with a copper–cadmium mixture in glycine buffer at pH 9.7 in an effort to reduce nitrate to nitrite. This way, nitrite concentrations in the specimens showed total nitrate + nitrite. In Griess reaction, a chromophore with a strong absorbance at 545 nm was formed by the reaction of nitrite with a mixture of sulphanilamide and naphthalenediamine.
Statistical analyses
Data analyses were performed by SPSS 15.0 (SPSS Inc. ), and mean and standard deviations were calculated. Whether there were differences between the groups or not was determined by means of Kruskal–Wallis test. Pairwise comparisons were made using Mann–Whitney U-test. For measures between the five different groups, Mann–Whitney U-test (Bonferroni correction) was used (as a post hoc test). Results were considered statistically significant at P-value <0.005. Bonferroni correction was used for repeated measures, and the predefined significance level (P<0.05) was reset at P<0.005 after correction.
Results
Histopathologic scores for all five groups are demonstrated in Table 1. The torsion and detorsion groups had higher scores in vascular congestion, hemorrhage, and inflammatory cell infiltration compared with the sham group (P<0.005). The mean cellular degeneration score, which indicated acute tissue damage, was significantly higher in the detorsion group compared with the sham group (Table 1). The total histologic evaluation score, which was obtained when all four ovarian damage parameters were added together, was significantly higher in the torsion and detorsion groups compared with the sham group (Table 1). The histologic ovarian morphologies for all the groups are demonstrated in Figure 4.
  | Table 1 Histopathologic evaluation scores of rat ovarian tissues in all groups (n=8) |
Plasma and ovarian tissue concentrations of MDA and NO are demonstrated in Table 2. Ovarian tissue concentrations of MDA were significantly higher in the torsion and detorsion groups compared with the sham and honokiol groups (P<0.005). On the one hand, ovarian tissue concentrations of NO were significantly higher in the torsion group compared with the sham, saline, and honokiol groups (Table 2). There was no significant difference between the groups in plasma concentrations of MDA. On the other hand, plasma concentrations of NO were significantly higher in the honokiol group compared with the detorsion group (P<0.005).
  | Table 2 MDA and NO concentrations in all groups (n=8) |
Discussion
The purpose of this study was to investigate the changes in plasma and ovarian tissue concentrations of MDA and NO as well as histopathologic changes occurring in response to the honokiol treatment in a rat ovarian torsion/detorsion model. Ovarian torsion/detorsion leads to morphological and biochemical changes in the ovarian tissue caused by I/R injury. Ischemia is a restriction in blood supply to organs which results in elevated concentrations of lactic acid and hypoxanthine caused by lipid peroxide accumulation and adenosine triphosphate degradation.25 Free oxygen radicals, which occur as a result of reperfusion injury, react with lipids, causing the formation of lipid peroxides. Lipid peroxidation impairs membrane functions and creates toxic substances such as aldehydes. Restriction in blood supply causes tissue hypoxia, resulting in the formation of lipid peroxides. MDA is a stable metabolite of the lipid peroxidation cascade. It is considerably elevated in I/R injury, which is believed to cause damages to the integrity and permeability of the cell wall.25–27 In their study on a rat ovarian I/R model, Ergun et al found that plasma and ovarian tissue concentrations of MDA were significantly higher in the torsion and detorsion groups compared with the sham group.28 In this study, as well, MDA concentrations were measured in an effort to determine and verify the degree of reperfusion injury in plasma and ovarian tissue, and significantly higher ovarian tissue concentrations of MDA were found in the torsion and detorsion groups compared with the sham group. This finding shows that I/R causes lipid peroxidation in the ovarian tissue, leading to oxidative damage.
One of the most important findings of this study was the significant reduction in ovarian tissue concentrations of MDA in the honokiol group, which shows that honokiol might prevent oxidative damage in I/R injury of the ovary. On the other hand, the absence of a significant difference between the groups in plasma concentrations of MDA might have been caused by the short torsion duration.
During the reperfusion of ischemic tissue, increased production of peroxynitrite, NO, and ROS including hydrogen peroxide, hydroxyl radicals, and superoxide anions is observed. These free radicals cause cellular damage via lipid peroxidation in the mitochondrial and cellular membranes.24–28 In previous studies, significantly increased tissue and plasma concentrations of NO were shown after I/R injury.27,28 In this study, as well, ovarian tissue concentrations of NO were significantly higher in the torsion group compared with the sham, saline, and honokiol groups. However, plasma concentrations of NO were significantly lower in the detorsion group compared with the honokiol group. This paradoxical result might be explained by the prevention of NO consumption by ROS in the ovary after the administration of honokiol. The increase in NO concentrations might be of protective importance in ovarian torsion. In addition, the absence of a significant difference in the other groups in respect of plasma concentrations of NO might be explained by the short torsion duration.
The basic functions of the human ovary include the nurture, development, and release of a mature oocyte that is ready for fertilization and successful propagation of the species. In this process, the ovary assumes the following roles: 1) secreting steroid hormones that induce the development and growth of secondary sexual characteristics; 2) playing a key role in the orderly, repetitive cyclic ovulation; and 3) providing support for the successful uterine implantation and the early phase of pregnancy.29 These functions are achieved by means of complex mechanisms under stable oxygen supply and consumption conditions requiring normal microcirculation. Oxidative damage induced by I/R might impair the ovarian functions. In a healthy individual, there is a balance between ROS and antioxidants. A shift in this balance in favor of ROS causes oxidative stress, which might affect the entire female reproductive life, even thereafter. ROS might affect a number of physiological processes, including oocyte maturation, fertilization, embryo development, and pregnancy.
There are a number of experimental drug studies on the protection of ovary from I/R injury.20,21,28 Anti-inflammatory and antioxidant effects of honokiol were investigated in a number of studies.15–17,30,31 In a molecular study, honokiol was reported to block lipopolysaccharide-induced cytotoxicity and the production of cytotoxic cytokines such as interleukin-1β and tumor necrosis factor-α, NO, and ROS.32 In addition, honokiol was shown to have a scavenging effect on both superoxide and peroxyl radicals.33 Besides, chronic treatment with honokiol in hypertensive rats was reported to decrease blood pressure due to its vasorelaxant and antioxidant effects.34 Furthermore, honokiol with its antioxidant effects was shown to provide protection against cerebral I/R injuries and dermatological disorders.31,35 In our study, we demonstrated the protective effects of honokiol on I/R injury in rat ovary. This is the first study in the literature to show the protective effects of honokiol on ovarian I/R injury. Ischemia that occurs in case of ovarian torsion causes oxidative damage. Even if the torsion is corrected, effects of this damage may persist. Therefore, early administration of such agents that could prevent oxidative damage or reverse the effects of the damage might prevent a potential future damage to the ovarian reserve, which might amount to fertility. Given the available clinical and experimental data, we are of the opinion that honokiol is a promising agent and a promising topic of investigation for future clinical trials.
The results of this study are short term. Therefore, we believe that there is a need for further studies to demonstrate the long-term results of honokiol on ovarian I/R injury as well as its fertility-preserving effects. Maybe, there should be clinical studies in humans to demonstrate the effects of honokiol on human ovary. We believe that such studies will shed light on the long-term protective effects of honokiol on ovarian follicular reserve and fertility.
In conclusion, honokiol can be administered prior to the treatment of ovarian torsion in order to prevent oxidative damage in I/R injury of the ovary. However, clinical studies are needed to verify the results of this study.
Acknowledgments
This research was funded by Dicle University Scientific Research Commission (grant number: 13-TF-100). The authors are grateful to the Department of Obstetrics and Gynecology and Department of Pathology, School of Medicine, Dicle University, for their valuable support.
Disclosure
The authors report no conflicts of interest in this work.
References
Houry D, Abbott JT. Ovarian torsion: a fifteen-year review. Ann Emerg Med. 2001;38:156–159. | ||
McWilliams GD, Hill MJ, Dietrich CS. Gynecologic emergencies. Surg Clin North Am. 2008;88:265–283. | ||
Chen M, Chen CD, Yang YS. Torsion of the previously normal uterine adnexa. Evaluation of the correlation between the pathological changes and the clinical characteristics. Acta Obstet Gynecol Scand. 2001;80:58–61. | ||
Oelsner G, Shashar D. Adnexal torsion. Clin Obstet Gynecol. 2006;49:459–463. | ||
Li C, Jackson RM. Reactive species mechanisms of cellular hypoxia-reoxygenation injury. Am J Physiol Cell Physiol. 2002;282:227–241. | ||
Sahin FK, Cosar E, Koken G, Toy H, Basarali K, Buyukbas S. Protective effect of aprotinin on ischemia-reperfusion injury in rat ovary. J Obstet Gynecol Res. 2008;34:794–800. | ||
Kuroda S, Siesjo BK. Reperfusion damage following focal ischemia: pathophysiology and therapeutic windows. Clin Neurosci. 1997;4:199–212. | ||
Land WG. The role of postischemic reperfusion injury and other non-antigen dependent inflammatory pathways in transplantation. Transplantation. 2005;79:505. | ||
Baker GL, Corry RJ, Autor AP. Oxygen free radical induced damage in kidneys subjected to warm ischemia and reperfusion: protective effect of superoxide dismutase. Ann Surg. 1985;202:628–641. | ||
McCord JM. Oxygen-derived free radicals in postischemic tissue injury. N Engl J Med. 1985;312:159–163. | ||
Gutteridge J. Free radicals in disease processes: a compilation of cause and consequence. Free Radic Res Commun. 1993;19:141–148. | ||
Del Rio D, Stewart AJ, Pellegrini N. A review of recent studies on malondialdehyde as toxic molecule and biological marker of oxidative stress? Nutr Metab Cardiovasc Dis. 2005;15:316–328. | ||
Lubos E, Handy DE, Loscalzo J. Role of oxidative stress and nitric oxide in atherothrombosis. Front Biosci. 2008;13:5323–5344. | ||
Hayashi T, Saito A, Okuno S, Ferrand-Drake M, Dodd RL, Chan PH. Damage to the endoplasmic reticulum and activation of apoptotic machinery by oxidative stress in ischemic neurons. J Cereb Blood Flow Metab. 2005;25:41–53. | ||
Liou KT, Lin SM, Huang SS, Chih CL, Tsai SK. Honokiol ameliorates cerebral infarction from ischemia-reperfusion injury in rats. Planta Med. 2003;69:130–134. | ||
Liou KT, Shen YC, Chen CF, Tsao CM, Tsai SK. Honokiol protects rat brain from focal cerebral ischemia-reperfusion injury by inhibiting neutrophil infiltration and reactive oxygen species production. Brain Res. 2003;992:159–166. | ||
Liou KT, Shen YC, Chen CF, Tsao CM, Tsai SK. The anti-inflammatory effect of honokiol on neutrophils: mechanisms in the inhibition of reactive oxygen species production. Eur J Pharmacol. 2003;475:19–27. | ||
Sheu ML, Liu SH, Lan KH. Honokiol induces calpain-mediated glucose-regulated protein-94 cleavage and apoptosis in human gastric cancer cells and reduces tumor growth. PLoS One. 2007;2(10):e1096. | ||
Tse AK, Wan CK, Shen XL, Yang M, Fong WF. Honokiol inhibits TNF-alpha-stimulated NF-kappaB activation and NF-kappaB-regulated gene expression through suppression of IKK activation. Biochem Pharmacol. 2005;70:1443–1457. | ||
Sak ME, Soydinc HE, Sak S, et al. The protective effect of curcumin on ischemia-reperfusion injury in rat ovary. Int J Surg. 2013;11: 967–970. | ||
Ozler A, Turgut A, Goruk NY, Alabalik U, Basarali MK, Akdemir F. Evaluation of the protective effects of CoQ10 on ovarian I/R Injury: an experimental study. Gynecol Obstet Invest. 2013;76:100–106. | ||
Yoshioka T, Kawada K, Shimada T, Mori M. Lipid peroxidation in maternal and cord blood and protective mechanism against active-oxygen toxicity in the blood. Am J Obstet Gynecol. 1979;135:372–376. | ||
Cortas NK, Wakid NW. Determination of inorganic nitrate in serum and urine by a kinetic cadmium-reduction method. Clin Chem. 1990;36:440–443. | ||
Somuncu S, Cakmak M, Dikmen G, Akman H, Kaya M. Ischemia-reperfusion injury of rabbit ovary and protective effect of trapidil: an experimental study. Pediatr Surg Int. 2008;24:315–318. | ||
Akgür FM, Kilinc K, Aktuğ T. Reperfusion injury after detorsion of unilateral testicular torsion. Urol Res. 1993;21:395–399. | ||
Vural H, Aksoy N, Ozbilge H. Alterations of oxidative-antioxidative status in human cutaneous leishmaniasis. Cell Biochem Funct. 2004;22:153–156. | ||
Carden DL, Granger DN. Pathophysiology of ischemia-reperfusion injury. J Pathol. 2000;190:255–266. | ||
Ergun Y, Koc A, Dolapcioglu K, et al. The protective effect of erythropoietin and dimethylsulfoxide on ischemia-reperfusion injury in rat ovary. Eur J Obstet Gynecol Reprod Biol. 2010;152:186–190. | ||
Kase N. The normal human ovary, part I. Reproductive and endocrine functions. In: Altchek A, Deligdish L, Kase NG, editors. Diagnosis and Management of Ovarian Disorders. San Diego, CA: Elsevier Science; 2003:11. | ||
Huang KH, Weng TI, Huang HY, et al. Honokiol attenuates torsion/detorsion-induced testicular injury in rat testis by way of suppressing endoplasmic reticulum stress-related apoptosis. Urology. 2012;79(4):967.e5–967.e11. | ||
Shen JL, Man KM, Huang PH, et al. Honokiol and magnolol as multifunctional antioxidative molecules for dermatologic disorders. Molecules. 2010;15(9):6452–6465. | ||
Lee SY, Cho JY. Inhibitory effects of honokiol on LPS and PMA-induced cellular responses of macrophages and monocytes. BMB Rep. 2009;42(9):574–579. | ||
Dikalov S, Losik T, Arbiser JL. Honokiol is a potent scavenger of superoxide and peroxyl radicals. Biochem Pharmacol. 2008;76(5):589–596. | ||
Zhang GS, Wang RJ, Zhang HN, Zhang GP, Luo MS, Luo JD. Effects of chronic treatment with honokiol in spontaneously hypertensive rats. Biol Pharm Bull. 2010;33(3):427–431. | ||
Zhang P, Liu X, Zhu Y, Chen S, Zhou D, Wang Y. Honokiol inhibits the inflammatory reaction during cerebral ischemia reperfusion by suppressing NF-κB activation and cytokine production of glial cells. Neurosci Lett. 2013;8(534):123–127. |
 © 2016 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2016 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.