Back to Journals » Drug Design, Development and Therapy » Volume 14
Protective Effect of Pravastatin on Myocardial Ischemia Reperfusion Injury by Regulation of the miR-93/Nrf2/ARE Signal Pathway
Authors Liu Z, Zhang F, Zhao L, Zhang X, Li Y, Liu L
Received 28 February 2020
Accepted for publication 5 August 2020
Published 22 September 2020 Volume 2020:14 Pages 3853—3864
DOI https://doi.org/10.2147/DDDT.S251726
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 2
Editor who approved publication: Professor Anastasios Lymperopoulos
Zhiqiang Liu, Fucheng Zhang, Lipei Zhao, Xueping Zhang, Yibo Li, Lingling Liu
Department of Cardiology, Xinxiang Central Hospital, Xinxiang, Henan Province, People’s Republic of China
Correspondence: Lingling Liu Department of Cardiology
Xinxiang Central Hospital, Weibin District, Xinxiang, Henan Province, People’s Republic of China
Tel +86-18603732581
Email [email protected]
Purpose: This research intended to study the mechanism of pravastatin in myocardial ischemia reperfusion (I/R) injury.
Patients and Methods: Altogether 70 male rats were selected and grouped into Sham operation group (Sham group), ischemia reperfusion group (I/R group), pravastatin pretreatment group (I/R+P group), I/R+miR-93-mimics, I/R+P+miR-93-mimics, I/R+Nrf2 siRNA, and I/R+P+Nrf2 siRNA group. The myocardial function of each group was detected.
Results: Myocardial I/R injury could lead to abnormal myocardial enzyme activity, inflammatory reaction and oxidative stress. However, pravastatin could significantly inhibit the activity of myocardial enzymes, alleviate inflammatory reaction and inhibit oxidative stress reaction, thus playing a protective role. Furthermore, cell experiments showed that pravastatin can alleviate the injury of H9C2 myocardial cells caused by I/R, inhibit the apoptosis of myocardial cells, and lead to a significant reduction in pro-apoptotic genes Bax, caspase-3 and caspase-9 transcription levels, an obvious increase in anti-apoptotic gene Bcl-2, and an increase in cell activity. After I/R induced injury, miR-93 level was significantly up-regulated and Nrf2 level was down-regulated. Over-expression of miR-93 or inhibition of Nrf2 expression would lead to further aggravation of I/R myocardial injury, increase the apoptosis rate of cells and decrease the activity of myocardial cells. Pravastatin administration could inhibit miR-93, activate and promote Nrf2 in myocardial tissue, and promote protein expression of downstream regulatory genes HO-1 and NQO1. In the I/R model, pravastatin was given. Over-expression of miR-93 or silencing Nrf2 could reverse the therapeutic effect of pravastatin on I/R.
Conclusion: Pravastatin acts as a protector on myocardial ischemia reperfusion injury by regulating miR-93/Nrf2/ARE signaling pathway.
Keywords: pravastatin, miR-93/Nrf2/ARE, myocardial ischemia reperfusion injury, protective effect
Core Tip
This study found that over-expression of miR-93 or silence of Nrf2 can reverse the therapeutic effect of pravastatin on I/R. In myocardial I/R injury, pravastatin could play a role through miR-93/Nrf2/ARE signaling pathway, thus regulating the activity and apoptosis of myocardial cells.
Introduction
Myocardial ischemia reperfusion injury (I/R) is a common clinical disease. Due to the extensive application of various cardiac treatment technologies such as medical intervention and surgical operation, the cure rate of cardiovascular diseases has also been greatly improved, but the resulting I/R injury is also increasing.1,2 Clinically, I/R is an inevitable myocardial injury in the treatment of restoring coronary blood flow, myocardial ischemia and narrowing the scope of myocardial infarction. It has high morbidity and mortality. About 3.8 million male and 3.4 million female die due to the disease each year.3–5 Statins are competitive inhibitors of 3-hydroxy-3-methylglutaryl coenzyme and can be applied to reduce the risk of all major vascular events. They are commonly applied in cardiovascular diseases.6 Moreover, more and more reports showed that statins can effectively resist myocardial infarction and improve survival rate, inhibit inflammation and apoptosis of cells, stabilize artery plaque, maintain endothelial function and eliminate free radicals.7–10 Pravastatin belongs to statins. Previous studies have shown that pravastatin can effectively prevent fatal ventricular fibrillation caused by ischemia reperfusion and reduce the mortality rate of cardiovascular diseases.10,11 The report showed that pravastatin pretreatment can play a protective role in fatal ventricular arrhythmia caused by reperfusion by inhibiting [Ca 2+] (i) overload and preventing δ Ψ (M) loss caused by H 2 O 2.11 Moreover, pravastatin may reduce the death rate of cardiovascular diseases by affecting eNOS expression, and is superior to fluvastatin in reducing infarct size.10
Related studies have suggested that cardiac damage caused by myocardial ischemia is related to the imbalance of various signal pathways, especially the imbalance of intracellular signal pathways.12 miRNA, as a small non-coding RNA, can participate in gene regulation and cell activities, such as development, metabolism, proliferation and differentiation.13 Many studies also indicated that miRNA acts on myocardial I/R and has become an important target of clinical treatment intervention. For example, miR-7a/b in I/R can protect myocardial cells and hinder apoptosis by slashing poly (ADP ribose) polymerase.14 Research have shown that miR-93 may acts on ischemic heart disease, and transfection of miR-93 mimetic can reduce myocardial injury and apoptosis induced by hypoxia/reoxygenation (H/R) in H9c2 cells.15 Transcription factor Nrf2 is the main regulator of cell and body defense against endogenous and exogenous stress by coordinating basic activation and stress-induced activation of various cell protection genes.16 And it can play a protective role in I/R injury by regulating Nrf2-ARE pathway.17 Yan18 et al found that miR-93 can mediate OGD/R-induced apoptosis of H/R injured cells by targeting Nrf2. And research have shown that miR-93 can improve the injury caused by cerebral ischemia reperfusion through Nrf2/HO-1 pathway.19
At present, there is little research on the regulation mechanism of pravastatin in I/R, and whether pravastatin can play a protective role by mediating miR-93/Nrf2/ARE pathway still needs further research.
Patients and Methods
Model Construction
Seventy healthy and clean SD male rats with body mass (200~250 g) were from Shanghai Institute of Materia Medica, with the production license number of SCXK [Shanghai] 2004–0002. The rats were grouped into Sham operation group (Sham group), ischemia reperfusion group (I/R group), pravastatin pretreatment group (I/R+P group), I/R+miR-93-mimics, I/R+P+miR-93-mimics, I/R+Nrf2 siRNA, I/R+P+Nrf2 siRNA group after adaptive feeding for one week, with 10 rats in each group. In the sham operation group, the thoracic cavity was opened and not ligated after needling. The other groups established models according to the method of ischemia reperfusion.20 The anesthetized rats were fixed on the operating table in supine position, and the endotracheal tube was inserted into the rats and connected to the ventilator. The rat’s right carotid artery was isolated and inserted into arterial cannula, and connected to Med-Lab system (Nanjing Medease Science and Technology Co., Ltd., Nanjing China). The chest of rats was cut, and an incision was made along the left edge of sternum to separate pericardium and expose the heart. A 5–0 filaments were used to ligate through the bottom of the anterior descending branch of the left coronary artery (ischemia 30 min/reperfusion 2 h), with cyanosis of left ventricular anterior wall and ST segment elevation as signs of successful ischemia. After reperfusion, the above changes disappeared, which meant the reperfusion is successful. Sham group, I/R group, I/R+P group, I/R+miR-93-mimics, I/R+P+miR-93-mimics, I/R+Nrf2 siRNA, I/R+P+Nrf2 siRNA group were given normal saline, pravastatin, miR-93-mimics, pravastatin +miR-93-mimics, Nrf2 siRNA, pravastatin +Nrf2 siRNA respectively 10min before ischemia. The above process was approved by the Xinxiang Central Hospital, Xinxiang, Henan Province, China,Ethics Committee, and the operation referred to the guidelines of Laboratory Animal Care and National Institutes of Health (NIH). miR-93-mimics and Nrf2 siRNA were purchased from Shanghai Sangon Biotech. Pravastatin was purchased from Sankyo Company (Tokyo, Japan) and administered orally for 2 mg/kg/day and 7 days before modeling.
Blood Specimen Acquisition and Related Indicators Detection
A 5mL of abdominal aortic blood was collected, left at room temperature for 30 min, centrifuged at 1006.2× g at 4°C for 10min to separate serum. Enzyme-linked immunosorbent assay kits were applied to detect LDH (RL008, Shanghai Gefan Biotechnology Co., Ltd.), CK-MB (RC044, Shanghai Gefan Biotechnology Co., Ltd.), CTnl (EKC40439, R&D Systems Inc.). The operation was strictly carried out in accordance with the instructions, and absorbance was detected at 450nm with Multiskan FC microplate reader (Thermo Fisher Scientific).
Myocardial Tissue Acquisition and Related Index Detection
After reperfusion, myocardial tissues of each group were washed with phosphate buffer solution (PBS), added into ice-cold potassium phosphate buffer solution to make 10% homogenate, centrifuged for 15 min. Supernatant was taken, myeloperoxidase (MPO) activity was detected by spectrophotometry, superoxide dismutase (SOD), glutathione peroxidase (GSH-Px), malondialdehyde (MDA) were detected according to the instructions of the kit. Kits were collected from NanJing JianCheng Bioengineering Institute.
Cell Grouping and Activity Detection
H9C2 cardiomyocytes purchased from ScienCell Company of the United States were adopted, with the article number of YBCC337726. Myocardial cells were incubated in DMEM medium (KL-P0032, German merck/sigma) including 10% fetal bovine serum with 5%CO2 at 37°C. Myocardial cells were randomly divided into control group, I/R+NC group, I/R+P group, I/R+miR-93-mimics, I/R+P+miR-93-mimics, I/R+Nrf2 siRNA, I/R+P+Nrf2 siRNA group. I/R models were established, while not in the control group. After the cells adhered to the wall and grew to a certain density, the culture solution was discarded. Ischemia solution (containing 0.9mmol/l of caCl2·2h2o, 20.49mmol/L of MgCl, 137mmol/L of NaCl, 12mmol/L of KCl, 4mmol/L of 4- hydroxyethylpiperazine ethanesulfonic acid (HEPES), 10mmol/L of deoxyglucose, 0.75mmol/L of sodium dithionite, 20mmol/L of sodium lactate, p H value adjusted to 6.5) was added, and placed in an incubator at 37°C with 5% CO2 for 45 min in dark. After the simulated ischemia process was completed, the normal culture solution was replaced and continued to culture in the incubator for 4 h to complete reperfusion. After the completion of the action, the cells were inoculated into 96-well plates. After transfection, the cells were cultured at 5% CO2 at 37°C for 24h, 48h, 72h and 96h respectively. The original culture medium on the upper layer was discarded. A 20 L of MTT reagent (Sigma-Aldrich Co.) was put into each well and cultivated at 37°C for another 4h in the dark. The culture medium was discarded, 150μL of dimethyl sulfoxide was put into each well, shaked for 5–10min to completely melt the purple crystals, and the absorbance value at 450nm was detected using Multiskan FC microplate reader (Thermo Fisher Scientific).
Detection of Apoptosis
According to Annexin V-FITC/PI double staining apoptosis kit instructions, flow cytometry was applied to study myocardial cell apoptosis rate in each group. Cells were digested with trypsin and rinsed twice with PBS after digestion. Cells were collected into centrifuge tubes. A 20μL Annexin-V-FITC labeling solution was put into 1mL buffer solution, and then 20μL PI reagent was added. The solution was cultivated at room temperature in dark for 5min. FACScan flow cytometer (Becton Dickinson Company, USA) system was used for detection. The experiment was repeated for 3 times to get the average value.
Western Blot Assay
A 50 mg of left ventricular ischemic myocardium was taken, 500 μL of lysate was added for lysis, the sample was homogenized in ice bath and centrifuged at 12,000×g for 20 minutes at 4°C. The supernatant was taken and the protein concentration was detected by BCA kit (Thermo Fisher Scientific). A total of 12% SDS-PAGE was used for electrophoresis separation. After ionization, it was moved to PVDF membrane (Millipore Company) and placed in 5% skim milk for sealing. Immune reaction was carried out, and the membrane was incubated with primary antibody (Santa Cruz Biotechnologies, USA) 1: 1000 overnight at 4°C. The membrane was washed to remove the primary antibody, goat anti-rabbit secondary antibody (Abcam, USA) 1: 1000 was added, cultivated at 37°C for 1 hour, rinsed with PBS 3 times for 5min each time. After completion, ECL luminescent reagent (Thermo) was developed, fixed and photographed by using the Quantity One infrared imaging system. The relative expression of the protein to be measured = gray value of the strip to be measured/gray value of the internal reference protein strip.
RT-qPCR Analysis
Part of myocardial tissue was ground and prepared into homogenate, and the homogenate total RNA was taken by Trizol kit (Invitrogen, USA). The purity, concentration and integrity of total RNA were determined by UV spectrophotometer and agarose gel electrophoresis. RNA was reverse transcribed into cDNA referring to the instruction manual of the reverse transcription kit (Thermo Fisher Scientific). SYBR Premix Ex Taq TM kit (Takara Biotechnology Co, ltd.) was used and GAPDH or U6 was used as internal reference, the reaction was carried out on a PCR instrument (ABI 7500 PCR instrument). The PCR amplification cycle were as follows: 95°C for 10min, 95°C for 15s, and 60°C for 60 seconds, for a total of 40 cycles. Data were obtained after three repeated experiments, and the relative expression was calculated using 2−ΔCT (Table 1).
 |
Table 1 Primer Sequence |
Luciferase Reporter Gene Assay
TargetScan was applied to predict the binding sites of miR-93 and Nrf2. Subsequently, a fragment of Nrf 23ʹ- UTR containing the 3ʹ-untranslated region (3ʹ-UTR) of the predicted binding site wild type (Nrf2 wt) or mutation (Nrf2 mut) was cloned on the vector. After DNA sequencing verification, the plasmid (miR-93-mimics, NC mimic) was transfected into cardiac muscle cells according to Lipofectamine™2000 instructions (Invitrogen Company of the United States). Cells were taken 48 h after transfection and determined with a dual luciferase reporting system (DLR ® Promega Corporation).
Statistical Treatment
In this study, SPSS20.0 was used to carry out statistical analysis on the collected data, GraphPad 7 software package to visualize the required pictures, t-test for pair-wise comparison, one-way ANOVA for comparison among multiple groups, LSD-t-test for post-even comparison. When P< 0.05, there was statistical difference.
Results
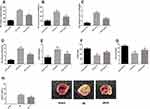
Role of Pravastatin on I/R-Induced Myocardial Cell Injury
The myocardial injury markers in each group were detected as shown in Figure 1. The results indicated that compared with sham group, the activities of myocardial enzyme LDH, CK-MB and CTnl in I/R group increased significantly (P<0.05), while compared with I/R group, the activities of myocardial enzyme LDH, CK-MB and CTnl in I/R+P group after administration could be significantly inhibited (P<0.05). Compared with sham group, the expression of inflammatory marker MPO and oxidative stress marker MDA in I/R group were significantly up-regulated, while oxidative stress markers SOD and GSH-Px were significantly down-regulated (P<0.05). After administration, I/R+P group could significantly inhibit the levels of MPO and MDA and increase the levels of SOD and GSH-Px compared with I/R group (P<0.05). The myocardial infarction area of I/R and I/R group was significantly higher than that of sham operation group (P < 0.05). Compared with I/R group, the myocardial infarction area decreased significantly after pravastatin administration (P<0.05).
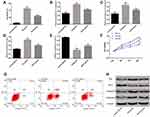
Effect of Pravastatin on Apoptosis and Activity of Myocardial Cells Induced by I/R
The apoptosis of each group was observed, as shown in Figure 2. The apoptosis rate in the I/R group increased significantly, while the cell activity decreased significantly (P<0.05). The I/R+P group could alleviate the occurrence of apoptosis, reduce the apoptosis rate and increase the proliferation activity of cells compared with I/R group (P< 0.05). The levels of apoptosis-related proteins in each group were detected. The expression and content of caspase-9, caspase-3 and Bax in the I/R group were up-regulated (P<0.05), while the expression and content of Bcl-2 were decreased (P<0.05). After administration, the I/R+P group could alleviate the above changes, making caspase-9, caspase-3 and Bax lower and Bcl-2 higher (P<0.05).
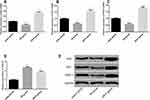
Effect of Pravastatin on miR-93/Nrf2/ARE
In order to explore whether pravastatin has influence on miR-93/Nrf2/ARE, the expression levels of miR-93, Nrf2 and ARE in sham group, I/R group and I/R+P group were detected respectively, as shown in Figure 3. The levels of Nrf2, HO-1 and NQO-1 in I/R group decreased compared with I/R group, while the contents of Nrf2, HO-1 and NQO-1 in I/R+P group after administration increased significantly (P< 0.05). miR-93 expression in I/R group was significantly up-regulated compared with I/R group, while miR-93 expression was inhibited in I/R+P group (P<0.05).
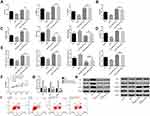
2.4 miR-93 Reverses Myocardial Injury Caused by Pravastatin
The above research found that pravastatin plays a protective role in the I/R model. After miR-93-mimics transfection, as shown in Figure 4, it was found that the over-expression of miR-93 can reverse the effects of pravastatin on myocardial injury, oxidative stress, inflammatory index, apoptosis and proliferation activity, resulting in an increase in the apoptosis rate and a decrease in the proliferation activity of cells. Moreover, there was no significant difference between the indicators of the I/R+miR-93-mimics group and the I/R+P+miR-93-mimics group (P> 0.05). The over-expression of miR-93 could reverse the effect of pravastatin on Nrf2/ARE pathway, and Nrf2, HO-1 and NQO-1 contents decreased compared with I/R group (P<0.05).
Nrf2 Reverses Myocardial Injury Caused by Pravastatin
However, after Nrf2 siRNA transfection, as shown in Figure 5, silencing Nrf2 treatment significantly inhibited the increase of Nrf2, HO-1, NQO-1 content caused by pravastatin, and Nrf2, HO-1, NQO-1 contents decreased compared with I/R+P group (P<0.05). Silence of Nrf2 could significantly reverse the effects of pravastatin on myocardial injury, oxidative stress, inflammatory indexes, apoptosis and proliferative activity, and reverse the protective effect of pravastatin in I/R model.
Targeting Relationship Between miR-93 and Nrf2
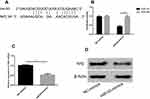
We suggested that miR-93 may mediate the effect of pravastatin on Nrf2 pathway according to the above results. Therefore, we predicted the possible targets using prediction software to explore the possible biological functions of miR-93. There was a complementary pairing region of miR-93 with Nrf2 sequence, which determined that Nrf2 is the target of miR-93. After transfection of miR-93- mimics, the luciferase activity of Nrf2-Wt was inhibited (P<0.05). Compared with NC, the expression and content of Nrf2 in miR-93- mimics group were significantly down-regulated (P<0.05) (Figure 6).
Discussion
Previous studies have shown that Nrf2-ARE signaling pathway can improve reactive oxygen species (ROS) scavenging ability and inhibit oxidative and inflammatory reactions. The activation of ARE is the key for Nrf2-ARE pathway to regulate cell antioxidant stress.21 Nrf2 is a kind of transcription factor, which can participate in the regulation of the generation and expression of antioxidant related genes. It is often combined with the upstream promoter region of the downstream regulatory antioxidant genes (such as HO-1, NQO1, SOD, GPX), and the polymer combined in the tissue can induce the up-regulation of the expression of endogenous cell antioxidant protection genes in the organism, so as to maintain the cells in the oxidation and antioxidant water in a state of equilibrium.17,19,22
Many research have proved that the generation of a large number of oxygen free radicals in myocardial cells, the changes of cell adhesion molecules, the overload of calcium ions, the dysfunction of myocardial energy metabolism, and the occurrence of apoptosis are all correlated with myocardial I/R injury. The correlation between these mechanisms constituted a complex signal network, which restricts and balances each other.5,23 Pravastatin is a compound with naphthalene skeleton and heptanoate, and has the ability to scavenge superoxide anion, hydroxyl radical and nitric oxide radical.24 Relevant studies showed that pravastatin can reduce myocardial infarction area and mortality rate of cardiovascular diseases, and has good application effect in I/R.10,11 In order to explore the regulation mechanism of pravastatin in I/R, this research examined the role of pravastatin in I/R. The results showed that pravastatin can significantly inhibit the activities of myocardial enzyme LDH, CK-MB, CTnl, reduce inflammatory reaction, inhibit oxidative stress reaction and thus play a protective role. We observed myocardial infarction through TTC, and found that pravastatin administration can significantly reduce myocardial infarction area caused by I/R, which is similar to the result of previous research10 that pravastatin can reduce myocardial infarction area caused by coronary artery ischemia reperfusion in rats. However, further cell experiments showed that pravastatin could alleviate the injury of H9C2 myocardial cells caused by I/R, inhibit the apoptosis of myocardial cells, and lead to a significant reduction in pro-apoptotic genes Bax, caspase-3 and caspase-9, an obvious increase in anti-apoptotic gene Bcl-2, and an increase in cell activity. Previous studies determined that pravastatin can enhance the proliferation by regulating the expression of apoptosis-related genes, thus participating in the protective effects of cardiovascular and cerebrovascular diseases.25,26
According to the research of Li27 et al, miR-93 participates in the process of cardiac remodeling, increases in blood of patients with cardiovascular diseases, and increases in miR-93 level after myocardial infarction. In addition, studies have evaluated the promising effect of miR-93 on I/R injury animal models. It was found that down-regulation of miR-93 level can reduce cerebral infarction volume, neuronal apoptosis, restore neurological function score, inhibit oxidative stress caused by I/R, and down-regulation of miR-93 can elevate Nrf2 and HO-1.19 There are more than 200 endogenous protective genes regulated by Nrf2-ARE signaling pathway. HO-1 and NQO1 are landmark target genes in a series of antioxidant enzyme and detoxifying enzyme genes regulated in the middle and lower reaches of Nrf2 pathway.28,29 NQO1 is ubiquitous in eukaryotic cells and has strong antioxidant properties, which can prevent oxidation-reduction reactions and oxidative stress reactions of quinones.30 HO-1 can inhibit various inflammation and oxidation reactions, maintain platelet aggregation and vascular tension, and has protective effect on I/R.31 At present, more and more researches have found that miR-93 participates in the biological development process of various diseases by mediating Nrf2. For example, miR-93 mediates endothelial glycolysis and proliferation by regulating Nrf2, and mediates the effect of oxidized phospholipids on endothelial activation.32 In breast cancer, miR-93 can target Nrf2 pathway and participate in disease process.13 miR-93 was significantly up-regulated and Nrf2 level is down-regulated after I/R induced injury. Over-expression of miR-93 or inhibition of Nrf2 expression will lead to further aggravation of myocardial injury of I/R, increase the apoptosis rate of cells and decrease the activity of myocardial cells.18 Moreover, the luciferase reporter gene confirmed that Nrf2 is a target gene of miR-93, and the over-expression of miR-93 after transfection can significantly reduce the content of Nrf2. It was suggested that miR-93 mediated Nrf2 pathway plays a role in I/R injury.
It also determined that drugs can play a protective role in I/R injury by regulating signal pathways. Chu33 et al Ginsenoside Rg1 plays a protective role in I/R injury and alleviates neuron injury by regulating miR-144/Nrf2/are pathway. Li34 et al Hydrogen rich water can alleviate I/R-induced cardiac injury and effectively reduce the occurrence of oxidative stress by regulating Nrf2/ARE signaling pathway. In this study, we speculated that pravastatin may alleviate I/R injury by regulating Nrf2/ARE signaling pathway. Hence, determined the changes of miR-93/Nrf2/ARE pathway after pravastatin, and found that pravastatin can inhibit miR-93, activate and promote Nrf2 protein in myocardial tissue, and promote the increase of protein expression of downstream regulatory genes HO-1 and NQO1, suggesting that the endogenous defense mechanism of the body against oxidative stress is activated after pravastatin is given, thus providing protective and preventive measures for the occurrence of oxidation resistance and anti-apoptosis of body cells. Moreover, in order to further explore this effect pathway, through transfection of miR-93 and Nrf2, it was found that over-expression of miR-93 or silencing Nrf2 can reverse the therapeutic effect of pravastatin on I/R in the I/R model. The results indicated that pravastatin acts on miR-93/Nrf2/ARE pathway during the treatment of I/R, and proved that pravastatin can reduce myocardial damage, reduce cell apoptosis and improve cell activity by regulating miR-93/Nrf2/ARE pathway during I/R injury.
This research found the regulatory mechanism of pravastatin in myocardial I/R injury, which provides some ideas for future research and treatment. However, there are certain limitations. As there are many regulatory pathways for myocardial injury, other regulatory pathways still needs further research and evidence. However, the effect of pravastatin on ROS and whether pravastatin has an effect on myocardial infarction area in rats through miR-93/Nrf2/ARE pathway need further research.
Conclusion
To sum up, pravastatin acts as a protector on myocardial ischemia reperfusion injury by regulating miR-93/Nrf2/ARE signaling pathway.
Disclosure
The authors report no conflicts of interest in this work.
References
1. Zhang S, Hou J, Wang S, Hui Y, Li J, Peng Y. miR-124 regulates myocardial ischemia reperfusion injury. Int J Clin Exp Med. 2019;12(11):12799–12807.
2. Xie Y, Li Y, Zhao J. Rosuvastatin reduces myocardial ischemia-reperfusion injury by inhibiting miR-155. Int J Clin Exp Med. 2019;12(7):7975–7984.
3. Sun MY, Ma DS, Zhao S, Wang L, Ma CY, Bai Y. Salidroside mitigates hypoxia/reoxygenation injury by alleviating endoplasmic reticulum stress induced apoptosis in H9c2 cardiomyocytes. Mol Med Rep. 2018;18(4):3760–3768. doi:10.3892/mmr.2018.9403
4. Mentzelopoulos SD, Myrianthefs P, Zakynthinos SG. Postcardiac arrest ischemia/reperfusion pathophysiology and functional outcome: can intra-aortic balloon counterpulsation confer any overall or patient-specific benefit? Resuscitation. 2019;143:214–216. doi:10.1016/j.resuscitation.2019.08.005
5. Morano M, Angotti C, Tullio F, et al. Myocardial ischemia/reperfusion upregulates the transcription of the Neuregulin1 receptor ErbB3, but only postconditioning preserves protein translation: role in oxidative stress. Int J Cardiol. 2017;233:73–79. doi:10.1016/j.ijcard.2017.01.122
6. Balan A, Szaingurten-Solodkin I, Swissa SS, et al. The effects of pravastatin on the normal human placenta: lessons from ex-vivo models. PLoS One. 2017;12(2):e0172174. doi:10.1371/journal.pone.0172174
7. Jones SP, Trocha SD, Lefer DJ. Pretreatment with simvastatin attenuates myocardial dysfunction after ischemia and chronic reperfusion. Arterioscler Thromb Vasc Biol. 2001;21(12):2059–2064. doi:10.1161/hq1201.099509
8. Birnbaum Y, Birnbaum GD, Birnbaum I, Nylander S, Ye Y. Ticagrelor and rosuvastatin have additive cardioprotective effects via adenosine. Cardiovasc Drugs Ther. 2016;30(6):539–550. doi:10.1007/s10557-016-6701-2
9. Wang ZX, Wang CQ, Li XY, Ding Y, Feng GK, Jiang XJ. Changes of naturally occurring CD4(+)CD25(+) FOXP3(+) regulatory T cells in patients with acute coronary syndrome and the beneficial effects of atorvastatin treatment. Int Heart J. 2015;56(2):163–169. doi:10.1536/ihj.14-245
10. Li H, Wang C, Sun J, Liu C, Li N, Chen J. Pravastatin decreases infarct size induced by coronary artery ischemia/reperfusion with elevated eNOS expression in rats. Int Heart J. 2018;59(1):154–160. doi:10.1536/ihj.16-607
11. Thuc LC, Teshima Y, Takahashi N, et al. Cardioprotective effects of pravastatin against lethal ventricular arrhythmias induced by reperfusion in the rat heart. Circ J. 2011;75(7):1601–1608. doi:10.1253/circj.CJ-10-1139
12. Ichimura K, Matoba T, Nakano K, et al. A translational study of a new therapeutic approach for acute myocardial infarction: nanoparticle-mediated delivery of pitavastatin into reperfused myocardium reduces ischemia-reperfusion injury in a preclinical porcine model. PLoS One. 2016;11(9):e0162425. doi:10.1371/journal.pone.0162425
13. Makhdoumi P, Roohbakhsh A, Karimi G. MicroRNAs regulate mitochondrial apoptotic pathway in myocardial ischemia-reperfusion-injury. Biomed Pharmacother. 2016;84:1635–1644. doi:10.1016/j.biopha.2016.10.073
14. Li B, Li R, Zhang C, et al. MicroRNA-7a/b protects against cardiac myocyte injury in ischemia/reperfusion by targeting poly(ADP-ribose) polymerase. PLoS One. 2014;9(3):e90096. doi:10.1371/journal.pone.0090096
15. Ke ZP, Xu P, Shi Y, Gao AM. MicroRNA-93 inhibits ischemia-reperfusion induced cardiomyocyte apoptosis by targeting PTEN. Oncotarget. 2016;7(20):28796–28805. doi:10.18632/oncotarget.8941
16. Liu L, Locascio LM, Dore S. Critical role of Nrf2 in experimental ischemic stroke. Front Pharmacol. 2019;10:153. doi:10.3389/fphar.2019.00153
17. Barancik M, Gresova L, Bartekova M, Dovinova I. Nrf2 as a key player of redox regulation in cardiovascular diseases. Physiol Res. 2016;65(Suppl 1):S1–S10. doi:10.33549/physiolres.933403
18. Yan LJ, Fan XW, Yang HT, Wu JT, Wang SL, Qiu CG. MiR-93 inhibition ameliorates OGD/R induced cardiomyocyte apoptosis by targeting Nrf2. Eur Rev Med Pharmacol Sci. 2017;21(23):5456–5461. doi:10.26355/eurrev_201712_13935
19. Wang P, Liang X, Lu Y, Zhao X, Liang J. MicroRNA-93 downregulation ameliorates cerebral ischemic injury through the Nrf2/HO-1 defense pathway. Neurochem Res. 2016;41(10):2627–2635. doi:10.1007/s11064-016-1975-0
20. Yang J, Yang C, Yang J, et al. Huibo wang 2RP105 alleviates myocardial ischemia reperfusion injury via inhibiting TLR4/TRIF signaling pathways. Int J Mol Med. 2018;41(6):3287–3295. doi:10.3892/ijmm.2018.3538
21. Gonchar OO, Maznychenko AV, Bulgakova NV, et al. C60 fullerene prevents restraint stress-induced oxidative disorders in rat tissues: possible involvement of the Nrf2/ARE-antioxidant pathway. Oxid Med Cell Longev. 2018;2018:2518676. doi:10.1155/2018/2518676
22. Sohel MMH, Amin A, Prastowo S, et al. Sulforaphane protects granulosa cells against oxidative stress via activation of NRF2-ARE pathway. Cell Tissue Res. 2018;374(3):629–641. doi:10.1007/s00441-018-2877-z
23. Rana A, Sharma S. Mechanism of sphingosine-1-phosphate induced cardioprotection against I/R injury in diabetic rat heart: possible involvement of glycogen synthase kinase 3beta and mitochondrial permeability transition pore. Clin Exp Pharmacol Physiol. 2016;43(2):166–173. doi:10.1111/1440-1681.12516
24. Umeda R, Takanari H, Ogata K, et al. Direct free radical scavenging effects of water-soluble HMG-CoA reductase inhibitors. J Clin Biochem Nutr. 2019;64(1):20–26. doi:10.3164/jcbn.18-48
25. Lemoine S, Allouche S, Coulbault L, et al. Mechanisms involved in cardioprotective effects of pravastatin administered during reoxygenation in human myocardium in vitro. Anesthesiology. 2012;116(4):824–833. doi:10.1097/ALN.0b013e31824be77c
26. Shiota M, Hikita Y, Kawamoto Y, et al. Pravastatin-induced proangiogenic effects depend upon extracellular FGF-2. J Cell Mol Med. 2012;16(9):2001–2009. doi:10.1111/j.1582-4934.2011.01494.x
27. Li K, Lin T, Chen L, Wang N. MicroRNA-93 elevation after myocardial infarction is cardiac protective. Med Hypotheses. 2017;106:23–25. doi:10.1016/j.mehy.2017.07.003
28. Kang S, Kim W, Jeong S, et al. Oxidized 5-aminosalicylic acid activates Nrf2-HO-1 pathway by covalently binding to Keap1: implication in anti-inflammatory actions of 5-aminosalicylic acid. Free Radic Biol Med. 2017;108:715–724. doi:10.1016/j.freeradbiomed.2017.04.366
29. Lv D, Zhou Q, Xia Y, et al. The association between oxidative stress alleviation via sulforaphane-induced Nrf2-HO-1/NQO-1 signaling pathway activation and chronic renal allograft dysfunction improvement. Kidney Blood Press Res. 2018;43(1):191–205. doi:10.1159/000487501
30. Aghagolzadeh P, Radpour R, Bachtler M, et al. Hydrogen sulfide attenuates calcification of vascular smooth muscle cells via KEAP1/NRF2/NQO1 activation. Atherosclerosis. 2017;265:78–86. doi:10.1016/j.atherosclerosis.2017.08.012
31. Zhao Y, Liu X, Fu X, Mo Z, Jiang Y, Yan Y. Protective effects of epigallocatechin gallate against ischemia reperfusion injury in rat skeletal muscle via activating Nrf2/HO-1 signaling pathway. Life Sci. 2019;239:117014. doi:10.1016/j.lfs.2019.117014
32. Kuosmanen SM, Kansanen E, Kaikkonen MU, et al. NRF2 regulates endothelial glycolysis and proliferation with miR-93 and mediates the effects of oxidized phospholipids on endothelial activation. Nucleic Acids Res. 2018;46(3):1124–1138. doi:10.1093/nar/gkx1155
33. Chu SF, Zhang Z, Zhou X, et al. Ginsenoside Rg1 protects against ischemic/reperfusion-induced neuronal injury through miR-144/Nrf2/ARE pathway. Acta Pharmacol Sin. 2019;40(1):13–25. doi:10.1038/s41401-018-0154-z
34. Li L, Liu T, Liu L, et al. Effect of hydrogen-rich water on the Nrf2/ARE signaling pathway in rats with myocardial ischemia-reperfusion injury. J Bioenerg Biomembr. 2019;51(6):393–402. doi:10.1007/s10863-019-09814-7
 © 2020 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2020 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.