Back to Archived Journals » Patient Intelligence » Volume 7
Prospective, open-label study to validate proper use of the Versacloz™ (clozapine) oral suspension kit by people with schizophrenia
Received 25 February 2015
Accepted for publication 20 April 2015
Published 27 May 2015 Volume 2015:7 Pages 3—8
DOI https://doi.org/10.2147/PI.S83489
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 2
Editor who approved publication: Dr Roger Pinder
Anthony D Andre
Interface Analysis Associates, Saratoga, CA, USA
Purpose: This study was designed to validate that people with schizophrenia can correctly, safely, and effectively prepare doses of Versacloz™ using the Versacloz oral suspension kit and instructions for use (IFU).
Materials and methods: This was a prospective, open-label, simulated-use validation study of 61 people with schizophrenia who were stabilized on clozapine or were clozapine-naive and stabilized on another antipsychotic treatment. Participants were randomized to one of two groups: untrained (n=46) and trained (n=15). Participants were asked to select the proper syringe and prepare two test doses of 1, 3.5, or 5 mL, as randomly assigned. Participants in the untrained group did not receive any training on using the kit, but had access to kit materials, including packaging and the IFU; both test dose preparations were unaided. Participants in the trained group received brief training from the moderator, and then prepared one test dose during training and one unaided test dose during the study period. Prepared placebo doses were not ingested. Performance and behavior were assessed in 14 critical tasks identified in the user failure mode and effects analysis. Test dose failures or dose errors (threshold ±0.1 mL) were assessed. Subjective participant assessments of usability were captured in interviews and IFU comprehension was probed.
Results: A total of 107 test doses were prepared: 92 and 15 by the untrained and trained groups, respectively. Overall success for unassisted dose preparation was 87.9%; all test failures (failure to shake the bottle or failure to obtain the correct test dose) occurred in the untrained group. All participants selected the correct syringe for their assigned dose.
Conclusion: This study shows that the Versacloz oral suspension kit and IFU can be correctly, safely, and effectively used to prepare doses by people with schizophrenia, with few instances of failure or errors observed.
Keywords: Versacloz, clozapine, test dose, oral suspension, treatment-resistant schizophrenia
Introduction
Schizophrenia is a chronic, severe mental illness characterized by delusions, hallucinations, and thought disorder. In the United States, the prevalence of schizophrenia is approximately 2.4 million adults, or approximately 1.1% of the population over 18 years of age.1 At present, there is no cure for schizophrenia. Drug therapies are aimed at reducing the frequency or intensity of symptoms, maximizing quality of life and enhancing the patient’s adaptation to life in the community, and promoting and maintaining recovery.2
Treatments of schizophrenia approved by the US Food and Drug Administration (FDA) include antipsychotics (first generation) and atypical antipsychotics (second generation). However, despite treatment, up to 30% of people with schizophrenia still experience symptoms and are considered treatment-resistant. Treatment-resistant schizophrenia is defined as little or no symptomatic response to at least two trials at least 6 weeks in duration using antipsychotic medications from at least two different chemical classes within the therapeutic dose range.2 Studies have shown that clozapine, an atypical antipsychotic, is an effective treatment for treatment-resistant schizophrenia.3,4 However, people taking clozapine must be monitored regularly for dose-related adverse effects, such as agranulocytosis, cardiomyopathy, myocarditis, and seizures.5
VersaclozTM (Jazz Pharmaceuticals, Inc., Palo Alto, CA, USA) is an oral clozapine suspension that is bioequivalent to marketed clozapine tablets and is the first and only approved antipsychotic oral suspension indicated for the treatment of severely ill people with schizophrenia who fail to respond adequately to standard antipsychotic treatment. Because of the significant risk of agranulocytosis and seizure associated with its use, Versacloz should be used only in those who have failed to respond adequately to standard antipsychotic treatment. Versacloz is also indicated for reducing the risk of recurrent suicidal behavior in people with schizophrenia or schizoaffective disorder who are judged to be at chronic risk for reexperiencing suicidal behavior, based on history and recent clinical state.5
Administration of an oral formulation may facilitate medically supervised titration to the minimum effective dose of clozapine because it involves a single vehicle formulation of which doses can be adjusted by altering the volume ingested. Moreover, it involves a consistent dosing formulation and appearance over time for a patient population that is sensitive to change.6 To fulfill the requirement by the FDA for a human factor engineering study needed for the registration of a drug–device combination,7 we conducted validation testing of the Versacloz oral suspension kit. Validation testing was requested to test the worst-case scenario of people with schizophrenia using the appropriately sized applicator (syringe) without being trained by their health care providers. The primary objective of this study was to validate that people with schizophrenia can correctly, safely, and effectively prepare doses using the Versacloz oral suspension kit and instructions for use (IFU) without patterns of preventable errors that would result in harm. Secondary objectives included validating that there were no aspects of the Versacloz oral suspension kit, IFU, or labeling that led to confusion, failures, or errors.
Methods
Design
This was a prospective, open-label, simulated-use validation study investigating potential errors by people with schizophrenia using the Versacloz oral suspension kit and its IFU. The study design conformed to existing FDA guidance for human factors validation testing and was approved by the FDA prior to conducting the study.8 Inclusion criteria were: United States citizenship; ability to understand, speak, and read English as the primary language; diagnosis of schizophrenia; stabilized on oral clozapine or other medications for schizophrenia; and normal or corrected vision. Those in the medical profession and those not meeting all criteria above were excluded from the study. All participants gave written informed consent.
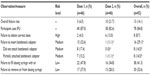
Sixty-one people with schizophrenia who were stabilized on clozapine or were clozapine-naive and stabilized on another antipsychotic treatment were randomized to a group that was untrained (n=46) but with access to the IFU, or to a group that was trained (n=15) by a study moderator and had access to the IFU. The kit contained the following: one 100 mL amber glass bottle with a child-resistant closure; one bottleneck adaptor; and two oral dosing syringes, 1 and 9 mL. To test the Versacloz oral suspension kit, each untrained or trained participant was randomly assigned to prepare one of three doses (1, 3.5, or 5 mL) using the IFU. All participants in both groups were instructed not to actually ingest the prepared placebo doses. Random volumetric doses were selected to ensure use of both sizes of oral syringes was tested. All test dose preparations in both groups were videotaped to facilitate poststudy analysis, and all prepared syringes were photographed. After the moderator had left the room, participants in the untrained group prepared the first test dose. The moderator returned to debrief them on any errors and to ask follow-up questions about their experience. After a short break, participants prepared the second test dose, and further follow-up questions were asked. For the trained group, moderators reviewed syringe preparation and explained the procedure using the kit components while following the IFU. Trained participants then prepared the first test dose under guidance and, if successful, the moderator certified that they had been successfully trained. After a break, this group prepared the second test dose without supervision. Including participants from the untrained and trained groups, a total of 107 unaided doses were prepared (Table 1).
  | Table 1 Number of prepared doses |
Participants
Participants were recruited prospectively through displays and Web announcements from four walk-in clinics: HELP Mental Health Parent Support Group, NAMI Support Group for Family and Friends North County, Schizophrenia for Family and Friends, and Grace Community Center. The validation study was performed at two US locations: San Jose and San Francisco, CA. A minimum of 45 untrained participants in the study was suggested by the FDA and the size of the trained group was selected based on FDA human factors guidance, which states that there needs to be at least 15 participants in each group for validation testing. Within these constraints, participants were randomly assigned a dose for simulated delivery.
Performance measures
The measurement plan included the following factors: performance, behavioral, and subjective measures. Performance and behavior were assessed during the preparation of test doses. Comprehension questions were formulated to probe participants’ understanding of aspects of the IFU not covered by test dose preparation during the study follow-up debriefing. Participants were asked to comment on the IFU and invited to suggest any changes. Errors at each step of the instructions were recorded. The IFU user failure mode and effects analysis for the kit is described in Table 2. Analysis of the steps in the IFU identified 14 critical tasks rated as being at low, medium, or high risk for test dose failure. Noncritical errors were those involving tasks that impart a low or medium risk of test dose failure; critical errors were those involving high-risk tasks. Test dose failure was defined as preparation of an incomplete or wrong dose, where the threshold for an incorrect dose was set at ±0.1 mL.
  | Table 2 User failure mode and effects analysis |
All results are reported using descriptive statistics and written narratives of all test dose failures.
Results
Participants
A total of 61 participants with schizophrenia were recruited from four walk-in clinics; of these, 46 were randomly assigned to the untrained group with access to the IFU and 15 to be trained on the IFU. Demographic data and schizophrenia status are described in Table 3. Most participants were clozapine-naive (33/61, 54.1%) and stabilized on other antipsychotic treatments/medications; the most commonly used in the total population were risperidone (13/61, 21.3%), quetiapine (12/61, 19.7%), aripiprazole (11/61, 18.0%), and olanzapine (6/61, 9.8%). The rest of the participants were stabilized on clozapine, alone or in combination with other antipsychotic medications (28/61, 45.9%). There were no significant differences in any of the demographic parameters between those in the untrained and trained groups. All participants were naive to the IFU and kit being tested. Participants were randomly assigned to prepare 1 mL (n=20), 3.5 mL (n=21), or 5 mL (n=20) test doses (Table 4).
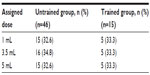  | Table 4 Dose assignments |
Performance, behavior, and subjective measures
Overall, the participants had a success rate of 87.9% (94/107) for all unassisted test doses. The important task of selecting the correct syringe was done successfully by all participants in the untrained (n=46) and trained (n=15) groups (total =107). In the untrained group, 85.9% (79/92) of test doses were successful; all 15 (100%) of the unassisted test dose attempts were successful in the trained group (Table 5).
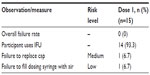  | Table 5 Summary of errors by risk level in trained participants |
All 13 trial failures occurred in the untrained group (Table 6); all would have resulted in doses lower than those assigned. There were no test dose failures resulting in an overdose. One participant committed two errors that led to a test dose failure: failure to draw the correct dose and failure to shake the bottle. Errors leading to failure were failing to draw out the correct test dose (n=8) and failing to shake the bottle before drawing the test dose (n=6). The eight participants who did not draw out the correct test dose left air in the syringe (n=3), used the wrong part of the plunger to measure their assigned dose (n=4), or placed a thumb on the plunger while attempting to remove the filled syringe from the bottle, resulting in partial evacuation of the prepared dose from the syringe (n=1). All six participants who failed to shake the bottle before drawing their assigned test dose stated during debriefing that they did not use the IFU provided, did not read the packaging, and did not read the instructions on the bottle.
Across all participants, the most common noncritical errors not leading to trial failure involved not drawing air into the syringe before withdrawing medication (39/107 unaided doses; 36.4%), not evacuating air from the syringe while dosing (30/107 unaided doses; 28.0%), and not inserting the bottleneck adaptor (16/70 unaided doses; 22.9%). Only one trained participant committed an error: failure to draw air into the syringe. In the trained group, only one participant did not use the IFU, but still successfully prepared the assigned test dose.
Subjective responses
Untrained participants stated that they were successful in 98.9% (91/92) of test dose attempts, reflecting a lack of awareness of the observed errors, as well as the importance of shaking the bottle before drawing the dose. When asked whether they experienced difficulty in preparing the assigned dose, 23.9% (11/46) of all untrained participants said they did on the first attempt, and 4.3% (2/46) said they did on the second test dose attempt. Of the untrained participants who used the IFU, 97% (76/78) reported that the IFU provided useful guidance, whereas only two participants (2/78; 3%) said the IFU was not helpful.
The trained group stated that they were successful in preparing the test doses in all cases. Only one participant reported having some difficulty; this participant volunteered that a learning disability, not the IFU, was responsible for the difficulty. All participants who used the IFU reported that it was helpful.
Across all participants in the study, only 9.8% (6/61) stated that they had difficulty with the IFU. Overall, 11.5% (7/61) of participants (all in the untrained group) suggested changes to the IFU, including more information about correctly inserting the bottleneck adaptor, an expanded explanation as to why air needs to be added to the syringe prior to attaching it to the bottleneck adaptor, and use of larger images. All participants correctly answered knowledge probe questions related to proper ingestion of the medication, proper device cleaning, and proper device storage.
Discussion
Because people with schizophrenia who take clozapine must be closely monitored for potentially severe dose-related adverse effects, the ability to properly prepare the required test dose with the Versacloz oral suspension kit may be critical to successfully managing these individuals. The results of the current study show that people with schizophrenia should be able to correctly, safely, and effectively use the Versacloz oral suspension kit to prepare a clozapine dose, even without the aid of health care providers. Use of the kit did not produce any significant pattern of confusion, failures, errors, or user safety risks. Even untrained participants performed relatively well, especially when they used the IFU. The procedural errors were a direct result of failure to read the IFU, and this ultimately had a minor or, in most cases, no impact on the measured dose. Therefore, even without training (the worst-case scenario), participants should be able to correctly, safely, and effectively use the Versacloz oral suspension kit and IFU to prepare and deliver a dose. The IFU proved to be sufficiently supportive of the procedure when it was used by the participants.
All dosing failures were due to one of two types of errors: failure to shake the bottle or failure to obtain the correct test dose, actions that imparted medium and high risk of failure, respectively. Among all unaided dose trials, untrained participants failed to shake the bottle on six of 92 trials. The IFU, writing on the packaging/carton, and writing on the bottle all explicitly state to shake the bottle prior to use. However, the six untrained participants did not utilize these resources during the study and engaged in the process without any attempt to learn the required steps. Thus, these failures were associated with not using the resource materials, and not to any flaws or shortcomings of the IFU, Versacloz carton labeling, or Versacloz bottle labeling. All participants who read the IFU, packaging, or bottle label successfully shook the bottle; thus, no changes to the IFU are necessary. Because the trials were completed in a short amount of time, it is also possible that the participants did not shake the bottle on their second trial as they had previously during their first trial. They may have been more likely to shake the bottle if they were using the bottle for the first time.
The second type of error was the failure to obtain the correct dose. All dosing errors were underdoses, most of which were by approximately 0.2–0.4 mL, committed by those in the untrained group. The two underlying causes of these underdoses were having too much air in the syringe and using the wrong part of the plunger head to measure the dose. The dosing error could be mapped to the syringe used, as all eight were committed by participants with a dose requiring the larger of the two syringes in the Versacloz oral suspension kit. Because of the width of the barrel of the larger syringe, any air left in the syringe or inaccurate measurement can have a greater impact on the amount of medication withdrawn into the syringe.
Conclusion
This study shows that the Versacloz oral suspension kit and IFU can be correctly, safely, and effectively used to prepare doses by people with schizophrenia with few instances of failure or errors observed. Even untrained participants performed relatively well, especially when they used the IFU.
Acknowledgments
This study was conducted under the direction of Douglas Pharmaceuticals on behalf of Jazz Pharmaceuticals plc or its subsidiaries. Presented in part as a poster (F14) at the Academy of Managed Care Pharmacy (AMCP) 2014 Nexus conference, October 7–10, 2014, in Boston, MA, USA. The author would like to thank Gerard D’Angelo, PhD, of The Curry Rockefeller Group, LLC, for editorial assistance that was funded by Jazz Pharmaceuticals plc or its subsidiaries. The author also thanks Kathleen Villa, MS, of Jazz Pharmaceuticals, Inc., for her assistance.
Disclosure
ADA is the Founding Principal of Interface Analysis Associates (Saratoga, CA, USA) and reports no conflicts of interest in this work.
References
The National Institute of Mental Health. Schizophrenia. Available from http://www.nimh.nih.gov/health/statistics/prevalence/schizophrenia.shtml. Accessed May 6, 2015. | |
Lehman AF, Lieberman JA, Dixon LB, et al. Practice Guideline for the Treatment of Patients with Schizophrenia. 2nd ed. American Psychiatric Association; 2010. Available from: http://psychiatryonline.org/pb/assets/raw/sitewide/practice_guidelines/guidelines/schizophrenia.pdf. Accessed November 7, 2014. | |
McEvoy JP, Lieberman JA, Stroup TS, et al; CATIE Investigators. Effectiveness of clozapine versus olanzapine, quetiapine, and risperidone in patients with chronic schizophrenia who did not respond to prior atypical antipsychotic treatment. Am J Psychiatry. 2006;163(4):600–610. | |
Souza JS, Kayo M, Tassell I, Martins CB, Elkis H. Efficacy of olanzapine in comparison with clozapine for treatment-resistant schizophrenia: evidence from a systematic review and meta-analyses. CNS Spectr. 2013; 18(2):82–89. | |
VERSACLOZ (clozapine) oral suspension [package insert]. Palo Alto, CA: Jazz Pharmaceuticals, Inc.; 2013. | |
Kamerow D. The pros and cons of generic drugs. BMJ. 2011;343:d4584. | |
US Food and Drug Administration. CFR – Code of Federal Regulations Title 21 [webpage on the Internet]. 21CFR820.30. Quality System Regulation. Design Controls. Silver Spring, MD: US Food and Drug Administration; 2014 [updated September 1, 2014]. Available from: http://www.accessdata.fda.gov/scripts/cdrh/cfdocs/cfcfr/cfrsearch.cfm?fr=820.30. Accessed October 29, 2014. | |
US Food and Drug Administration. Draft Guidance for Industry and Food and Drug Administration Staff – Applying Human Factors and Usability Engineering to Optimize Medical Device Design [webpage on the Internet]. Silver Spring, MD: US Food and Drug Administration; 2011 [updated September 4, 2014]. Available from: http://www.fda.gov/medicaldevices/deviceregulationandguidance/guidancedocuments/ucm259748.htm. Accessed May 6, 2015. |
 © 2015 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2015 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.