Back to Journals » Integrated Pharmacy Research and Practice » Volume 4
Promoting evidence-based practice in pharmacies
Authors Toklu H
Received 9 May 2015
Accepted for publication 12 June 2015
Published 3 September 2015 Volume 2015:4 Pages 127—131
DOI https://doi.org/10.2147/IPRP.S70406
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 3
Editor who approved publication: Professor Jonathan Ling
Hale Zerrin Toklu
Department of Pharmacology and Therapeutics, College of Medicine, University of Florida, Gainesville, FL, USA
Abstract: Evidence-based medicine aims to optimize decision-making by using evidence from well-designed and conducted research. The concept of reliable evidence is essential, since the number of electronic information resources is increasing in parallel to the increasing number and type of drugs on the market. The decision-making process is a complex and requires an extensive evaluation as well as the interpretation of the data obtained. Different sources provide different levels of evidence for decision-making. Not all the data have the same value as the evidence. Rational use of medicine requires that the patients receive “medicines appropriate to their clinical needs, in doses that meet their own individual requirements, for an adequate period of time, and at the lowest cost to them and their community.” Pharmacists have a crucial role in the health system to maintain the rational use of medicine and provide pharmaceutical care to patients, because they are the drug experts who are academically trained for this purpose. The rational use of the pharmacist's workforce will improve the outcome of pharmacotherapy as well as decreasing the global health costs.
Keywords: pharmacist, rational use of medicine, pharmacotherapy, pharmaceutical, outcome
The concept of evidence-based medicine
Evidence-based medicine (EBM) aims to optimize decision-making by using evidence from well-designed and conducted research. The concept of reliable evidence is essential, since the number of electronic information resources is increasing in parallel to the increasing number and type of drugs on the market. Decision-making process is a complex process and requires an extensive evaluation as well as the interpretation of the data obtained. The evidences obtained from different sources provide different levels of evidence for decision-making.1,2
Levels of evidence
Not all the data have the same value as the evidence.3–6 Figure 1 shows the evidence pyramid according to the study designs in the order of their importance and strength to provide the evidence.
  | Figure 1 Levels of evidence in the database. |
- Meta-analysis: a meta-analysis combines the results of similar studies by searching the literature and systematically assesses previous research studies to derive conclusions.3
- Systematic reviews: systematic reviews are similar to meta-analyses. It is a literature review focused on a research question that tries to identify, appraise, select, and synthesize all high-quality research evidence relevant to that question. Often, systematic reviews include a meta-analysis component which involves using statistical techniques to synthesize the data from several studies into a single quantitative estimate or summary effect size.4
- Randomized controlled clinical trials: a randomized controlled trial is a type of scientific experiment, where the people being studied are randomly allocated one or two different treatments (or placebo) under study. Subjects are randomly allocated to a control group (no intervention) or to an experimental group (those receiving treatment or having a diagnostic test performed). Blinding is conducted to prevent bias; ie, the patients do not know whether they are receiving the active treatment or a placebo. If there is double-blinding, neither the researcher nor the study participants know who is receiving the treatment or the placebo.
- Cohort studies: a cohort study is a form of observational study. It analyzes the risk factors and follows a group of people who do not have the disease, and uses correlations to determine the absolute risk of subject contraction, or some populations are observed over time to determine whether exposure to something will result in a certain effect. Cohort studies are not randomized, and there may be other confounding conditions affecting the results. For these reasons, cohort studies are less reliable than randomized controlled clinical trials. Cohort studies may be prospective and retrospective. A prospective study follows the population from present to the future, while the retrospective study collects data from the past by evaluating hospital records.
- Case–control studies: the case–control is a type of epidemiological observational study. An observational study is a study in which subjects are not randomized to the exposed or unexposed groups, rather the subjects are observed in order to determine both their exposure and their outcome status and the exposure status is thus not determined by the researcher.
- Case series/case reports: a case series is simply a report describing many case reports on the same subject. A case report is important to initiate a signal about an effect.
- Editorials and expert opinions: editorials and expert opinions have a weaker role in EBM, since they are not studies. However, they are based on knowledge and experience of the experts in the field.
- In vivo (animal) research: basic science studies using animals also build some evidence up to some extent. On the other hand, the controlled conditions in the laboratory setting may not always reflect the effects on humans.
- In vitro research: in vitro studies are performed with cells or biological molecules done outside their normal biological context; ie, in a solution or culture medium.
As mentioned earlier, not all the medical evidence has the same power. It is important to use the well-designed, objective studies as a reliable source of evidence. The number of patients, health condition, other accompanying disease conditions, inclusion/exclusion criteria, methodology, statistical analysis, bias, and conclusions should be carefully evaluated. Then the critical question should be asked “Is that applicable to this patient?”
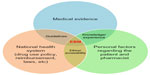
EBM (Figure 2) is a final decision-making process for implementing the best treatment plan for the patient according to the external medical evidence that is compatible with national health policy and patient factors.3–6
Evidence-based pharmacy
Traditional pharmacy practice usually focuses on the order (prescription/over the counter), which is fulfilled on demand, and the pharmacist gives a drug-oriented service. On the other hand, pharmaceutical care emphasizes establishing the pharmacist–patient relationship and adding additional value to the clinical outcome by being actively involved in the treatment procedure.7,8
For the delivery of pharmaceutical care, the training of the pharmacist should be in line for the development of skills in patient assessment, education and counseling, the development of patient-specific pharmacist care plans, treatment protocols, dosage adjustments, selection of therapeutic alternatives, and preventive therapies.8
EBM in pharmacy practice is essential for providing pharmaceutical care. The concept of evidence for clinical decision-making was recognized in the early 1990s and drove a major challenge for the adaptation of clinical guidelines in daily practice in settings including the pharmacy.9,10 Evidence suggests that the patients receive irrational care at times beyond the scope of the providers.11–15 One of the challenges is to keep oneself abreast with the latest developments in the field of medical science with so many new specialties and sub-specialties. The basic principle of EBM is to make all practical decisions based on the literature, which is selected due to the standards pertaining to quantitative, qualitative, and theoretical studies.2 It is believed that EBM is difficult to practice, and one of the ways is to bridge the gaps in the knowledge of the practitioners by enhancing supportive information systems, which will help to prevent errors.16 There is a need to develop a curriculum that is sensitive to the skill development in the area of evidence-based pharmacy.17 A recent survey showed that more than 80% of pharmacists believed that EBM will improve patient care. But they also stated that the patient overload and limited access to EBM sources were barriers to practicing EBM.18 On the other hand, there are limited studies that evaluate EBM practice among pharmacists. In a study in Canada, family physicians had a positive attitude toward the collaborative practice with pharmacists. Some early concerns were resolved as they discovered the benefits of working with pharmacists, such as increased safety in prescribing.19,20
Promoting evidence-based pharmacotherapy among pharmacists
Although hospital and community pharmacists have different responsibilities in regard to the settings in which they practice their profession, they still share a common role in maintaining EBM practice for their patients. EBM is a way to promote rational use of medicine, ie, ensuring that patients receive “medicines appropriate to their clinical needs, in doses that meet their own individual requirements, for an adequate period of time, and at the lowest cost to them and their community”. For rational consumption of medicine, the cooperation of the pharmacist is as crucial as the prescriptions given by the doctors.21–23 However, pharmacists’ advisory activities must not interfere with the doctor–patient relationship. A safe drug dispensing process requires knowledge about different drugs and good communication/consultation skills.24 It is the pharmacist’s responsibility to detect problems besides informing/educating the patient to maintain the appropriate use of medicines.25,26 Misinforming/disinforming or uninforming the patient causes legal liability, and sometimes it is a breach of duty of the pharmacist if the patient is harmed as a consequence.
The role of today’s pharmacists needs to be expanded to include pharmaceutical care concepts, making the pharmacist a health care professional rather than a medicine dispenser in a commercial enterprise.25–28
However, good pharmacy practice/pharmaceutical care concept is new to many of the community pharmacists working in many of the developing countries. Moreover, there are many barriers to implementing this concept into practice.29 Lack of time, lack of understanding, lack of training, and resource constraints are the main barriers. At times, the curriculums are not competent with daily practice. Every country will have to come up with their own models in order to identify the problems and actions required.
Mandatory continuing pharmacy education has an important role to ensure that pharmacists’ knowledge is updated. Regular training on the advances of treatment guidelines provide a valuable source of up-to-date information. Also, workshops on the use of electronic data bases may have beneficial effects.30,31 Hanna and Hughes have shown that EBM training improved decision-making with regards to over-the-counter products.32 Such an improvement was also demonstrated with problem-based pharmacotherapy courses for pharmacists.25
Continuing pharmacy education
As pharmacists are health professionals rather than businessman, the goal must be to develop pharmacists who are well educated, responsible, competent, and committed to improving the health outcome of the patient and society.
Pharmacy schools should prepare a program that is competent regarding the changing role of the pharmacist. The education should provide an ability for critical thinking, improve problem-solving skills and decision-making during pharmacotherapy.33 The student should be trained to create, transmit, and apply new knowledge based on cutting-edge research in the pharmaceutical, social, and clinical sciences and should collaborate with other health professionals and learn to enhance the quality of life through improved health for people of local society and the global community.8
Conclusion
The steps for evidence-based pharmacy practice can be summarized as identification of the problem, evaluating the evidence to choose the appropriate treatment for the patient, personalization of the therapy for the specific patient in regard to the experiences and patient preferences, and decision-making to initiate the treatment.
Pharmacists have a crucial role in the health system to maintain the rational use of medicine and provide pharmaceutical care to patients, because they are the drug experts who are academically trained for this purpose. The “rational use of the pharmacist’s workforce” will lead to “rational use of medicines”, thus improving the outcome of pharmacotherapy as well as decreasing the global health costs.
Disclosure
The author has no conflicts of interest to disclose.
References
Albrecht S. Evidence-based Medicine in Pharmacy Practice. US Pharmacist. 2009;34(10):HS14–HS18. | |
Schindler B, Gointher J, Suter K. Qualitätsbewertung klinischer Studien [Evidence-based pharmacy – assessment of study quality]. Med Monatsschr Pharm. 2014;37(11):413–418. German. | |
Mulrow CD, Cook DJ, Davidoff F. Systematic reviews: critical links in the great chain of evidence. Ann Intern Med. 1997;126(5):389–391. | |
Uman LS. Systematic reviews and meta analyses. J Can Acad Child Adolesc Psychiatry. 2011;20(1):57–59. | |
Malta College of Pharmacy Practice. Cordina M. Evidence-Based Pharmacy Practice. Journal of the Malta College of Pharmacy Practice; 2000. Available from: http://www.mcppnet.org/publications/ISSUE04-1.PDF. Accessed June 26, 2015. | |
Strite Sheri A, Stuart ME. The Pharmacy and Therapeutics Committee Evidence-Based Decision-Making Handbook: Practical Guidance, Advice, Strategies, Tips and Efficiencies. Delfini Group Evidence-based Practice Series. CreateSpace Independent Publishing Platform; 2015. | |
Wiedenmayer K, Summers RS, Mackie CA, Gous AG, Everard M, Tromp D. Developing Pharmacy Practice – Focus Patient Care. World Health Organization and International Pharmaceutical Federation; 2006. Netherlands. | |
Toklu HZ, Hussain A. The changing face of pharmacy practice and the need for a new model of pharmacy education. J Young Pharm. 2013; 5(2):38–40. | |
Grol R, Grimshaw J. From best evidence to best practice: effective implementation of change in patients’ care. Lancet 2003;362(9391):1225–1230. | |
Astier A. Pour une “evidence-based pharmacy”. [For an evidence-based pharmacy]. Ann Pharm Fr. 2012; 70(4):179–181. French. | |
Gokcekus L, Toklu HZ, Demirdamar R, Gumusel B. Dispensing practice in the community pharmacies in the Turkish Republic of Northern Cyprus. Int J Clin Pharm. 2012;34(2):312–324. | |
Akici A, Kalaca S, Ugurlu MU, Toklu HZ, Iskender E, Oktay S. Patient knowledge about drugs prescribed at primary healthcare facilities. Pharmacoepidemiol Drug Saf. 2004;13(12):871–876. | |
Toklu HZ, Uysal MK. The knowledge and attitude of the Turkish community pharmacists toward pharmacovigilance in the Kadikoy district of Istanbul. Pharm World Sci. 2008;30(5):556–562. | |
Toklu HZ, Akici A, Oktay S, Cali S, Sezen SF, Keyer Uysal M. The pharmacy practice of community pharmacists in Turkey. Marmara Pharm J. 2010;14:53–60. | |
Toklu HZ, Dülger GA, Hidiroglu S, et al. Knowledge and attitudes of the pharmacists, prescribers and patients towards generic drug use in Istanbul – Turkey. Pharm Pract (Granada). 2012;10(4):199–206. | |
Bates DW, Kuperman GJ, Wang S, et al. Ten commandments for effective clinical decision support: making the practice of evidence-based medicine a reality. J Am Med Inform Assoc. 2003;10(6):523–530. | |
Wiffen P, Eriksson T, Lu H. Introduction to evidence-based practice in evidence-based pharmacy 2nd edition. Eur J Hosp Pharm. 2013;20:324–327. | |
Abu Farha R, Alefishat E, Suyagh M, Elayeh E, Mayyas A. Evidence-based medicine use in pharmacy practice: a cross-sectional survey. J Eval Clin Pract. 2014;20(6):786–792. | |
Pottie K, Farrell B, Haydt S, et al. Integrating pharmacists into family practice teams: physicians’ perspectives on collaborative care. Can Fam Physician. 2008;54(12):1714–1717. e5. | |
Dolovich L, Pottie K, Kaczorowski J, et al. Integrating family medicine and pharmacy to advance primary care therapeutics. Clin Pharmacol Ther. 2008;83(6):913–917. | |
National databases of Indian medical journals. Gupta MC, Verma S. Drug Use at the Level of Primary Health Centres – A Critical Appraisal. Health Administrator; 2006. Available from: http://medind.nic.in/haa/t06/i1/haat07i1p8.pdf. Accessed June 10, 2015. | |
Hawksworth GM, Chrystyn H. Clinical pharmacy in primary care. Br J Clin Pharmacol. 1998;46(5):415–420. | |
World Health Organization. The World Health Report 2008: Primary Health Care Now More than Ever. WHO; 2008. Available from: http://www.who.int/whr/2008/whr08_en.pdf. Accessed June 10, 2015. | |
Palaian S, Prabhu M, Shankar PR. Patient counseling by pharmacist – a focus on chronic illness. Pak J Pharm Sci. 2006;19(1):62–65. | |
Toklu HZ. Problem based pharmacotherapy teaching for pharmacy students and pharmacists. Curr Drug Deliv. 2013;10(1):67–70. | |
Toklu HZ. Rational Use of Medicine in Pharmacy Practice. Turkiye Klinikleri Journal of Pharmacology Special Topics. 2015; 3(1):74–83. | |
Abduelkarem A. Extending the Role of Pharmacists in Patient Care: Are Pharmacists in Developing Nations Ready to Change? Pharmacology and Pharmacy. 2014;5:865–875. | |
Al-Quteimat OM, Amer MA. Evidence-based pharmaceutical care: The next chapter in pharmacy practice. Saudi Pharmaceutical Journal. Epub August 4, 2014. | |
Burkiewicz JS, Zgarrick DP. Evidence-based practice by pharmacists: utilization and barriers. Ann Pharmacother. 2005;39(7–8):1214–1219. | |
Burkiewicz JS, Vesta KS, Hume AL. Update in handheld electronic resources for evidence-based practice in the community setting. Ann Pharmacother. 2005;39(12):2100–2104. | |
Tiğ EÖ, Dülger GA, Hidiroğlu S, Toklu HZ. Serbest Eczacilarin Elektronik Bilgi Kaynaği Kullanimi. [The use of electronic information resources by community pharmacists.] Marmara Pharm J. 2012; 16(1):29–35. Turkish. | |
Hanna LA, Hughes C. The influence of evidence-based medicine training on decision-making in relation to over-the-counter medicines: a qualitative study. Int J Pharm Pract. 2012;20(6):358–366. | |
Council on Credentialing in Pharmacy, Albanese NP, Rouse MJ. Scope of contemporary pharmacy practice: Roles, responsibilities, and functions of pharmacists and pharmacy technicians. J Am Pharm Assoc (2003). 2010;50(2):e35–e69. |
 © 2015 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2015 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.