Back to Journals » Drug Design, Development and Therapy » Volume 13
Progestin-Primed Ovarian Stimulation with Dydrogesterone versus Medroxyprogesterone Acetate in Women with Polycystic Ovarian Syndrome for in vitro Fertilization: A Retrospective Cohort Study
Authors Huang J, Xie Q, Lin J, Lu X, Zhu J, Gao H, Cai R, Kuang Y
Received 6 September 2019
Accepted for publication 29 November 2019
Published 3 January 2020 Volume 2019:13 Pages 4461—4470
DOI https://doi.org/10.2147/DDDT.S230129
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 2
Editor who approved publication: Professor Anastasios Lymperopoulos
Jialyu Huang,* Qin Xie,* Jiaying Lin, Xuefeng Lu, Jing Zhu, Hongyuan Gao, Renfei Cai, Yanping Kuang
Department of Assisted Reproduction, Shanghai Ninth People’s Hospital, Shanghai Jiao Tong University School of Medicine, Shanghai 200011, People’s Republic of China
*These authors contributed equally to this work
Correspondence: Renfei Cai; Yanping Kuang
Department of Assisted Reproduction, Shanghai Ninth People’s Hospital, Shanghai Jiao Tong University School of Medicine, 639 Zhizaoju Road, Shanghai 200011, People’s Republic of China
Tel +86-21-2327 1699 Ext 5539
Fax +86-21-6313 6856
Email [email protected]; [email protected]
Purpose: Dydrogesterone (DYG) is an alternative progestin in progestin-primed ovarian stimulation (PPOS) protocol with weaker pituitary suppression than medroxyprogesterone acetate (MPA) in normal ovulatory women. However, the endocrinological characteristics, oocyte retrieval and pregnancy outcomes of DYG application in polycystic ovarian syndrome (PCOS) patients undergoing in vitro fertilization (IVF) remain unclear.
Patients and methods: This retrospective cohort study included 420 PCOS patients who underwent controlled ovarian stimulation with human menopausal gonadotropin (hMG) and DYG (n=105) or MPA (n=315) from January 2014 to December 2017. Baseline characteristics of the two groups were balanced with propensity score matching using the nearest-neighbor random matching algorithm in a ratio of 1:3. The primary outcome measure was the number of oocytes retrieved. Other main outcome measures included the number of viable embryos, incidence of premature luteinizing hormone (LH) surge and live birth rate per frozen-thawed embryo transfer (FET) cycle.
Results: A similar number of oocytes was retrieved in the two protocols (16.1±6.5 vs 15.1±10.0, P=0.342). Patients in both groups achieved consistent LH suppression with no premature LH surge detected. In the DYG + hMG group, the mean LH levels were significantly higher than the MPA + hMG group on cycle day 9–11 and trigger day (all P<0.001), and the dose of hMG was significantly lower (1710.7±431.6 vs 1891.3±402.2 IU, P<0.001). No significant between-group differences were found in the number of viable embryos (5.3±3.1 vs 5.0±4.1, P=0.139) and live birth rate per FET cycle (43.5% vs 47.7%, P=0.383). None of the participants experienced moderate-to-severe ovarian hyperstimulation syndrome in either group.
Conclusion: Our results showed that the application of DYG in PPOS protocol could achieve comparable oocyte retrieval and pregnancy outcomes to MPA, but significantly reduce the consumption of gonadotropins in PCOS women for IVF treatment.
Keywords: polycystic ovary syndrome, dydrogesterone, medroxyprogesterone acetate, progestin-primed ovarian stimulation, in vitro fertilization
Introduction
Polycystic ovarian syndrome (PCOS), categorized as a heterogeneous syndrome of ovulatory dysfunction, polycystic ovarian morphology and hyperandrogenism, is a highly prevalent endocrine and reproductive disorder.1 It is estimated to affect 5% to 20% of the reproductive-age female population worldwide and approximately 80% of women who suffer from anovulatory infertility have PCOS.1,2
As recommended by the European Society of Human Reproduction and Embryology, in vitro fertilization (IVF) or intracytoplasmic sperm injection (ICSI) serves as a third-line treatment to infertile PCOS patients who fail to respond to first- or second-line ovulation therapies.3 By promoting the recruitment and development of multiple dominant follicles within one cycle, controlled ovarian stimulation (COS), using either gonadotropin-releasing hormone (GnRH) agonist or antagonist, is considered to be a key factor in IVF/ICSI success. While GnRH agonist desensitizes pituitary to facilitate antral follicle synchronization, final oocyte maturation triggering by human chorionic gonadotropin (hCG) increases the risk of ovarian hyperstimulation syndrome (OHSS). GnRH antagonist suppresses luteinizing hormone (LH) secretion rapidly and reversibly without initial flare effects, but a varied percentage of patients would experience premature LH rise.4,5 Compared with normal ovulatory women, patients with PCOS are also more frequently confronted with problems such as poor oocyte quality, fertilization failure and pregnancy complications.1,6 Therefore, the current unsatisfactory status encourages researchers to explore safer and more efficacious COS regimens.
As the freeze-all strategy avoids the detrimental effect of progestin on endometrial receptivity, a novel COS protocol named progestin-primed ovarian stimulation (PPOS) was proposed by our group in 2015.7 Oral administration of exogeneous progesterone (P) from the early follicular phase, combined with human menopausal gonadotropin (hMG), is able to effectively prevent the activation and transmission phases of estradiol (E2)-induced LH surges and thus serves as an alternative to conventional GnRH analogues.8,9 Prior studies have shown that the PPOS protocol with medroxyprogesterone acetate (MPA) could produce competent oocytes/embryos and achieve comparable pregnancy outcomes to standard GnRH agonist short protocol.7,10,11 Coupled with the application of frozen-thawed embryo transfer (FET) and dual trigger of GnRH agonist with low-dose hCG, the PPOS protocol also allows for nearly complete avoidance of OHSS occurrence.12
Nevertheless, use of MPA in the PPOS regimen tends to inhibit pituitary in a more profound manner and therefore requires higher doses of hMG than the GnRH agonist short protocol in patients with or without PCOS.7,10 On the contrary, due to the differences in chemical structure, pharmacodynamics and pharmacokinetics, dydrogesterone (DYG) has been recently demonstrated to be another viable progestin in PPOS protocol with weaker pituitary suppression strength than MPA, as reflected by consistently higher LH levels, lower hMG doses and shorter stimulation duration in normal ovulatory women.13
In consideration of distinct endocrine characteristics and ovarian responses to COS between women with normal ovulation and PCOS,14,15 we designed this retrospective cohort study aiming to comprehensively compare the application of DYG versus MPA in PPOS protocol regarding the endocrinological profiles, oocyte retrieval and pregnancy outcomes in PCOS patients for IVF treatment.
Materials and Methods
Study Design and Patients
This retrospective cohort study was conducted at the Department of Assisted Reproduction of Shanghai Ninth People’s Hospital affiliated with Shanghai Jiao Tong University School of Medicine. The study was approved by the Institutional Review Board of the hospital, and was carried out in accordance with the principles of the Declaration of Helsinki.
Women with PCOS who underwent IVF/ICSI cycles using DYG or MPA co-treatment with hMG from January 2014 to December 2017 were screened. Eligible women were 20 to 40 years of age, had a normal ovarian reserve (basal follicle-stimulating hormone [FSH] level <10 mIU/mL), and had an infertile history of over 1 year. Patients were diagnosed with PCOS according to the revised 2003 Rotterdam consensus and met 2 out of 3 criteria as follows:16 1) oligo- and/or anovulation; 2) biochemical and/or clinical evidence of hyperandrogenism; 3) polycystic ovarian morphology on ultrasound. Other etiologies of hyperandrogenism and ovulatory dysfunction were excluded, including androgen-secreting tumors, congenital adrenal hyperplasia, hyperprolactinemia and thyroid disease.
Patients were excluded from the study if they met one of the following criteria: 1) endometriosis grade 3 or higher; 2) use of hormonal contraceptives for pretreatment before the study cycle; 3) documented history of ovarian surgery including laparoscopic ovarian drilling, ovarian endometrioma stripping and unilateral oophorectomy; 4) previous diagnosis of congenital (septate uterus, duplex uterus, uterus bicomis and uterus unicornis) or acquired (intrauterine adhesion, submucosal myomas and adenomyosis) uterine anomalies; 5) history of recurrent spontaneous abortion, defined as three or more previous spontaneous pregnancy losses; 6) abnormal chromosomal karyotype in either of the partners. In cases of more than one IVF/ICSI cycle from the same patient using the same ovarian stimulation protocol during the study period, only the first cycle was included for analysis.
Ovarian Stimulation and Laboratory Procedures
As described previously in detail,7,13,17 patients were co-administrated with hMG (Anhui Fengyuan Pharmaceutical Co., China) 150–225 IU/d via intramuscular injection and oral DYG (Duphaston; Abbott Biologicals B.V., the Netherlands) 20mg/d or MPA (Shanghai Xinyi Pharmaceutical Co., China) 10mg/d from spontaneous menstrual cycle day 3 (MC3) to the day of triggering. For both groups, the hMG doses were adjusted depending on the number and size of developing follicles on ultrasound as well as serum concentrations of sexual hormones including FSH, LH, E2 and P. Once the leading follicle exceeded 20 mm or at least three follicles exceeded 18 mm in diameter, the final stage of oocyte maturation was induced by the combination of 0.1 mg triptorelin (Decapeptyl; Ferring Pharmaceuticals, Germany) and 1000 IU hCG (Lizhu Pharmaceutical Trading Co., China).
Oocyte retrieval was performed 34–36 hrs after trigger and guided by transvaginal ultrasound with a double-lumen aspiration needle. Follicles with a diameter over 10 mm were all aspirated and flushed up to three times to obtain any available cumulus-oocyte complexes. Approximately 4–6 hrs after follicular aspiration, oocytes were fertilized by either standard IVF or ICSI according to semen quality. Embryos were cultured consistently in the Continuous Single Culture (Irvine Scientific, USA) and scored on day 3 according to Cummins’s criteria.18 High-quality embryos (grade I and II) were selected for cryopreservation by means of vitrification, while suboptimal embryos (grade III and IV) were subjected to extended culture until day 5 or 6, of which morphologically good blastocysts (grade ≥3BC) based on the Gardner and Schoolcraft system were frozen.19
Endometrial Preparation and Embryo Transfer
A detailed description of the endometrial preparation protocols has been presented in our previous publications.7,13 Mild stimulation with letrozole was recommended for patients in general, while hormone replacement therapy (HRT) was performed for those with a history of thin endometria (endometrial thickness ≤6 mm). In the mild stimulation cycle, a bolus of urinary hCG (5000 IU) was injected for ovulation trigger when the dominant follicle was ≥17 mm in diameter with endometrial lining >8 mm, E2 >150 pg/mL and P <1 ng/mL. The hCG administration was set in the same afternoon when LH ≥20 IU/L, or at night (9:00 p.m.) if LH <20 IU/L. Respectively, oral DYG (40 mg/d) and vaginal micronized P (400 mg/d; Utrogestan, Besins Manufacturing, Belgium) were commenced 2 and 3 days later, followed by day 3 embryo transfer 4 and 5 days later or blastocyst transfer 6 and 7 days later. For patients undergoing HRT cycles, the timing of embryo transfer was scheduled on the 3rd or the 5th day after P administration depending on the embryo stage. Embryo transfer was all conducted via the guidance of abdominal ultrasound in our center, although some evidences are emerging in favor of transvaginal ultrasound.20,21 Once a pregnancy was achieved, luteal-phase support was continued until 10 weeks of gestation.
Hormone Measurement
Serum FSH, LH, E2 and P levels were analyzed on MC3, MC9-11, the trigger day and the day after trigger using chemiluminescence (Abbott Biologicals B.V., the Netherlands). Intra- and inter-assay coefficients of variation were as follows, respectively: FSH, 2.6% and 5.8%; LH, 5.9% and 8.1%; E2, 6.3% and 6.4%; and P, 7.9% and 10%. The lower limits of sensitivity were 0.06 mIU/mL for FSH, 0.09 mIU/mL for LH, 10 pg/mL for E2 and 0.1 ng/mL for P. The upper limit for E2 measurement was 5000 pg/mL. If higher, the E2 level was recorded as 5000 pg/mL without repeating the assay after sample dilution.
Follow-Up of Pregnancy and Neonatal Outcomes
All participants were routinely followed up by blood tests and ultrasound examinations at our center until the end of the first trimester. Information of pregnancy complications, delivery and neonates were then collected by telephone surveys at every trimester until one week after delivery. The standardized interview questionnaire included a wide range of preconception and pregnancy exposures, pregnancy-related complications, gestational age, mode of delivery, birth date, birth weight and length, infant gender, perinatal mortality and major congenital malformations. In cases of failed attempts to contact the couples, the follow-up data were further gathered through the local family planning service agencies. For babies born with congenital malformations, every case information was re-examined by a professionally trained nurse to ensure that it met the definition of the Chinese Birth Defects Monitoring Program. The details of our follow-up system have been presented elsewhere.22–24
Outcome Measures and Definitions
The primary outcome measure was the number of oocytes retrieved. Other analyzed outcomes in the present study mainly included the number of viable embryos, hMG dose and duration, dynamic characteristics of endocrinological profiles, incidence of premature LH surge, profound LH suppression and moderate-to-severe OHSS, as well as pregnancy and neonatal outcomes after FET cycles.
Premature LH surge was defined as a serum LH level of more than 10 IU/L before trigger. Profound LH suppression was defined as a serum LH level of less than 1 IU/L during ovarian stimulation.25 The viable embryo rate per oocyte was calculated as the number of viable embryos divided by the number of oocytes retrieved. The definition of cycle cancelation referred to the completion of oocyte retrieval but without viable embryos. Moderate or severe OHSS was defined according to the guideline issued by the Practice Committee of the American Society for Reproductive Medicine in 2016.26 Biochemical pregnancy was defined as a serum β-hCG level ≥5 IU/L at 14 days after FET. The implantation rate was calculated as the number of gestational sacs visualized on transvaginal ultrasound divided by the number of embryos transferred. Clinical pregnancy was defined as the presence of at least one gestational sac with or without fetal heart activity at 7 weeks after FET. The miscarriage rate was defined as the proportion of eventuated pregnancies in spontaneous or therapeutic abortion throughout. Live birth was identified as delivery of any viable infant at ≥28 weeks of gestation.
Statistical Analysis
The normality of continuous variables was tested by the Shapiro–Wilk test as well as the graphical illustration of histograms and Q–Q plots. The data were described as mean with standard deviation and between-group differences were compared by the Student’s t-test or Mann–Whitney U-test. For categorical variables, the data were presented as number with percentage, and Chi-square test or Fisher exact test was used for comparison, as appropriate.
A propensity score matching (PSM) model was established to balance differences in baseline characteristics between the two groups. We selected 12 covariates to estimate the propensity score by logistic regression, including age (continuous), body mass index (BMI) (continuous), type of infertility (primary or secondary), infertility duration (continuous), infertility diagnosis (PCOS only, PCOS + tubal factor, PCOS + male factor or PCOS + mixed/other factors), previous IVF attempts (0, 1–2 or ≥3), antral follicle count (AFC) (continuous) as well as basal levels of FSH, LH, E2, P and testosterone (all continuous). Patients using DYG were matched with the MPA group using the nearest-neighbor random matching algorithm in a ratio of 1:3.
Data analysis was performed with the Statistical Package for the Social Sciences (version 20.0; SPSS Inc., USA), while PSM was conducted using R statistical programming language (version 3.6.0; R Foundation for Statistical Computing, Vienna, Austria). All P values were based on two-sided tests and P <0.05 was considered to be statistically significant.
Results
Patients Characteristics
The flow chart of the study design and patient selection is shown in Figure 1. Briefly, a total of 1055 IVF/ICSI cycles were screened from our database and 266 cycles were excluded as described in the Materials and methods section. Of the remaining 789 patients, 105 patients undergoing the DYG + hMG protocol were matched with 315 patients who used MPA in PPOS protocol. No significant between-group differences were found in post-matching analysis with regard to all baseline characteristics, including age, BMI, infertility type, duration and diagnosis, previous IVF attempts, AFC as well as basal endocrine profiles (all P >0.05) (Table 1). The distributions of propensity scores before and after matching were demonstrated in Supplementary Figure 1.
 |
Table 1 Baseline Characteristics by Regimen Group |
Ovarian Stimulation, Follicle Development, and Oocyte Performance
Table 2 presents the cycle characteristics and outcomes by the regimen group. The number of oocytes retrieved (16.1 ± 6.5 vs 15.1 ± 10.0, P = 0.342) and viable embryos (5.3 ± 3.1 vs 5.0 ± 4.1, P = 0.139) were comparable in both protocols. The mean hMG doses in the DYG + hMG group were significantly lower than the MPA + hMG group (1710.7 ± 431.6 vs 1891.3 ± 402.2 IU, P <0.001), while the duration of ovarian stimulation failed to reach statistical difference (9.1 ± 1.6 vs 9.0 ± 1.6 days, P = 0.670). There were no significant between-group differences when the mature oocyte rate, fertilization rate and cleavage rate were analyzed (P = 0.635, 0.760 and 0.474, respectively). The rate of cycle cancellation for no viable embryos did not differ between the two groups, and no moderate-to-severe OHSS was observed during the study.
 |
Table 2 Cycle Characteristics and Outcomes by Regimen Group |
Hormone Profile
The endocrine dynamics of FSH, LH, E2 and P during ovarian stimulation are illustrated in Figure 2. The FSH levels increased after hMG administration and then maintained stable from MC9-11 to the trigger day. After dual trigger by GnRH agonist and hCG, the FSH levels increased dramatically to 16.90 ± 4.37 and 16.98 ± 5.19 mIU/mL in the DYG + hMG group and MPA + hMG group, respectively. No statistical differences in FSH levels were observed at any time point between the two groups.
Serum LH concentrations were well controlled by DYG and MPA, and no premature LH surge was detected in either protocol. In both groups, the LH levels decreased gradually after progestin treatment and increased dramatically upon triggering. However, the mean LH levels of the DYG + hMG group were significantly higher than the MPA + hMG group on MC9-11 (4.33 ± 2.67 vs 3.38 ± 2.37 mIU/mL, P <0.001) and trigger day (2.74 ± 1.50 vs 2.16 ± 1.68 mIU/mL, P <0.001), while the values on the day after trigger remained similar (P = 0.256). In addition, compared with the MPA group, there were significantly fewer patients experiencing a profound LH suppression in the DYG group (71/315 [22.5%] vs 13/105 [12.4%], P = 0.024).
Serum E2 levels increased constantly with multiple follicle development from MC3 to the day after trigger in both protocols. The P levels in both groups increased slightly before trigger and dramatically after triggering final oocyte maturation. No significant between-group differences were found regarding E2 and P levels at each observation point.
Pregnancy and Neonatal Outcomes
Table 3 presents the pregnancy outcomes of frozen-thawed embryos originating from the two regimens. A total of 95 patients completed 154 FET cycles in the DYG + hMG group and 261 patients completed 367 FET cycles in the MPA + hMG group. There were no significant differences between the two groups in the number of transferred embryos, embryo stage, endometrial preparation protocol and endometrial thickness.
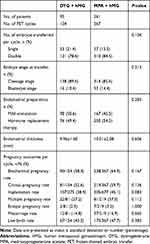 |
Table 3 Pregnancy Outcomes of Frozen-Thawed Embryos Originating from the Two Regimens |
The live birth rate per FET cycle was 43.5% and 47.7% in the DYG and MPA groups, respectively (P = 0.383). Similarly, the two groups were comparable in other pregnancy parameters regarding the rates of biochemical pregnancy, clinical pregnancy, implantation, multiple pregnancy, ectopic pregnancy and miscarriage (all P >0.05).
As demonstrated in Supplementary Table 1, between-group comparisons in both singletons and twins did not reveal any significant differences in gestational age, birthweight and length at birth. In terms of adverse neonatal outcomes, the two groups were also similar in the proportion of early neonatal death, preterm birth, low birthweight and major congenital malformations.
Discussion
In this propensity-matched retrospective cohort study, we demonstrated for the first time that the application of DYG in PPOS protocol could achieve comparable oocyte retrieval and pregnancy outcomes to MPA, but significantly reduce the gonadotropin consumption in PCOS women undergoing IVF treatment.
Consistent with previous studies,7,10,13 oral delivery of both DYG and MPA displayed effective blocking effects in premature LH surges and produced oocytes/embryos with similar developmental competence in patients with PCOS. Nevertheless, compared with the conventional short protocol, application of MPA in PPOS regimen tends to suppress pituitary in a stronger manner and results in higher gonadotropin doses and longer stimulation duration.7,10 In the present study, higher circulating LH levels in the mid- and late-follicular phase of ovarian stimulation, along with a lower incidence of profound LH suppression, indicated that the DYG had a weaker strength than MPA in pituitary inhibition of PCOS women. Accordingly, the total hMG doses in the DYG group were reduced for approximately 10% in comparison with the MPA group, while the hMG duration remained comparable. This result also concurred with our recent study finding in normal ovulatory women.13
The phenomenon could be partially elucidated from the differences in chemical structure, pharmacodynamics and pharmacokinetics between DYG and MPA.27,28 The MPA was derived from P acetylation of a hydroxyl group at carbon 17 and the addition of a methyl group at carbon 6, while DYG, a group member of retroprogesterone, has a methyl group at carbon 10 but is not acetylated. This structural alternation leads to a lower binding affinity of DYG with P receptor in both in vitro tests and animal bioassays, thus causing lower effective concentrations in the peripheral circulation.28 It may also explain why the use of MPA 10 mg/d, a half dose of DYG, is sufficient to exhibit equivalent or even deeper pituitary suppressive effects. Secondly, frequent blood sampling during 24 h after oral dosing shows that MPA has the highest bioavailability (>90%) among several progestins, whereas the bioavailability of DYG is only 28%.28 The half-life of MPA (24 h) also lasts much longer than that of DYG (14–17 h), which implies a longer-acting period with serum drug level above its threshold. Finally, due to the homology of significant amino acid in certain regions and conservatism of overall domain structure among members of the steroid receptor family, many progestins could display off-target influences by binding to androgen, glucocorticoid and mineralocorticoid receptors associated with follicle sensitivity and ovarian response.27,28 However, the binding affinities of DYG and MPA with these receptors exhibit considerable variations and this may, therefore, contribute to different clinical features when they are applied in PPOS protocol.
OHSS is an iatrogenic complication of ovarian hyperstimulation, which, in its moderate-to-severe form, may result in morbidity and even mortality. The prevalence of OHSS ranges from 1% to 5% in all IVF cycles.26 However, because of higher ovarian response to gonadotropins and higher E2 concentrations from multiple follicular development, the risk is especially greater for patients with PCOS.1,2 In the present study, approximately half patients had more than 20 follicles larger than 10 mm in diameter on trigger day and the average number of oocytes retrieved was more than 15. On the contrary, it is notable that none of these patients experienced moderate or severe OHSS. The application of dual trigger, as we speculate, is one of the main contributive factors.29 Conventionally, a high-dose hCG is used to trigger final oocyte maturation by activating the same receptor as LH, but its prolonged half-life and sustained luteotropic activity also increase the chance of OHSS. As the PPOS protocol does not down-regulate and desensitize pituitary as in GnRH agonist regimen, the partial replacement of long-acting hCG by short-acting GnRH agonist to elicit an endogenous LH release was made applicable. Another key strategy is that all viable embryos are cryopreserved after ovarian stimulation for later thawing and transfer.29 This freeze-all policy avoids the deleterious effects of supraphysiological hormonal milieu during COS and instead, embryos are transferred into a more physiological intrauterine environment for implantation in FET cycles. As a consequence, a complete avoidance of OHSS occurrence is made possible even in the high-risk PCOS population.
An issue debated for the past years is the potential adverse effects of progestin exposure on oocyte quality and offspring health. By retrospectively analyzing 1931 newborn follow-up data, our group has previously demonstrated that the MPA + hMG protocol was a safe option without compromising neonatal outcomes or elevating birth defect risks.23 DYG was first introduced in the 1960s and has been widely utilized in the treatment of various diseases such as endometriosis, menstrual disorders, recurrent miscarriage as well as luteal insufficiency in assisted reproductive settings.30 Although the safety of DYG on neonates has been verified by the Lotus I and II Phase III studies on luteal support,31,32 it is still unclear whether the new application of DYG for ovarian stimulation remains to be the same situation as its use in women whose embryos have been produced, transferred and implanted in the uterus beforehand. In the present study, the two groups were comparable in neonatal outcomes and congenital malformations in both singletons and twins, suggesting that the DYG may be as safe as MPA for the newborn population when used in PPOS protocol. However, the low incidence of congenital malformations may lead to inadequate statistical power to detect subtle differences between DYG and MPA. Therefore, future investigations with larger sample size and longer follow-up duration are warranted to validate this finding.
One major advantage of the study is that we matched the DYG and MPA groups using propensity scores. Selection bias and an imbalance of baseline characteristics between the groups are unignorable problems in observational studies.33 By calculating the combined action of multiple covariates as an accurate estimated value, the PSM has been demonstrated to be an effective technique in balancing the confounders for a similarly randomized treatment and minimizing selection bias.34 Furthermore, our study was strengthened by the meticulous patient screening with strict inclusion criteria, consistent routine clinical and laboratory procedures during the study period, as well as highly specialized pregnancy and newborn follow-up system at our center.
Several limitations have to be acknowledged for the present study. Firstly, potential unknown or unmeasured covariates may lead to incomplete or inexact matching and thus confound the robustness of the study findings. Secondly, the GnRH antagonist protocol has been recommended as the first choice for PCOS women undergoing IVF treatment owing to its advantage in reducing OHSS rate.2 However, given the proportional use of fresh embryo transfer in this regimen, we did not make a direct comparison with the DYG + hMG protocol for the favorable pregnancy outcomes of FET over fresh embryo transfer could result in mistaken interpretation of results.14,15 Thirdly, the starting dose of hMG was 150 IU for patients with high AFC (>20) or slightly increased FSH level (7–10 IU/L), whereas 225 IU hMG was applied initially for all other PCOS patients.10 Nonetheless, similar to other clinical trials,14,35,36 this tailored starting dose was based on single center’s experience and was not evidently validated in comparison with the standard starting dose. Considering the limited number of individualized gonadotropin dosing studies for the PCOS subgroup,37 this program deserves further investigation in future studies. Finally, cumulative live birth rate was unavailable because of incomplete FET cycles with surplus embryos for transfer. Although DYG use in PPOS protocol appears to be more cost-effective than MPA based on its fewer gonadotropin consumption, a comprehensive health economic evaluation still deserves to be performed considering the differences in progestin doses and prices.
Conclusion
In summary, our study showed that the application of DYG in PPOS protocol could significantly decrease the gonadotropin doses while producing comparable oocyte retrieval and pregnancy outcomes to MPA use in PCOS women for IVF treatment. However, further randomized controlled trials are still needed to confirm this conclusion.
Acknowledgments
This study was funded by the National Key Research and Development Program of China (2018YFC1003000) and National Natural Science Foundation of China (81571397, 81771533).
Disclosure
The authors report no conflicts of interest in this work.
References
1. Azziz R, Carmina E, Chen Z, et al. Polycystic ovary syndrome. Nat Rev Dis Primers. 2016;2:16057. doi:10.1038/nrdp.2016.57
2. Balen AH, Morley LC, Misso M, et al. The management of anovulatory infertility in women with polycystic ovary syndrome: an analysis of the evidence to support the development of global WHO guidance. Hum Reprod Update. 2016;22(6):687–708. doi:10.1093/humupd/dmw025
3. Thessaloniki ESHRE/ASRM-Sponsored PCOS Consensus Workshop Group. Consensus on infertility treatment related to polycystic ovary syndrome. Hum Reprod. 2008;23(3):462–477. doi:10.1093/humrep/dem426
4. Reichman DE, Zakarin L, Chao K, Meyer L, Davis OK, Rosenwaks Z. Diminished ovarian reserve is the predominant risk factor for gonadotropin-releasing hormone antagonist failure resulting in breakthrough luteinizing hormone surges in in vitro fertilization cycles. Fertil Steril. 2014;102(1):99–102. doi:10.1016/j.fertnstert.2014.04.010
5. Bosch E, Valencia I, Escudero E, et al. Premature luteinization during gonadotropin-releasing hormone antagonist cycles and its relationship with in vitro fertilization outcome. Fertil Steril. 2003;80(6):1444–1449. doi:10.1016/j.fertnstert.2003.07.002
6. Sterling L, Liu J, Okun N, Sakhuja A, Sierra S, Greenblatt E. Pregnancy outcomes in women with polycystic ovary syndrome undergoing in vitro fertilization. Fertil Steril. 2016;105(3):791–797. doi:10.1016/j.fertnstert.2015.11.019
7. Kuang Y, Chen Q, Fu Y, et al. Medroxyprogesterone acetate is an effective oral alternative for preventing premature luteinizing hormone surges in women undergoing controlled ovarian hyperstimulation for in vitro fertilization. Fertil Steril. 2015;104(1):62–70. doi:10.1016/j.fertnstert.2015.03.022
8. Harris TG, Dye S, Robinson JE, Skinner DC, Evans NP. Progesterone can block transmission of the estradiol-induced signal for luteinizing hormone surge generation during a specific period of time immediately after activation of the gonadotropin-releasing hormone surge-generating system. Endocrinology. 1999;140(2):827–834. doi:10.1210/endo.140.2.6490
9. Wildt L, Hutchison JS, Marshall G, Pohl CR, Knobil E. On the site of action of progesterone in the blockade of the estradiol-induced gonadotropin discharge in the rhesus monkey. Endocrinology. 1981;109(4):1293–1294. doi:10.1210/endo-109-4-1293
10. Wang Y, Chen Q, Wang N, Chen H, Lyu Q, Kuang Y. Controlled ovarian stimulation using medroxyprogesterone acetate and hMG in patients with polycystic ovary syndrome treated for IVF: a double-blind randomized crossover clinical trial. Medicine (Baltimore). 2016;95(9):e2939. doi:10.1097/MD.0000000000002939
11. Guo H, Wang Y, Chen Q, et al. Use of medroxyprogesterone acetate in women with ovarian endometriosis undergoing controlled ovarian hyperstimulation for in vitro fertilization. Sci Rep. 2017;7(1):11927. doi:10.1038/s41598-017-12151-7
12. Lu X, Hong Q, Sun L, et al. Dual trigger for final oocyte maturation improves the oocyte retrieval rate of suboptimal responders to gonadotropin-releasing hormone agonist. Fertil Steril. 2016;106(6):1356–1362. doi:10.1016/j.fertnstert.2016.07.1068
13. Yu S, Long H, Chang HY, et al. New application of dydrogesterone as a part of a progestin-primed ovarian stimulation protocol for IVF: a randomized controlled trial including 516 first IVF/ICSI cycles. Hum Reprod. 2018;33(2):229–237.
14. Chen ZJ, Shi Y, Sun Y, et al. Fresh versus frozen embryos for infertility in the polycystic ovary syndrome. N Engl J Med. 2016;375(6):523–533. doi:10.1056/NEJMoa1513873
15. Shi Y, Sun Y, Hao C, et al. Transfer of fresh versus frozen embryos in ovulatory women. N Engl J Med. 2018;378(2):126–136. doi:10.1056/NEJMoa1705334
16. Rotterdam ESHRE/ASRM-Sponsored PCOS Consensus Workshop Group. Revised 2003 consensus on diagnostic criteria and long-term health risks related to polycystic ovary syndrome. Fertil Steril. 2004;81(1):19–25. doi:10.1016/j.fertnstert.2003.10.004
17. Huang J, Lin J, Gao H, et al. Anti-mullerian hormone for the prediction of ovarian response in progestin-primed ovarian stimulation protocol for IVF. Front Endocrinol (Lausanne). 2019;10:325.
18. Cummins JM, Breen TM, Harrison KL, Shaw JM, Wilson LM, Hennessey JF. A formula for scoring human embryo growth rates in in vitro fertilization: its value in predicting pregnancy and in comparison with visual estimates of embryo quality. J in vitro Fert Embryo Transf. 1986;3(5):284–295. doi:10.1007/BF01133388
19. Gardner DK, Schoolcraft WB. In vitro culture of human blastocyst. In: Jansen R, Mortimer D, editors. Towards Reproductive Certainty: Infertility and Genetics Beyond 1999. Carnforth: Parthenon Press; 1999:378–388.
20. Larue L, Keromnes G, Massari A, et al. Transvaginal ultrasound-guided embryo transfer in IVF. J Gynecol Obstet Hum Reprod. 2017;46(5):411–416. doi:10.1016/j.jogoh.2017.02.015
21. Cozzolino M, Vitagliano A, Di Giovanni MV, et al. Ultrasound-guided embryo transfer: summary of the evidence and new perspectives. A systematic review and meta-analysis. Reprod Biomed Online. 2018;36(5):524–542. doi:10.1016/j.rbmo.2018.01.015
22. Chen H, Wang Y, Lyu Q, et al. Comparison of live-birth defects after luteal-phase ovarian stimulation vs. conventional ovarian stimulation for in vitro fertilization and vitrified embryo transfer cycles. Fertil Steril. 2015;103(5):1194–1201. doi:10.1016/j.fertnstert.2015.02.020
23. Zhang J, Mao X, Wang Y, et al. Neonatal outcomes and congenital malformations in children born after human menopausal gonadotropin and medroxyprogesterone acetate treatment cycles. Arch Gynecol Obstet. 2017;296(6):1207–1217. doi:10.1007/s00404-017-4537-z
24. Huang J, Xie Q, Lin J, et al. Neonatal outcomes and congenital malformations in children born after dydrogesterone application in progestin-primed ovarian stimulation protocol for IVF: a retrospective cohort study. Drug Des Devel Ther. 2019;13:2553–2563. doi:10.2147/DDDT.S210228
25. Liu Y, Chen Q, Yu S, et al. Progestin-primed ovarian stimulation with or without clomiphene citrate supplementation in normal ovulatory women undergoing in vitro fertilization/intracytoplasmic sperm injection: a prospective randomized controlled trial. Clin Endocrinol (Oxf). 2018;88(3):442–452. doi:10.1111/cen.2018.88.issue-3
26. Practice Committee of American Society for Reproductive Medicine. Prevention and treatment of moderate and severe ovarian hyperstimulation syndrome: a guideline. Fertil Steril. 2016;106(7):1634–1647. doi:10.1016/j.fertnstert.2016.08.048
27. Schindler AE, Campagnoli C, Druckmann R, et al. Classification and pharmacology of progestins. Maturitas. 2003;46(Suppl 1):S7–S16. doi:10.1016/j.maturitas.2003.09.014
28. Stanczyk FZ, Hapgood JP, Winer S, Mishell DR
29. Mourad S, Brown J, Farquhar C. Interventions for the prevention of OHSS in ART cycles: an overview of cochrane reviews. Cochrane Database Syst Rev. 2017;1:Cd012103.
30. Griesinger G, Tournaye H, Macklon N, et al. Dydrogesterone: pharmacological profile and mechanism of action as luteal phase support in assisted reproduction. Reprod Biomed Online. 2018.
31. Tournaye H, Sukhikh GT, Kahler E, Griesinger G, Phase A. III randomized controlled trial comparing the efficacy, safety and tolerability of oral dydrogesterone versus micronized vaginal progesterone for luteal support in in vitro fertilization. Hum Reprod. 2017;32(5):1019–1027. doi:10.1093/humrep/dex023
32. Griesinger G, Blockeel C, Sukhikh GT, et al. Oral dydrogesterone versus intravaginal micronized progesterone gel for luteal phase support in IVF: a randomized clinical trial. Hum Reprod. 2018;33(12):2212–2221.
33. Sturmer T, Joshi M, Glynn RJ, Avorn J, Rothman KJ, Schneeweiss S. A review of the application of propensity score methods yielded increasing use, advantages in specific settings, but not substantially different estimates compared with conventional multivariable methods. J Clin Epidemiol. 2006;59(5):437–447. doi:10.1016/j.jclinepi.2005.07.004
34. Austin PC. An introduction to propensity score methods for reducing the effects of confounding in observational studies. Multivariate Behav Res. 2011;46(3):399–424. doi:10.1080/00273171.2011.568786
35. Fischer D, Reisenbuchler C, Rosner S, Haussmann J, Wimberger P, Goeckenjan M. Avoiding OHSS: controlled ovarian low-dose stimulation in women with PCOS. Geburtshilfe Frauenheilkd. 2016;76(6):718–726. doi:10.1055/s-00000020
36. Di Paola R, Garzon S, Giuliani S, et al. Are we choosing the correct FSH starting dose during controlled ovarian stimulation for intrauterine insemination cycles? Potential application of a nomogram based on woman’s age and markers of ovarian reserve. Arch Gynecol Obstet. 2018;298(5):1029–1035. doi:10.1007/s00404-018-4906-2
37. Broekmans FJ. Individualization of FSH doses in assisted reproduction: facts and fiction. Front Endocrinol (Lausanne). 2019;10:181.
 © 2020 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2020 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.


