Back to Journals » Drug Design, Development and Therapy » Volume 14
Pretreatment with Lactobacillus fermentum XY18 Relieves Gastric Injury Induced by HCl/Ethanol in Mice via Antioxidant and Anti-Inflammatory Mechanisms
Authors Wang R, Zhou K, Xiong R, Yang Y, Yi R, Hu J, Liao W, Zhao X
Received 4 September 2020
Accepted for publication 12 December 2020
Published 30 December 2020 Volume 2020:14 Pages 5721—5734
DOI https://doi.org/10.2147/DDDT.S280429
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 3
Editor who approved publication: Professor Anastasios Lymperopoulos
Ranran Wang,1– 4,* Kexiang Zhou,5,* Rongrong Xiong,1– 4 Yi Yang,1– 4 Ruokun Yi,1– 3 Jing Hu,1– 4 Wei Liao,1,6 Xin Zhao1– 3
1Chongqing Collaborative Innovation Center for Functional Food, Chongqing University of Education, Chongqing, People’s Republic of China; 2Chongqing Engineering Research Center of Functional Food, Chongqing University of Education, Chongqing, People’s Republic of China; 3Chongqing Engineering Laboratory for Research and Development of Functional Food, Chongqing University of Education, Chongqing, People’s Republic of China; 4College of Biological and Chemical Engineering, Chongqing University of Education, Chongqing, People’s Republic of China; 5Gastroenterology, The Third Affiliated Hospital, Chongqing Medical University, Chongqing, People’s Republic of China; 6Department of Public Health, Our Lady of Fatima University, Valenzuela, Philippines
*These authors contributed equally to this work
Correspondence: Xin Zhao Tel +86-23-6265-3650
Email [email protected]
Aim: Lactobacillus fermentum XY18 (LF-XY18) is a bacterial strain with satisfactory antioxidant properties in vitro that we previously isolated from Xinjiang yogurt. This article will explore the preventive effect of LF-XY18 on acute gastric injury and provide the basis for the innovative development and application of lactic acid bacteria (LAB).
Methods: Kunming mice underwent gastric injury induced by hydrochloric acid and ethanol. LF-XY18 isolated from yogurt in Xinyuan County in the Yili region of Xinjiang was subsequently administered intragastrically to mice for 2 weeks to explore the mechanism of LF-XY18 in preventing gastric injury via its antioxidant effects.
Results: There was decreased gastric juice volume, gastric injury area, and formation of gastric mucosal lesions in the LF-XY18 mice as compared to those in the control mice, while LF-XY18 prevented the decrease in the gastric juice pH value in mice. Compared with the gastric injury model group mice, LF-XY18 reduced the serum levels of motilin, substance P, interleukin-6, interleukin-12, tumor necrosis factor-α, and interferon-γ but increased the serum levels of somatostatin and vasoactive intestinal peptide. The activities of superoxide dismutase, glutathione peroxidase, glutathione, and nitric oxide were increased in the gastric tissue of the LF-XY18 mice compared with the control mice, but malondialdehyde activity was decreased in the LF-XY18 mice. Quantitative polymerase chain reaction analysis illustrated that in the gastric tissue of LF-XY18 mice, the messenger RNA (mRNA) expression of occludin, epidermal growth factor (EGF), EGF receptor, vascular EGF, inhibitor kappa-B-α, neuronal nitric oxide synthase, endothelial nitric oxide synthase, cuprozinc superoxide dismutase, manganese superoxide dismutase, and catalase was stronger than that in the control mice, but the mRNA expression of activated B cells (NF-κB), inducible nitric oxide synthase, and cyclooxygenase-2 was weaker than in the control mice.
Conclusion: These results indicate that LF-XY18 has a potential role in the prevention of gastric injury through antioxidant effects, and a high concentration (1.0 × 109 CFU/kg b.w.) of LF-XY18 has a stronger anti-gastric injury effect than a low concentration (1.0 × 108 CFU/kg b.w.).
Keywords: Lactobacillus fermentum XY18, gastric injury, HCl/ethanol-induced, mice, antioxidant
Introduction
Alcohol production and consumption occur worldwide, and the intake of alcohol per capita in 2016 was approximately 6.4 L.1 Excessive alcohol intake has become a global danger to human health that cannot be ignored, with the death toll having reached approximately 3 million.2 Alcohol is one of the main exogenous invasive factors that cause inflammatory damage to the gastric mucosa in vivo. Excessive alcohol consumption can result in acute gastric mucosal damage, with symptoms such as hemorrhage, necrosis, edema, and microcirculation damage.3 In addition, long-term alcohol consumption can give rise to gastric ulcers, gastritis, gastric cancer, and other severe gastric mucosal lesions.4 Until now, there has been a gap to be filled in terms of the mechanism of alcohol-induced acute gastric injury, but research has confirmed that oxidative stress and inflammatory responses are strongly linked to the development of acute gastric mucosal lesions.2 The stomach is stimulated by external factors such as the invasion of excessive ethanol, which activates the transient receptor potential vanilloid 1. Substance P (SP) can be released as a result, activating the neurokinin type 1 receptor in gastric epithelial cells, which leads to an increase in reactive oxygen species that can bring about the lipid peroxidation of gastric mucosal cell membranes.5 Additionally, ethanol can cause DNA strand breakage, and DNA bases can combine with the malondialdehyde (MDA) produced by the lipid peroxidation of oxygen free radicals, causing mutational damage to the gastric mucosa.6 The release of lysosomal enzymes caused by the lipid peroxidation of cell membranes also aggravates gastric mucosal injury. High-dose ethanol destroys the defense barrier of the gastric mucosal antioxidant system, decreases nitric oxide (NO) and glutathione (GSH) activities, and exacerbates acute gastric mucosal injury.7,8 Hydrochloric acid (HCl) can also induce acute gastric mucosal injury and decrease gastric mucosal blood flow.4
Acute alcoholic gastric injury can primarily be alleviated by reducing the ethanol and oxygen free radical levels in vivo, which will subsequently reduce the oxidative damage that they cause.9 At present, ranitidine, omeprazole, and other drugs have obvious curative effects on gastric ulcers, although various adverse effects are often observed due to long-term use of these drugs.10 Therefore, the development of functional health foods that prevent and treat acute gastric mucosal injury induced by ethanol is particularly important. The antioxidant properties of probiotics act upon the gastrointestinal tract,11 reducing lipid peroxidation and SP levels and alleviating the symptoms of alcohol-induced acute gastric mucosal injury, and very few side effects have been observed due to the use of probiotics.12
Xinyuan County in the Yili region of Xinjiang is located in northwest China, with a temperate climate and abundant rainfall. The abundance of forage grass resources can be used for livestock breeding. The yogurt produced in the Yili region is a traditional fermented dairy product that is rich in essential nutrients, such as essential amino acids, lactose, lactic acid, fats, and minerals. Its nutritional value and health effects are far superior to those of ordinary yogurt, and its pH, fermentation time, and the natural fermentation temperature are suitable for microbial growth.13 Our research team isolated a new strain of lactic acid bacteria (LAB) from yogurt originating from Xinyuan County, Yili, Xinjiang, China, and we named it Lactobacillus fermentum XY18 (LF-XY18). Our early research demonstrated that the LAB isolated from Xinjiang Kashi yogurt and Qinghai-Tibet Plateau yak yogurt could more effectively through its antioxidant properties prevent acute gastric injury induced by HCl/ethanol as compared to Lactobacillus delbrueckii subsp. bulgaricus (LB), which is commonly used in commerce.14,15 Probiotics can be used as a substitute for antibiotics to eliminate Helicobacter pylori in vivo, while protecting gastric mucosal cells from invasion and causing minimal side effects.16 Lactobacillus reuteri DSM 17938, which has been applied in the field of functional food research and development for many years, has many beneficial gastrointestinal tract functions, such as alleviating human gastrointestinal diseases, ameliorating children’s colic, and reducing constipation and diarrhea.17 The probiotic mixture VSL #3® (containing 8 probiotic bacteria) lessens the release of pro-inflammatory factors and enhances the expression of vascular regeneration proteins to relieve alcohol-induced gastric ulcers.18 Lactobacillus plantarum LC27 and Bifidobacterium longum LC67 can prevent and treat acute gastric mucosal damage and liver injury caused by alcohol through their antioxidant effects.19
At present, there has been insufficient domestic and foreign research performed using LF-XY18. Because our team holds the independent intellectual property rights for LF-XY18, we treated HCl/ethanol-induced mice with this bacterial strain and determined its ability to prevent and alleviate gastric mucosal damage through its antioxidant effects. The results of this study will help to further our understanding of the mechanisms involved in preventing and alleviating acute gastric injury and pave the way for the application of LF-XY18 in the field of functional food.
Materials and Methods
Isolation and Preliminary Identification of LAB
A 1-mL yogurt sample obtained from Hongyuan County, Yili, Xinjiang, was added to 9 mL of sterile saline solution, and the yogurt sample was diluted by gradient. Then, 100 μL of 10−4, 10−5, 10−6, and 10−7 gradient dilution samples was evenly coated onto sterilized plates containing Man, Rogosa, and Sharpe (MRS) agar medium (Becton, Dickinson and Company, USA). After culturing at 37°C for 24–48 h, the morphology of the LAB colonies was observed and recorded. LAB with different morphologies were selected, and after culturing in a constant temperature incubator at 37°C for 48 h, different LAB colony shapes were selected again for streaking on plates. This process was repeated until pure single colonies with the same shape were obtained. The pure LAB colonies were selected and then inoculated into 5 mL of MRS liquid medium. After culturing at 37°C for 24 h, 1 mL of the MRS liquid medium was immediately centrifuged at 12,000 rpm for 1 min. After discarding the supernatant, 200–500 μL of sterile normal saline was added, and the bacteria were microscopically examined by Gram stain. Finally, the strain was re-inoculated into 5 mL of MRS liquid medium, and after 24 h of incubation at 37°C, the DNA of the LAB was extracted (Beijing Tianjin Biotechnology Co., Ltd., Beijing, China). The 16S ribosomal DNA (rDNA) gene from the LAB strain was amplified by PCR (upstream primer: 27F (5ʹ-AGA GTT TGA TCC TGG CTC AG-3ʹ); downstream primer: 1495R (5ʹ-CTA CGG CTA CCTTGT TAC GA-3ʹ)). The size range of the MW ladder (Thermo Fisher Scientific, Waltham, USA) used was 14.4 to 116 kDa, and agarose gel electrophoresis was used to detect the product. The 16S rDNA method was used to successfully sequence the PCR products detected, and NCBI’s BLAST (Basic Local Alignment Search Tool) was used to compare and analyze the sequences.20
Determination of LAB Tolerance to 0.3% Bile Salts
For preparation of 0.3% bile salts MRS-THIO culture medium, porcine bile salts and 0.2% sodium thioglycollate MRS broth were added to MRS-THIO culture medium, and then sterilized at 121°C for 15 min. The sample group received MRS-THIO culture medium with 0.3% bile salts, the control group received MRS-THIO culture medium without bile salts (0.0%), and the blank group received MRS-THIO culture medium without LAB. Next, 5 mL of 2% (v/v) activated strain was inoculated into the sample group and the control group media, respectively. After culturing for 24 hours at 37°C, the OD600 nm values of the three groups were determined, and the bile salt tolerance (%) of the strain was calculated according to the formula: bile salt tolerance (%)=(OD600 of the sample group-OD600 of the blank group)/(OD600 of the control group-OD600 of the blank group)×100.21
Determination of the LAB Tolerance to Artificial Gastric Juice
The artificial gastric juice was prepared with 0.2% NaCl and 0.35% pepsin. Then, the pH was adjusted to 3.0 with 1 M HCl, sterile-filtered with a 0.22-µm filter membrane, and set aside. To isolate the bacteria, 5 mL of bacteria-containing medium was centrifuged at 3000 rpm for 10 minutes. The supernatant was discarded, and then, 5 mL of sterile normal saline was added and evenly mixed with the LAB. Next, 1 mL bacterial suspension was added to 9 mL pH 3.0 artificial gastric juice, mixed evenly, and then, 1 mL of the mixture was treated for 0 h as an artificial gastric juice sample and the remaining mixture was maintained at 37°C and 150 rpm for 3 h in a water bath shaker. The 0 and 3 h samples were sequentially diluted 10 times and then cultured at 37°C for 48 h by spreading on agar plates. The appropriate gradient was selected to determine the survival rate according to the formula: survival rate (%)=(3 h viable count)/(0 h viable count)×100.22
Detection of DPPH Radical Scavenging Ability of LAB
To measure the ability of LAB to scavenge DPPH radicals, 0.2 mM DPPH solution was prepared with absolute ethanol. Next, 1 mL of the sample to be tested was added to 1 mL 0.2 mM DPPH solution, thoroughly mixed, incubated in the dark for 30 min at room temperature, and then centrifuged at 6000 rpm for 10 min. The supernatant was removed for the determination of absorbance at the wavelength of 517 nm, namely A1. The DPPH solution was replaced with absolute ethanol to measure the absorbance value, namely A2. The sample solution was replaced with sterile distilled water to measure the absorbance value, namely A3. The DPPH clearance was determined according to the formula: DPPH clearance (%)=(1-(A1-A2)/A3)×100.23
The Culture and Preparation Conditions for the in vivo Assay of LAB Strains
L. fermentum XY18 (LF-XY18) was preserved in the China General Microbiological Culture Collection (CGMCC No. 16279, Beijing, China). After two generations of activation of the LF-XY18 strain previously frozen at −80°C, 100 μL of culture medium containing the second-generation activated LF-XY18 strain was placed in 10 mL of MRS medium and cultured at 37°C for 24 hours. Then, the supernatant of the MRS medium was removed and added to 10 mL of normal saline in a centrifuge tube containing LF-XY18 at the bottom, shaken well, centrifuged at 4000 rpm for 10 min, and then, the bacteria were repeatedly washed using this protocol. Finally, 5 mL normal saline was added, and the bacterial suspension was ready to be used after shaking as a gavage for mice.
Establishment of Acute Gastric Injury in Mice
Under the condition of moving, drinking, and eating freely, 6-week-old, male, 23–25 g Kunming mice, which were purchased from the Experimental Animal Center of Chongqing Medical University (Chongqing, China), were maintained in laboratory mouse cages for 1 week and then divided into 5 groups at random, each with 10 mice. The groups were normal, gastric injury model, ranitidine, LF-XY18-L, and LF-XY18-H. The normal and control mice received intragastric doses of 0.1 mL/10 g body weight (b.w.) of distilled water daily, and the mice in the ranitidine group received intragastric doses of 30 mg/kg b.w. of ranitidine solution daily. The LF-XY18-L and LF-XY18-H mice were gavaged with 1.0 × 108 CFU/kg b.w. and 1.0 × 109 CFU/kg b.w. of L. fermentum liquid, respectively. After 14 days of gavage, all mice were fasted for 24 h but were permitted to drink and move freely. Except for the normal mice, after fasting, the mice in the other groups were treated with a stomach damage inducer (0.1 mL HCl/ethanol/10 g b.w., 60% in 150 mM HCl) administered intragastrically.14 The normal group receive an equivalent amount of distilled water. After 0.5 h, the mice were killed with CO2. Retro-orbital sinus blood collection was immediately performed, and the gastric tissue was then dissected. Finally, mouse serum (obtained by centrifugation of blood at 4000 rpm for 10 min), gastric juice, and stomach tissue were measured and examined. The protocol for these experiments was approved by the Ethics Committee of Chongqing Collaborative Innovation Center for Functional Food (202001029B), Chongqing, China, and the guidelines followed for the welfare of the laboratory animals was GB/T 35892–2018 Laboratory Animal Guidelines for Ethical Review of Animal Welfare.24
Observation of the Degree of Gastric Injury
A 10-mL graduated cylinder and pH test paper were used to determine the gastric fluid volume and the gastric juice pH, respectively. Then, the entire stomach of each mouse was fixed in 10 mL of 1% formalin tissue fixative for 10 min. The entire stomach was then cut along the great curvature of the stomach, rinsed with cold physiological saline, and spread out and flattened. The morphology and degree of damage to the stomach were photographed. ImageJ v1.44 software and Equation 1 were used to measure the area of stomach injury (mm2).25 Finally, using 1% formalin as a tissue fixative, a piece of mung bean-sized stomach tissue was cut to prepare and observe pathological stomach sections. The gastric injury inhibitory rate (%)=(1-gastric injury area of sample treated mice/gastric injury area of injury group mice)×100.
Histopathological Observation of the Stomach
After fixing the stomach tissue with 10% formalin solution for 48 h, the stomach tissue was dehydrated with 95% ethanol for 24 h, and then, hematoxylin and eosin (H&E) slices were prepared through a series of waxing, embedding, slicing, dewaxing, dyeing, dehydration, and sealing. Finally, the morphology of the stomach tissue was observed using a light microscope (BX43, Olympus, Tokyo, Japan). Ulcer and inflammation scores were used to observe the histopathology of the stomach. The specific method, with appropriate modifications, was based on the study of Cheng et al.26
Determination of Motilin (MOT), Somatostatin (SS), Substance P (SP), and Vasoactive Intestinal Peptide (VIP) Levels in Serum
Using an enzyme-linked immunosorbent assay (ELISA) kit (Nanjing Jiancheng Bioengineering Institute, Nanjing City, China), the MOT, SS, SP, and VIP serum levels were tested according to the instructions provided with the kit.
Determination of Cytokine Levels in Serum
The serum levels of cytokines (interleukin (IL)-6, IL-12, tumor necrosis factor (TNF)-α, and interferon (IFN)-γ) were detected using an ELISA kit (Abcam, Cambridge, MA, USA) according to the instructions provided with the kit.
Determination of Superoxide Dismutase (SOD), Glutathione Peroxidase (GSH-Px), Glutathione (GSH), Nitric Oxide (NO), and Malondialdehyde (MDA) Activity in Stomach Tissue
The activity of SOD, GSH-Px, GSH, NO, and MDA in stomach tissue was determined using a biochemical kit (Nanjing Jiancheng Bioengineering Institute, Nanjing, China).
Determination of Related mRNA Expression in Stomach Tissue
For the total RNA extraction, 100 mg of gastric tissue was placed in a homogenization tube with 1 mL of TRIzol reagent (Invitrogen, Carlsbad, CA, USA). After homogenization, 200 μL of chloroform (Chongqing Xinjian Chemical Reagents Co., Ltd., Chongqing, China) was added to the tissue homogenate, and then it was incubated at 4°C for 5 min. At the same temperature, centrifugation was performed at 14,000 rpm for 15 min. The supernatant was then centrifuged, and isopropanol (Chongqing Xinjian Chemical Reagent Co., Ltd., Chongqing, China) of equal volume was added. After centrifugation under the same conditions, the yellow-white precipitate in the lower layer was the total RNA extracted from the stomach tissue. It was then diluted to 1 μg/μL. Using the total RNA as a template, complementary DNA (cDNA) derived from reverse transcription was mixed by centrifugation with 10 μL Master Mix, 1.0 μL upstream primer, 1.0 μL downstream primer, 1.0 μL cDNA template, and 7.0 μL sterile ultrapure water (purified in-house). Finally, an automatic thermocycler (QuantStudio 6 Flex Real-Time PCR; Life Technologies, Gaithersburg, MD, USA) was used to perform qPCR for 40 cycles at 95°C for 15 min, 60°C for 1 h, and 95°C for 15 min. GAPDH was chosen as an internal reference, and the relative expression level for each gene was calculated by the 2−ΔΔCT method.20 In Table 1, the related gene primer sequences are shown.
 |
Table 1 Sequences of Primers Used in the qPCR Assay |
Statistical Analysis
SPSS Statistics 19 software (SPSS Inc., Chicago, IL, USA) was used to perform one-way analysis of variance (ANOVA) and Student-Newman-Keuls test (S-N-K) multiple range tests for all data. The results are expressed as the mean ± standard deviation, with P < 0.05 indicating a statistical difference. The graphics were drawn using Origin 8.0 software (MicroCal Software, Northampton, MA, USA).
Results
Isolation and Identification of LF-XY18
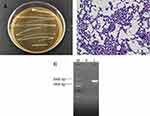
The vast majority of the colonies were round and white or milky white with regular edges and moist, smooth surfaces (Figure 1A). Gram staining was used to preliminarily identify the LAB. Under the microscope at 100× oil immersion, the observed cell morphologies of the strain were long and short rods. Importantly, no bud propagation was detected (Figure 1B), and therefore, it was determined that the isolated strain was a Gram-positive bacillus. Agarose gel electrophoresis was used to detect the 16S rDNA amplification products of this strain (Figure 1C). Sterile ultrapure water was used as the negative control. There were no bands in lane 0, which indicated that there was no contamination in the PCR amplification process. The specific amplified fragment length of the band in lane 1 was consistent with the expectation, approximately 1500 bp. The 16S rDNA method was used to examine the sequence, and the BLAST program was used for comparison and analysis. The results showed that the strain exhibited 99.93% homology with known Lactobacillus fermentum (Limosilactobacillus fermentum) in the GenBank database (GenBank No. CP034193.1). Therefore, it pertained to Lactobacillus fermentum and was named L. fermentum XY18.
Resistance of LAB to Bile Salts, Artificial Gastric Juice, and DPPH Free Radical
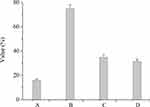
Figure 2 shows that the survival rates of LF-XY18 in 0.3% bile salts and pH 3.0 artificial gastric juice were 15.90% and 74.79%, respectively. The DPPH free radical scavenging rates of LF-XY18 complete cells and non-cell extracts were 34.92% and 31.52%, respectively. The above experimental results indicated that LF-XY18 was strongly tolerant of bile salts and gastric acid, and because it also showed strong in vitro antioxidant properties, it has the potential to be used as a probiotic strain.
Volume and pH of Gastric Juice
As seen in Table 2, the mice in the gastric injury model group produced the largest gastric juice volume (0.42 mL) and the lowest gastric juice pH (1.57) (P < 0.05). However, these 2 indexes exhibited the opposite tendency in normal mice compared with the control mice (P < 0.05). Compared with the gastric injury model group, LF-XY18 effectively prevented the increase in gastric juice volume and the decrease in gastric juice pH. In addition, the effect of LF-XY18-H was similar to that of ranitidine and significantly better than that of LF-XY18-L (P < 0.05).
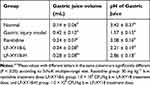 |
Table 2 Gastric Secretion Volume and pH in Mice Gastric Juice (n = 10) |
Observation of Gastric Morphology
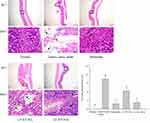
Figure 3 and Table 3 show that the normal mice had ruddy, smooth gastric mucosa without any signs of injury. The surface of the gastric mucosa showed the largest area of black hemorrhagic injury in the gastric injury model group mice, who were not subject to any preventive measures (0.47 ± 0.07 cm2) (P < 0.05). Except for the ranitidine group, the gastric injury inhibition rate of the LF-XY18-H group was closest to that of the normal group, which was 61.0 ± 6.01% (0.18 ± 0.03 cm2 of the gastric injury area) (P < 0.05). In summary, LF-XY18 effectively inhibited gastric injury in mice, and there was a relationship between its preventive effect and its concentration.
 |
Table 3 Gastric Morphology of Mice (n = 10) |
Histopathology of Stomach Tissue
The cells in the stomach tissue of the normal mice (score: 0.00 ± 0.00) were tightly arranged without becoming dislodged, the structures were relatively complete, and the stomach glands were clearly visible (Figure 4). In the gastric injury model group mice (score: 10.5 ± 1.08), the cells in the stomach tissue were very disorderly, and severe shedding occurred. Additionally, there was inflammatory cell infiltration, the submucosal blood vessels were hyperemic with symptoms of edema, and large-area focal ulcers were noted in the gastric mucosa. The mice in the ranitidine group (score: 1.90 ± 0.57) exhibited only slight tissue damage, and the cell arrangement and structural integrity were similar to those of the normal mice. Importantly, LF-XY18-H (score: 2.60 ± 0.70) exhibited a preventative effect on gastric damage that was comparable to ranitidine; the gastric tissue cells were tightly arranged and slightly loose, there were no obvious symptoms of hyperemia or edema in submucosal blood vessels, and the gland structure was relatively complete. Under the preventive effect of LF-XY18-L (score: 6.40 ± 0.84), gastric tissue cells were loosely arranged, focal ulcers appeared, submucosal blood vessels were dilated and edematous, and inflammatory cell infiltration was noted. Therefore, it was observed that LF-XY18-H effectively prevented gastric injury in mice.
MOT, SS, SP, and VIP Levels in Serum
Table 4 shows that the mice in the gastric injury model group had the highest MOT and SP serum levels (137.27 µg/L and 146.11 µg/L, respectively), while the SS and VIP serum levels were the lowest (45.73 µg/L and 39.04 µg/L, respectively) (P < 0.05). The preventive treatment of ranitidine and LF-XY18-H significantly lessened the MOT and SP serum levels in the mice and increased the levels of SS and VIP (P < 0.05). In addition, the MOT, SS, SP, and VIP levels of these 2 treatment groups were similar to those of the normal group. The preventive treatment of LF-XY18-L also decreased the MOT and SP serum levels and increased the SS and VIP serum levels, but its effect was far less than that of LF-XY18-H.
 |
Table 4 Serum Levels of MOT, SS, SP and VIP in Mice (n = 10) |
Cytokine Levels in Serum
The lowest IL-6, IL-12, TNF-α, and IFN-γ serum levels were measured in the normal mice, while these serum cytokine levels in the gastric injury model group mice were the highest (Table 5). After the preventive treatment, compared with the gastric injury model group mice, there were different degrees of reduction in the IL-6, IL-12, TNF-α, and IFN-γ serum levels in each treatment group. According to the treatment effects, they were arranged from strong to weak as follows: ranitidine > LF-XY18-H > LF-XY18-L. Therefore, LF-XY18-H strongly inhibited the increase in IL-6, IL-12, TNF-α, and IFN-γ serum levels, with values of 81.13 pg/mL, 418.30 pg/mL, 71.26 pg/mL, and 62.95 pg/mL, respectively (P < 0.05).
 |
Table 5 Serum Cytokine IL-6, IL-12, TNF-α and IFN-γ Levels in Mice (n = 10) |
SOD, GSH-Px, GSH, NO, and MDA Activity in Gastric Tissue
The gastric tissue of the normal mice exhibited the highest SOD, GSH-Px, GSH, and NO activity, at 218.35 U/mg prot, 241.32 U/mg prot, 7.49 mg/g prot, and 11.85 mmol/g prot, respectively, and the lowest MDA activity, at 3.45 nmol/mg prot (P < 0.05) (Table 6). The activities of SOD, GSH-Px, GSH, and NO in the gastric tissues of mice treated with LF-XY18-H were higher than those in the LF-XY18-L and gastric injury model group mice, and they was similar to those in the ranitidine mice. However, the opposite trend was observed for MDA activity (P < 0.05). LF-XY18-H more effectively increased the activities of SOD, GSH-Px, GSH, and NO, and decreased MDA activity in the gastric tissue of mice so as to be similar to those of normal mice.
 |
Table 6 Gastric-Tissue Activities of SOD, GSH-Px, GSH, NO and MDA in Mice (n = 10) |
Occludin, EGF, EGFR, and VEGF mRNA Expression in Gastric Tissue
The gastric tissue of the gastric injury model group mice had the weakest occludin, epidermal growth factor (EGF), EGF receptor (EGFR), and vascular EGF (VEGF) mRNA expression intensities (Figure 5A) (P < 0.05). After the effects of ranitidine and LF-XY18, the mRNA expression of occludin, EGF, EGFR, and VEGF in the treatment-of-gastric-injury mice were markedly enhanced (P < 0.05), with the preventive effect of ranitidine (3.33, 5.26, 3.20, and 2.55 fold greater than that of the control) altering the mRNA expression of occludin, EGF, EGFR, and VEGF in mice with gastric injury so that it was similar to that of normal mice (3.96, 6.45, 3.94, and 2.88 fold of the control) (P < 0.05). The effect of LF-XY18-H (2.87, 4.15, 2.86, and 2.07 fold of the control) was stronger than that of LF-XY18-L (1.46, 2.61, 1.63, and 1.46 fold of the control), and its effect was similar to that of ranitidine.
NF-κB and IκBα mRNA Expression in Gastric Tissue
As shown in Figure 5B, the gastric tissue of normal mice (0.12 and 2.78 fold of the control) exhibited the lowest mRNA expression of activated B cells (NF-κB) and the highest inhibitor kappa-B-α (IκBα) mRNA expression. However, the mRNA expression of NF-κB and IκBα in the gastric tissue of the gastric injury model group mice was the opposite (P < 0.05). Under the preventive effect of ranitidine (0.28 and 2.21 fold of the control), the mRNA expression of NF-κB and IκBα in the mice with gastric injury was similar to that of the normal group (P < 0.05). The mRNA expression of NF-κB decreased with increasing LF-XY18 concentration, and the mRNA expression of IκBα increased with increasing LF-XY18 concentration.
nNOS, eNOS, iNOS, and COX-2 mRNA Expression in Gastric Tissue
The expression of neuronal nitric oxide synthase (nNOS) and endothelial nitric oxide synthase (eNOS) mRNA (5.47 and 4.79 fold of the control) in the normal mice was significantly stronger as compared to the other groups, while the mRNA expression of inducible nitric oxide synthase (iNOS) and cyclooxygenase-2 (COX-2) (0.39 and 0.33 fold of the control) was significantly weaker than in the other groups (P < 0.05) (Figure 5C). The expression of these mRNAs in the stomach tissue of the gastric injury model group mice showed an opposite trend as compared to the normal mice (P < 0.05). LF-XY18 and ranitidine (4.04, 3.63, 0.53, and 0.49 fold of the control) significantly increased the mRNA expression of nNOS and eNOS and significantly decreased the mRNA expression of iNOS and COX-2 (P < 0.05). Importantly, the mRNA expression of nNOS, eNOS, iNOS, and COX-2 in the gastric tissue of the LF-XY18-H (3.54, 2.42, 0.61, and 0.62 fold of the control) and ranitidine groups was similar to that in the normal group (P < 0.05).
Cu/Zn-SOD, Mn-SOD, and CAT mRNA Expression in Gastric Tissue
As shown in Figure 5D, the mRNA expression of cuprozinc superoxide dismutase (Cu/Zn-SOD), manganese superoxide dismutase (Mn-SOD), and catalase (CAT) in mice of the gastric injury treatment groups was lower than that in the normal group (4.91, 4.73, and 5.19 fold of the control) and higher than that in the gastric injury model group mice (P < 0.05). In particular, ranitidine (4.38, 4.01, and 4.38 fold of the control) and LF-XY18-H (3.99, 3.30, and 3.70 fold of the control) were more effective than LF-XY18-L (2.54, 2.18, and 2.04 fold of the control) in promoting the mRNA expression of Cu/Zn-SOD, Mn-SOD, and CAT (P < 0.05).
Discussion
Our research showed that ethanol and HCl can easily induce acute and chronic gastric injury models, and then, we evaluated the potential efficacy of drugs for the prevention and treatment of acute and chronic gastric injury.25,27 Our team owns the independent intellectual property rights to Lactobacillus fermentum XY18 (LF-XY18). This study first proposed that LF-XY18 could effectively prevent HCl/ethanol-induced acute gastric injury. The international standard limits the concentration of LAB in Lactobacillus beverages to 107 CFU kg−1, while the concentration of Lactobacillus used in living mouse models is generally 109 CFU mL−1.28 When the concentration of Lactobacillus plantarum KSFY06 is 1.0×109 CFU kg−1 b.w., it can more optimally prevent acute gastric injury in mice.14 The present study selected 1.0×108 CFU kg−1 b.w. and 1.0×109 CFU kg−1 b.w. as the study concentrations of LF-XY18.
The treatment of acute gastric mucosal inflammatory injury mainly focuses on 2 aspects: (i) to inhibit gastric acid secretion and (ii) to enhance the resistance of the gastric mucosa. In the acute gastric injury model with HCl/ethanol as an inducer, Na+ is pumped out and K+ is pumped in, thereby reducing the blood flow of the gastric mucosa, increasing gastric acid secretion, and reducing the gastric juice pH, thus exacerbating gastric mucosal injury.25 Consistent with the results of this study, acute gastric injury can produce focal ulcers in the gastric mucosa, leading to symptoms such as hemorrhage of the blood vessels with edema and cell scattering.29 In this study, LF-XY18 decreased the amount of gastric juice secretion, prevented the decrease in the gastric juice pH value, and ameliorated the symptoms of hyperemia or edema in the submucosal blood vessels. Overall, a stronger effect was observed by LF-XY18-H as compared to LF-XY18-L.
MOT and SP are both gastrointestinal hormones. After being stimulated by various factors, the secretion of MOT and SP increases, leading to increased gastric acid secretion and decreased gastric acid pH, which aggravates the severity of acute gastric mucosal injury.30 Acute stomach injury caused by alcohol reduces SS and VIP levels in vivo.31 In addition, reducing MOT and SP levels and increasing SS and VIP levels can effectively treat gastric injury, gastritis, and gastric ulcers.32 The results of this study showed that LF-XY18 reduced MOT and SP levels and increased SS and VIP levels. In addition, these effects by LF-XY18-H are more effective than LF-XY18-L in preventing and treating acute gastric injury.
Unrestrained consumption of alcohol can activate the body’s immune system, causing great fluctuation in the serum levels of pro-inflammatory cytokines such as IL-6, interleukin-1β, and IFN-γ.33 Gastric lesions continue to deteriorate in an inflammatory environment, which is closely related to the production of inflammatory mediators.34 LAB can inhibit the rise of TNF-α and IFN-γ and increase the serum concentration of secretory immunoglobulin A, which is beneficial for alleviating gastric injury.35 TNF-α is a multidirectional functional pro-inflammatory cytokine that activates related pro-inflammatory cytokines, such as IL-6, which all promote the inflammatory response process. IL-12 can affect the immune system, and its serum level is positively correlated with the degree of inflammation in gastric tissue.36 In addition, both IL-12 and IFN-γ are pro-inflammatory cytokines, and the two are closely related.37 IFN-γ can weaken the body’s antiviral effect on non-target cell damage, while TNF-α participates in and promotes the inflammation and damage response in vivo.17 Compared with LF-XY18-L, LF-XY18-H more strongly inhibited the increase in IL-6, IL-12, TNF-α, and IFN-γ levels.
An important inducement of gastric mucosal injury is oxidative stress caused by external stimulation.38 There are enzymatic and non-enzymatic antioxidant defense systems in the body, including SOD, GSH, and GSH-Px, which scavenge superoxide, hydrogen peroxide, and hydroxyl, thus reducing the oxidative damage of tissues.39 SOD is the main enzyme that removes free radicals in the body due to its antioxidant and anti-inflammatory properties and other physiological activities.36 GSH and NO can react with lipid peroxides to reduce tissue oxidative damage and gastric ulcer formation, and they can also accelerate the healing of gastric ulcers.40 However, MDA activity is often used to measure the degree of lipid peroxidation, mainly because it is the final product of lipid oxidation.41 In this study, compared with the gastric injury model group, LF-XY18 increased SOD, GSH-Px, GSH, and NO activities while reducing MDA activity, thus inhibiting gastric injury through antioxidant effects.
Occludin is distributed in the epithelium of the gastric mucosa, and the decrease in its expression increases the permeability of the gastric mucosa, further aggravating the oxidative damage to the gastric mucosa resulting from alcohol, drugs, and other external factors.42 EGF is an antioxidant in the body that can stabilize mast cells, and LAB can enhance the mRNA expression of EGF, which in turn promotes the repair and regrowth of damaged cells in the gastric mucosa.43 EGFR located on the cell membrane surface can be activated by the binding of ligands (including EGF and TGF-α). Then, after EGFR binds to the cell membrane, EGF can exert an antioxidant effect on target cells, thus alleviating gastric injury.44 Similar to EGF, VEGF is a small peptide in the body. Enhanced EGFR expression can promote VEGF and angioprotein 1 expression, thus promoting the formation of blood vessels.45 The anti-inflammatory effect of LF-XY18 can increase the occludin, EGFR, and VEGF expression compared with the gastric injury model group and effectively alleviate gastric injury.
NF-κB, which is a transcription factor that can regulate the expression of genes related to oxidative stress, inflammation, and anti-apoptosis, is widely present in various cells.46 The combination of NF-κB and IκBα is relatively stable under normal conditions, but when the body is affected by external factors, the activated NF-κB is isolated from the phosphorylated IκBα and binds to specific κ gene sites, thus enhancing the expression of inflammatory factors and inflammatory mediator genes.47 Activation of the NF-κB pathway may be the main cause of gastric mucosal damage.48 In the current study, LF-XY18 decreased NF-κB expression and increased IκBα expression, thereby playing an antioxidant and inflammatory role to prevent and alleviate gastric injury.
NO is synthesized by the catalysis of NOS, of which there are 2 types: (i) constitutive NOS (cNOS, including nNOS and eNOS) and (ii) iNOS. NO from cNOS has certain preventive and alleviating effects on gastric injury. Reducing the expression of nNOS and increasing iNOS will worsen the degree of gastric tissue damage.49 NO synthesized by eNOS can reduce gastric acid volume, enhance the protective barrier function of gastric mucosa, increase the repair of epithelial tissue cells, participate in and promote the regeneration of blood vessels, and accelerate the blood flow of the gastric mucosa.50 COX-2 is a typical pro-inflammatory mediator in gastric injury, and it is also the inducible subtype of COX.28 Increasing the expression of iNOS can activate the gene expression of COX-2, thereby aggravating the degree of inflammation of stomach injury.51 This study showed that LF-XY18 protected the gastric mucosa by regulating NOS and COX-2 expression in gastric tissue.
In addition, increased levels of active oxygen in the body exacerbate the degree of alcohol-induced gastric damage. There are two isomers of SOD: Cu/Zn-SOD and Mn-SOD. Both are free radical scavengers. Cu/Zn-SOD is mainly active in the cytoplasm, while Mn-SOD is mainly distributed in the mitochondria of cells, with both having the function of protecting tissue cells from oxidative damage.52 Maintaining normal Cu/Zn-SOD and Mn-SOD levels in the body could efficaciously inhibit the spread of gastric lesions.33 As an antioxidant enzyme, CAT has the function of removing hydrogen peroxide in the body and can play a role in resisting the gastric lesions caused by alcohol-induced oxidative stress.53 The oxidative damage to gastric tissues caused by free radicals could be alleviated by maintaining the expression of active enzyme genes such as Cu/Zn-SOD, MN-SOD, and CAT in vivo.51 LF-XY18 demonstrated a satisfactory antioxidant effect through the expression of these antioxidant-related genes to prevent alcohol-induced gastric damage, and the higher the concentration, the stronger the antioxidant effect.
Conclusions
This study explored the preventive and alleviating effects of L. fermentum XY18 (LF-XY18) on gastric injury by establishing an acute gastric injury mouse model induced by HCl/ethanol and measuring the relevant indexes in the serum and gastric tissue. The results showed that LF-XY18 effectively prevents and relieves acute gastric injury, and the preventive effects were related to its concentration. That is, the higher the concentration of LF-XY18, the stronger the prevention and relief effects against acute gastric injury. In the gastric injury mice, LF-XY18 enhanced the defense function of enzymatic and non-enzymatic antioxidant systems in vivo, inhibited oxidative stress in serum and tissues, and decreased pro-inflammatory cytokine levels and tissue oxidative damage. LF-XY18 thereby restored normal gastric function in the gastric injury mice. LF-XY18 can be used to develop functional foods that will prevent gastric injury and will provide a theoretical basis for knocking out gastric injury-related genes to treat gastric injury.
Acknowledgments
This research was funded by the Chongqing University Innovation Research Group Project (CXQTP20033) and the Science and Technology Project of the Chongqing Education Commission (KJQN202001604), China.
Disclosure
The authors declare no conflicts of interest.
References
1. World Health Organization. Global Status Report on Alcohol and Health 2018. Geneva, Switzerland: World Health Organization; 2018:38–84.
2. Huh K, Kwon TH, Shin US, et al. Inhibitory effects of DA-9601 on ethanol-induced gastrohemorrhagic lesions and gastric xanthine oxidase activity in rats. J Ethnopharmacol. 2003;88(2–3):269–273. doi:10.1016/S0378-8741(03)00235-6
3. Li Y, Zhang Y, Meng H, et al. Efficacy and safety of acupuncture therapy for chronic atrophic gastritis: A meta-analysis and trial sequential analysis protocol. Medicine. 2019:98. doi:10.1097/MD.0000000000017003
4. Asmari AA, Shahrani HA, Masri NA, Faraidi AA, Elfaki I, Arshaduddin M. Vanillin abrogates ethanol induced gastric injury in rats via modulation of gastric secretion, oxidative stress and inflammation. Toxicol Rep. 2016;3:105–113. doi:10.1016/j.toxrep.2015.11.001
5. Zeze K, Hirano A, Torisu T, et al. Mucosal dysbiosis in patients with gastrointestinal follicular lymphoma. Hematol Oncol. 2020;38(2):181–188. doi:10.1002/hon.2717
6. Ajiboye TO. Standardized extract of Vitex doniana Sweet stalls protein oxidation, lipid peroxidation and DNA fragmentation in acetaminophen-induced hepatotoxicity. J Ethnopharm. 2015;164:273–282. doi:10.1016/j.jep.2015.01.026
7. Huang Z, Luo X, Liu M, et al. Function and regulation of apelin/APJ system in digestive physiology and pathology. J Cell Physiol. 2019;234(6):7796–7810. doi:10.1002/jcp.27720
8. Zhao F, Su J-F, Lun S-M, et al. Association between polymorphisms in the CYP1A1, CYP2E1 and GSTM1 genes, and smoking, alcohol and upper digestive tract carcinomas in a high-incidence area of northern China.. Oncol Lett. 2019;18(2):1267–1277. doi:10.3892/ol.2019.10455
9. Amato A, Serio R, Mulè F. Involvement of cholinergic nicotinic receptors in the menthol-induced gastric relaxation. Eur J Pharmacol. 2014;745:129–134. doi:10.1016/j.ejphar.2014.10.012
10. Simeoni M, Citraro ML, Cerantonio A, et al. An open-label, randomized, placebo-controlled study on the effectiveness of a novel probiotics administration protocol (ProbiotiCKD) in patients with mild renal insufficiency (stage 3a of CKD). Eur J Nutr. 2019;58(5):2145–2156. doi:10.1007/s00394-018-1785-z
11. Azmi L, Gupta SS, Shukla I, Kant P, Upreti DK, Rao CV. Gastro protective effects of usnea longissima metabolites on probiotic Lactobacillus casei. Int J Pharmacogn. 2016;3:140–148. doi:10.13040/IJPSR.0975-8232
12. Suo H, Zhao X, Qian Y, et al. Lactobacillus fermentum Suo attenuates HCl/Ethanol induced gastric injury in mice through its antioxidant effects. Nutrients. 2016;8(3):155. doi:10.3390/nu8030155
13. Chunyan W, Yuhui LI, Yingbiao LI, et al. Microbial Diversity and Gene Function Analysis in Traditional Hand-Made Cheese from Pastoral Areas of Yili in Xinjiang. Food Sci. 2019;24(7):147–154.
14. Wang R, Zeng X, Liu B, et al. Prophylactic effect of Lactobacillus plantarum KSFY06 on HCl/ethanol-induced gastric injury in mice. Food Funct. 2020;11(3):2679–2692. doi:10.1039/c9fo02474c
15. Yi RK, Tan F, Liao W, Wang Q, Mu J, Zhou X. Isolation and Identification of Lactobacillus plantarum HFY05 from Natural Fermented Yak Yogurt and Its Effect on Alcoholic Liver Injury in Mice. Microorganisms. 2019;7(11):530. doi:10.3390/microorganisms7110530
16. Zhao L, Jiang Y, Ni Y, et al. Protective effects of Lactobacillus plantarum C88 on chronic ethanol-induced liver injury in mice. J Funct Foods. 2017;35:97–104. doi:10.1016/j.jff.2017.05.017
17. Fatheree NY, Liu Y, Taylor CM, et al. Lactobacillus reuteri for infants with colic: A double-blind, placebo-controlled, randomized clinical trial. The Journal of Pediatrics. 2017;191:170–178. doi:10.1016/j.jpeds.2017.07.036
18. Dharmani P, De Simone C, Chadee K, Wallace J. The probiotic mixture VSL#3 accelerates gastric ulcer healing by stimulating vascular endothelial growth factor. PLoS One. 2013;8(3):e58671. doi:10.1371/journal.pone.0058671
19. Kwon EK, Kang G-D, Kim W-K, Han MJ, Kim D-H. Lactobacillus plantarum LC27 and Bifidobacterium longum LC67 simultaneously alleviate ethanol-induced gastritis and hepatic injury in mice. J Funct Foods. 2017;38:389–398. doi:10.1016/j.jff.2017.09.036
20. Wang R, Yang Z, Zhang J, Mu J, Zhou X, Zhao X. Liver Injury Induced by Carbon Tetrachloride in Mice Is Prevented by the Antioxidant Capacity of Anji White Tea Polyphenols. Antioxidants. 2019;8(3):64. doi:10.3390/antiox8030064
21. Zhao X, Zhang J, Yi S, et al. Lactobacillus plantarum CQPC02 prevents obesity in mice through the PPAR-α signaling pathway. Biomolecules. 2019;9:407.
22. Liu LL, Liu WS, Han BQ, Hu JB. Protective effects of glucosamine and chitooligosaccharide in alcohol liver injury mice. Period Ocean Univ China. 2010;40:73–76.
23. He Z, Wang X, Li G, et al. Antioxidant activity of prebiotic ginseng polysaccharides combined with potential probiotic Lactobacillus plantarum C88. Int J Food Sci Tech. 2015;50(7):1673–1682. doi:10.1111/ijfs.12824
24. GB/T 35892-2018. Laboratory animal—Guideline for Ethical Review of Animal Welfare. Beijing: China Standard Press; 2018.
25. Zhao X, Wang Q, Qian Y, Song J-L. Ilex kudingcha C.J. Tseng (Kudingcha) prevents HCl/ethanol-induced gastric injury in Sprague-Dawley rats.Mol Med Rep. 2013;7(5):1613–1616. doi:10.3892/mmr.2013.1402
26. Mao-Cheng S. Pretreatment with Lactobacillus reuteri F-9-35 attenuates ethanol-induced gastric injury in rats. Food Nutr Res. 2018;62:1469. doi:10.2921/fnr.v62.1469
27. Lam EKY, Yu L, Wong HPS, et al. Probiotic Lactobacillus rhamnosus GG enhances gastric ulcer healing in rats. Eur J Pharmacol. 2007;565(1–3):171–179. doi:10.1016/j.ejphar.2007.02.050
28. Guerin J, Burgain J, Borges F, et al. Use of imaging techniques to identify efficient controlled release systems of Lactobacillus rhamnosus GG during in vitro digestion. Food Funct. 2017;8(4):1587–1598. doi:10.1039/c6fo01737a
29. Liu P, Chen L, Zhang H. Natural killer cells in liver disease and hepatocellular carcinoma and the NK cell-based immunotherapy. J Immunol Res. 2018;2018:1206737. doi:10.1155/2018/1206737
30. Depoortere I, Thijs T, Janssen S, de Smet B, Tack J. Colitis affects the smooth muscle and neural response to motilin in the rabbit antrum. Br J Pharm. 2010;159(2):384–393. doi:10.1111/j.1476-5381.2009.00537.x
31. Zhou Y-L, Wang R, Feng X, Zhao X. Preventive effect of insect tea against reserpine-induced gastric ulcers in mice.Exp Ther Med. 2014;8(4):1318–1324. doi:10.3892/etm.2014.1859
32. Feng X, Wang M, Zhao Y, Han P, Dai Y. Melatonin from different fruit sources, functional roles, and analytical methods. Trends Food Sci Tech. 2014;37(1):21–25. doi:10.1016/j.tifs.2014.02.001
33. Beeskow AB, Meyer H-J, Schierle K, et al. Heterotopic gastric mucosa in gallbladder—A rare differential diagnosis to gallbladder masses. Medicine. 2018;97(10):e0058. doi:10.1097/MD.0000000000010058
34. Chang X, Luo F, Jiang W, et al. Protective activity of salidroside against ethanol-induced gastric ulcer via the MAPK/NF-κB pathway in vivo and in vitro. Int Immunopharmacol. 2015;28(1):604–615. doi:10.1016/j.intimp.2015.07.031
35. Banerjee M, Vats P. Reactive metabolites and antioxidant gene polymorphisms in Type 2 Diabetes Mellitus. Redox Biol. 2014;2:170–177. doi:10.1016/j.redox.2013.12.001
36. Khalaf RT, Ruan W, Orkin S, et al. Gastric injury secondary to button battery ingestions: a retrospective multicenter review. Gastrointest Endosc. 2020;92(2):276–283. doi:10.1016/j.gie.2020.04.037
37. Bagheri V, Memar B, Momtazi AA, et al. Cytokine networks and their association with Helicobacter pylori infection in gastric carcinoma. J Cell Phys. 2018;233(4):2791–2803. doi:10.1002/jcp.25822
38. Alhakamy NA, Badr-Eldin SM, Ahmed OAA, Halwani AA, Aldawsari HM, El-Moselhy A. Optimized Ellagic Acid–Ca Pectinate Floating Beads for Gastroprotection against Indomethacin-Induced Gastric Injury in Rats. Biomolecules. 2020;10(7):1006. doi:10.3390/biom10071006
39. Qian Y, Zhang J, Fu X, et al. Preventive effect of raw Liubao tea polyphenols on mouse gastric injuries induced by HCl/ethanol via anti-oxidative stress. Molecules. 2018;23(11):2848. doi:10.3390/molecules23112848
40. Palle S, Kanakalatha A, Kavitha CN. Gastroprotective and Antiulcer Effects of Celastrus paniculatus Seed Oil Against Several Gastric Ulcer Models in Rats. J Diet Suppl. 2018;15(4):373–385. doi:10.1080/19390211.2017.1349231
41. Reyes‐Reyes M, Salazar‐Montoya JA, Rodríguez‐Páez LI, et al. In vitro fermentation of oligosaccharides obtained from enzymatic hydrolysis of Opuntia streptacantha mucilage. J Sci Food Agric. 2019;99(6):2883–2891. doi:10.1002/jsfa.9501
42. Song J-L, Zhou YL, Feng X, Zhao X. White tea (Camellia sinenesis (L.)) ethanol extracts attenuate reserpine-induced gastric ulcers in mice. Food Sci Biotechn. 2015;24(3):1159–1165. doi:10.1007/s10068-015-0148-2
43. Long X, Zhao X, Wang W, et al. Protective effect of silkworm pupa oil on hydrochloric acid/ethanol-induced gastric ulcers. J Sci Food Agric. 2019;99(6):2974–2986. doi:10.1002/jsfa.9511
44. Taha MS, El-Sherbiny EM, Osman HF. Anti-ulcerogenic activity of Gum Arabic in gastric mucosal injury induced by ethanol in male albino rats. Applied Physiol Nutrition Metab. 2020;45(7):731–736. doi:10.1139/apnm-2018-0233
45. Chen S, Zhao X, Sun P, Qian J, Shi Y, Wang R. Preventive effect of Gardenia jasminoides on HCl/ethanol induced gastric injury in mice. J Pharmacol Sci. 2017;133(1):1–8. doi:10.1016/j.jphs.2016.05.011
46. Dembinski HE, Wismer K, Vargas JD, et al. Functional importance of stripping in NFκB signaling revealed by a stripping-impaired IκBα mutant. Proce Nat Acad Sci. 2017;114(8):1916–1921. doi:10.1073/pnas.1610192114
47. Zhang B, Wang L-S, Zhou Y-H. Elevated microRNA-125b promotes inflammation in rheumatoid arthritis by activation of NF-κB pathway. Biomed Pharmacother. 2017;93:1151–1157. doi:10.1016/j.biopha.2017.07.042
48. Liu H, Wang J, Wang J, Wang P, Xue Y. Paeoniflorin attenuates Aβ1-42-induced inflammation and chemotaxis of microglia in vitro and inhibits NF-κB- and VEGF/Flt-1 signaling pathways. Brain Res. 2015;1618:149–158. doi:10.1016/j.brainres.2015.05.035
49. El-Gohary OA. Obestatin improves hepatic injury induced by ischemia/reperfusion in rats: role of nitric oxide. Gen Physiol Biophys. 2017;36(1):109–115. doi:10.4149/gpb_2016030
50. Bachmann M, Waibler Z, Pleli T, Pfeilschifter J, Mühl H. Type I interferon supports inducible nitric oxide synthase in murine hepatoma cells and hepatocytes and during experimental acetaminophen-induced liver damage. Front Immunol. 2017;8:890. doi:10.3389/fimmu.2017.00890
51. Chen L-W, Chien C-H, Kuo S-F, et al. Low vitamin D level was associated with metabolic syndrome and high leptin level in subjects with nonalcoholic fatty liver disease: a community-based study. BMC Gastroenterol. 2019;19(1):125. doi:10.1186/s12876-019-1040-y
52. Gottfredsen RH, Larsen UG, Enghild JJ, Petersen SV. Hydrogen peroxide induce modifications of human extracellular superoxide dismutase that results in enzyme inhibition. Redox Biol. 2013;1(1):24–31. doi:10.1016/j.redox.2012.12.004
53. Sayed DA, Soliman AM, Fahmy SR. Echinochrome pigment as novel therapeutic agent against experimentally - induced gastric ulcer in rats. Biomed Pharmacother. 2018;107:90–95. doi:10.1016/j.biopha.2018.07.173
 © 2020 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2020 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.