Back to Journals » Clinical Interventions in Aging » Volume 14
Prediction of chemotherapy adverse reactions and mortality in older patients with primary lung cancer through frailty index based on routine laboratory data
Authors Wang Y, Zhang R, Shen Y, Su L , Dong B , Hao Q
Received 17 January 2019
Accepted for publication 9 June 2019
Published 5 July 2019 Volume 2019:14 Pages 1187—1197
DOI https://doi.org/10.2147/CIA.S201873
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 2
Editor who approved publication: Dr Zhi-Ying Wu
Yuting Wang,*,1 Rui Zhang*,2 Yanjiao Shen,1,3 Lin Su,1 Birong Dong,1 Qiukui Hao1
1The Center of Gerontology and Geriatrics/National Center for Geriatric Clinical Research, West China Hospital, Sichuan University, Chengdu, People’s Republic of China; 2Health Informatics Center, West China Hospital, Sichuan University, Chengdu, People’s Republic of China; 3Sleep Medicine Center, West China Hospital, Sichuan University, Chengdu, People’s Republic of China
*These authors contributed equally to this work
Objectives: To assess the role of a pre-chemotherapy frailty index based on routine laboratory data in predicting mortality and chemotherapy adverse reactions among older patients with primary lung cancer.
Design: Retrospective cohort study
Setting: West China Hospital, Chengdu, China
Participants: We included patients aged ≥60 years with primary lung cancer receiving the first course of chemotherapy.
Measurements: Data were collected from medical records, local government death databases or telephone interviews. Outcomes included chemotherapy adverse reactions and all-cause mortality. We constructed a frailty index based on 44 laboratory variables (FI-LAB) before chemotherapy, and chose the following cutoff points: robust (0.0–0.2), pre-frail (0.2–0.35) and frail (≥0.35).
Results: We included 1,020 patients (71.4% male; median age: 65 years old). Both pre-frailty and frailty was associated with any chemotherapy adverse reactions and infections during chemotherapy (OR=3.48, 95%CI: 1.77–6.87; OR=3.58, 95%CI: 1.55–8.26, respectively). Frail patients had a shorter median overall survival rate compared to robust patients (18.05 months vs 38.89 months, log-rank p<0.001). After adjusting for some potential confounding variables, the risk of all-cause mortality was dramatically increased in frail patients (HR:2.13, 95% CI:1.51–3.00) with an average follow-up of 3.9 years. Each 0.01 or per standard deviation (SD) increase in the FI-LAB value significantly increased the HR of death by 2.0% (HR:1.02, 95% CI: 1.01–1.03) and 23.0% (HR: 1.23, 95% CI: 1.13–1.34), respectively.
Conclusions: Frailty assessed by routine laboratory data indicates increased risks of chemotherapy adverse reactions and death in older patients with primary lung cancer receiving the first course of chemotherapy.
Keywords: frailty, prognosis, older patients, lung cancer, chemotherapy
Introduction
Rapid population ageing is closely linked with an increased incidence of age-related chronic diseases, leading to a large social and economic burden. Accordingly, the majority of the chronic disease burden is attributable to health problems amongst adults aged ≥60 years.1 Among these chronic diseases, cancer is a leading contributor.1 Lung cancer is the most commonly diagnosed cancer and the primary cause of cancer-related death among elders.2Lung cancer is generally diagnosed at an advanced stage3 so that chemotherapy is often one of the main interventions for primary lung cancer.4 However, chemotherapy often causes severe adverse reactions among lung cancer patients. Even though pharmacovigilance studies are significant tools to oversee these adverse reactions,5 physicians frequently express concerns regarding the benefits of chemotherapy for older patients, as advanced age with co-morbidities significantly increases chemotherapy intolerance and mortality risks.6 Thus, exploring the risk factors affecting the benefits of chemotherapy is necessary and could help physicians facilitate lung cancer management.
Frailty is a common geriatric syndrome that manifests as an impaired homeostasis in which minor stressor events trigger disproportionate harm to individuals. This syndrome has recently been widely introduced into oncology.7,8 Some studies have highlighted the significant associations between frailty and tumor progression and tumor-related death in elders.9 Harvey et al indicated the potential predictive value of frailty on prognosis. They suggest that frailty assessed by comprehensive geriatric assessments (CGA) could strongly contribute to the adverse outcomes after chemotherapy among older patients with cancer.10 The role of the Frailty index based on CGA or self-reported data in predicting adverse outcomes was validated among old people in the community, in acute care settings and in long-term care institutions.11–13 However, to construct a frailty index based on CGA is time-consuming. Howlett and colleagues constructed a frailty index based on laboratory variables (FI-LAB) and found their FI-LAB to be closely associated with frailty indexes based on CGA data and to be able to identify older people with an increased mortality risk in the Canadian Study of Health and Aging (CSHA) cohort.14 These results have since been replicated by other studies.15–18
The role of the FI-LAB in predicting mortality and chemotherapy adverse reactions in older patients with primary lung cancer has not been reported until now. Furthermore, considering that Harvey et al’ s study was limited because of the complex and time-consuming process of CGA and the heterogeneity of cancer types, we constructed a FI-LAB, which can be routinely collected during clinical practice according to previous studies.14,19 We conducted this study to explore the role of the FI-LAB in predicting the risks of chemotherapy adverse reactions and all-cause mortality in older patients with primary lung cancer.
Methods
Study design and participants
This study was designed as a single-institution retrospective study. We consecutively recruited patients aged ≥60 years with primary lung cancer pathologically proven to be adenocarcinoma, squamous carcinoma, small cell carcinoma and others who received a first course of chemotherapy in West China Hospital, Sichuan University between January 2010 and December 2017. We excluded patients missing medical records and routine laboratory variables (here, routine blood tests, blood biochemistry and coagulation tests). Baseline characteristics and health-related variables were obtained from the anonymous electronic medical record system by two independent study investigators (YW and RZ).
Ethics
The study was a retrospective study using the medical record. The Health Informatics Center anonymize all related data and oversee the study protocol. In the process of the study, researchers covering all data confidentiality and compliance with the Declaration of Helsinki. Thus, patient consent to review their medical records was not required in this retrospective study. The Research Ethics Committee of West China Hospital, Sichuan University approved this study (No.2018–94).
Frailty assessment
The FI-LAB was constructed to assess a patient’s frailty status at baseline according to a standard procedure.14,19 In this study, 44 variables from three types of tests-routine blood tests, blood biochemistry and coagulation tests-were used to construct the FI-LAB (Table S1). All 44 variables could be measured from a fasting blood sample taken prior to the first course of chemotherapy. Each item was coded “1” if the value exceeded the normal range or cut-offs (deficits) and “0” if within range (Table S1). For each patient, the FI-LAB was calculated as the sum of all presented deficits (number of variables coded as “1”) divided by the sum of the considered deficits. Thus, the FI-LAB was a continuous score ranging between 0 and 1 for each individual, theoretically. The cut-off points of the FI-LAB were robust/non-frail (<0.2), pre-frail (0.2–0.35) and frail (≥0.35) according to a previous study.10
Short-term chemotherapy-related outcomes: adverse reactions
Chemotherapy adverse reactions were reviewed following chemotherapy from the medical record, including digestive reactions (nausea or vomiting or diarrhea), infections at any sites (bacteria or fungus infection) during the chemotherapy and other adverse reactions (non-infection fever or rash). Adverse reactions related to chemotherapy were assessed by the attending physicians. Since the FI-LAB includes blood and hepatic indicators, we did not consider hematologic toxicities and hepatic dysfunction as short-term outcomes.
All-cause mortality
First, we obtained the death information from local government death databases. For patients we did not have the access to the information from the database, we conducted telephone interviews. The death information including the status of survival and the time of death until April 1st, 2018. The average follow-up period is 3.9 years. Overall survival (OS) was defined as the time from the treatment start date until the date of death due to any cause, or the last date the patient was known to be alive.
Covariates
We retrospectively collected the following baseline data prior to chemotherapy: age, sex, marital status, occupation, health insurance, body mass index (BMI), smoking history, drinking history and tumor-related characteristics, including histology, clinical stage, metastasis, regimen of chemotherapy, radiotherapy and lung cancer surgery. The following smoking history was reviewed: smoking status, such as current smoker, former smoker or non-smoker, and number of pack-year of cigarettes smoked.
Statistical analysis
The results were statistically analyzed using SPSS 19.0 (IBM Corp., Somers, NY). All statistical tests were two-sided with an acceptable level of significance deemed as p<0.05. Variables were reported as numbers (percentage) or means ± standard deviation according to types of data. Significance testing of the differences between groups was carried out using Kruskal-Wallis test, Pearson’s chi-square analysis, Fisher’s exact test or one-way analysis of variance as appropriate. Binary logistic regression was used to explore the associations between frailty and short-term outcomes. Three models were employed to calculate odds ratio and 95% confidence intervals: first, a non-adjusted model; second, a model adjusting demographic covariates (age, sex, occupation, health insurance, BMI, pack-year of cigarettes smoked, drinking history); third, a model further adjusting number of chronic diseases (except the primary lung cancer) and tumor-related covariates (histology, clinical stage, metastasis, regimen of chemotherapy, radiotherapy and lung cancer surgery). Survival curves were plotted using the Kaplan–Meier method, and significant differences were tested using a log-rank test. The hazard ratio of frailty on all-cause mortality was assessed by Cox proportional hazards models. The following confounding factors were adjusted in the multivariate Cox regression models: age, sex, occupation, health insurance, BMI, pack-year of cigarettes smoked, drinking history, number of chronic disease (except the primary lung cancer) and tumor-related covariates (histology, clinical stage, metastasis, regimen of chemotherapy, radiotherapy and lung cancer surgery).
Results
Prevalence of frailty among older patients with lung cancer
Of the 1,263 patients enrolled, 243 were excluded from the study, of which 78 and 165 patients lacked necessary laboratory variables or medical records respectively (Figure 1). In this study, we included 1,020 patients (71.4% male; median age: 65 years old) with primary lung cancer who received a first course of chemotherapy. Of the 1,020 eligible participants, 50 (4.9%) were categorized as frail, 269 (26.4%) as pre-frail and 701 (68.7%) as robust. The maximum, minimum, and median FI scores of the participants were 0.61, 0, and 0.14, respectively. The 99th percentile of the FI was 0.45.
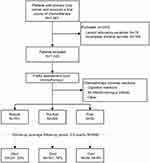 |
Figure 1 Study profile including patients’ selection and mortality information. |
Characteristics of the patients
The baseline characteristics are shown in Table 1. Frail patients were older than pre-frail or robust patients (median age 67, 65, 64, respectively, p=0.136). Robust patients had a lower median pack-year of cigarettes smoked than pre-frail and frail groups (15 vs 24 vs 20, p=0.013). Other sociodemographic factors did not differ significantly within groups, including gender, marriage, occupation, health insurance, smoking status, drinking history and BMI. The patients in the three groups suffered similar number of chronic diseases (p>0.05). More patients received lung cancer surgery in robust group (80.4%) than pre-frail (18.3%) or frail group (1.3%) (p<0.05). The most common histological subtype was adenocarcinoma (70.6%), followed by squamous cell carcinoma (15.4%) and small cell carcinoma (14.0%). Compared to robust patients, pre-frail and frail patients were more likely to suffer from metastasis and to be diagnosed at an advanced stage (p<0.05). Most patients were treated with multiple chemotherapy drugs, however, prefrail and frail patients had a higher rate of receiving single-drug treatments and radiotherapy (p<0.05).
 |
Table 1 Characteristics of the study population according to frailty assessed by FI-LAB |
Frailty and short-term chemotherapy
We observed a higher rate of chemotherapy-related adverse reactions among frail and prefrail patients compared to robust patients (30.0% vs 19.7% vs 11.8%, p=0.001). More patients suffered infection in the frail group (18.0%) than the patients in prefrail (12.3%) and robust (5.7%) groups (p<0.001). We did not find significant difference within groups for digestive reactions (p=0.935). Table 2 shows the prevalence of the participants according to their frailty status.
 |
Table 2 The differences of short-term chemotherapy adverse reactions according to baseline frailty status |
Binary logistic regression was performed to assess the associations between frailty and risk of short-term adverse outcomes (Table 3). Both the prefrail and frail patients had higher risks of any adverse reactions (OR=1.86, 95%CI: 1.25–2.77, p=0.002; OR=3.48, 95%CI: 1.77–6.87, p<0.001, respectively) and all infections (OR=2.24, 95%CI: 1.35–3.74, p=0.002; OR=3.58, 95%CI: 1.55–8.26, p=0.003, respectively) even after adjusting for multiple covariates. At the same time, frail patients were more likely to suffer other adverse reactions (non-infection fever or rash) (OR=5.65, 95%CI: 1.52–20.93, p=0.010) following adjustments (Table 3).
 |
Table 3 The associations between frailty and risk of short-term chemo adverse reactions |
Mortality and survival after chemotherapy according to the presence of frailty
Amongst the 1,020 patients, 94 patients (9.2%) were lost to follow-up due to non-response. Therefore, 926 patients were used to assess the association between frailty and all-cause mortality. A total of 563 patients died, of whom 337 (53.0%) were in the robust group, 187 (76.0%) in the pre-frail group and 39 (88.6%) in the frail group (p<0.001). The median OS of frail (18.05 months, 95% CI: 16.89 −19.21) and prefrail (26.93 months, 95% CI: 24.11 −29.75) patients was significantly shorter than robust patients (38.89 months, 95%CI: 34.64–43.15). Five-year OS rates were also lower among frail and prefrail groups compared to the robust group (10.9%, 14.5% and 35.6%, respectively). Figure 2 shows the survival curves of the study population according to their pre-chemotherapy frailty status and the curves differ significantly among the three groups (log-rank p<0.001).
Frailty was independently associated with overall survival with an average follow-up of 3.9 years (Table 4). The risk of all-cause mortality was dramatically increased in frail patients (HR=3.00, 95% CI=2.15–4.18, p<0.001) and prefrail patients (HR =1.66,95% CI=1.39–1.98, p<0.001) compared to robust patients. The prognostic role of frailty and pre-frailty for OS were still significant after adjusting for some potential confounding variables (HR=2.13, 95% CI=1.51–3.00, p<0.001; HR=1.30, 95% CI=1.08–1.57, p=0.006; respectively, Table 3). Moreover, higher FI-LAB values were also associated with a higher risk of death. Each 0.01 or per standard deviation (SD) increase in the FI-LAB value significantly increased the HR of death by 2.0% (HR: 1.02; 95% CI: 1.01–1.03, p<0.001) and 23.0% (HR: 1.23; 95% CI: 1.13–1.34, p<0.001), respectively (Table 4).
 |
Table 4 The prognostic role of frailty in predicting all-cause mortality |
Discussion
In this study, we included 1,020 consecutive participants aged ≥60 years with primary lung cancer receiving chemotherapy, and constructed a FI-LAB based on 44 laboratory variables to assess frailty status prior to chemotherapy. To the best of our knowledge, this is the first study to explore a frailty index only based on laboratory data containing the prognostic value to predict short-term chemotherapy adverse reactions as well as all-cause mortality in older patients with primary lung cancer. To some extent, it is reasonable to regard FI-LAB as a risk indicator of poor outcomes after chemotherapy. Simply including frailty assessment in the risk assessment before chemotherapy may help to facilitate appropriate treatment planning for older patients with lung cancer.20–22
FI-LAB quantifies frailty based on routine laboratory data, which meets the criteria of ideal the frailty screening tool in clinical practice.15,16,19 In this regard, assessing frailty using FI-LAB before chemotherapy is intriguing and convenient in routine practice for older patients with primary lung cancer. In previous studies, FI-LAB also has been demonstrated to display comparable outputs to previously validated FI assessments based on the CGA among community-dwelling old people.15,17,23 The present study extends these conclusions to lung cancer patients and employs a wide range of variables in routine blood tests, blood biochemistry and coagulation tests.
Large heterogeneity always occurs within older patients with cancer due to different onsets of the aging process and the complexity of co-morbidities.24,25 Therefore, for older patients, a carefully tailored assessment that should be adapted to life expectancy and risk of toxicity is required in cancer treatment.24,26 One such tool could be the frailty assessment since frailty can reflect a loss of functional reserve of multiple organ systems of elders.7 According to previous studies, frailty can be used to assess the risk of postoperative complications and survival in cancer patients.27,28 Furthermore, frailty is strongly associated with the progression of tumors and has the properties to predict poor treatment tolerance.10,25 Consistently, our study revealed that frailty can be used to identify the risk of chemotherapy-related intolerance and mortality in older patients with primary lung cancer. Concerning the prognostic significance of frailty, the frailty assessment could be a clinically applicable method of risk assessment for cancer treatment, which could help physicians to facilitate the optimization of cancer care.
There are some potential mechanisms for the frailty assessed by FI-LAB which may be useful in predicting chemotherapy adverse reactions and mortality in our study. First, a higher score in FI-LAB usually represents the coexistence of more uncontrolled diseases. We adjusted the number of chronic diseases in the multi-variable analysis. Second, FI-LAB was objective and more sensitive than the frailty index based on CGA in predicting mortality.14–16 Third, abnormal routine blood count parameters, such as platelet distribution width and fluctuations in routine blood count, were useful in predicting adverse events among cancer patients.29,30 Our study summarizes the abnormal variables in the FI-LAB, which was proven to be a useful way to predict the prognosis of old patients with lung cancer.
This study has some limitations. The study was performed at a single institution with a limited sample size and is a retrospective study with inevitable selection bias. The economic conditions in West China, where more frail patients gave up treatment in hospital, may lead to the underestimation of the prevalence of frailty in this cohort. In addition, only Chinese ethnicities were included meaning that conclusions cannot be generalized to other races or countries. We did not include chemotherapy-associated hematologic toxicities as adverse outcomes, like myelosuppression, because FI-LAB has included hematologic indicators. However, during our data analysis, we found that frailty assessed by FI-LAB was also associated with a higher risk of myelosuppression (data not shown). Finally, the choice of the FI-LAB cutoff used to define frailty refers to the study from Harvey et al,10 in which the frailty index is constructed based on comprehensive geriatric assessments. Nonetheless, the cutoffs for the frailty index are controversial now10,31,32 and regardless of the FI-LAB cutoffs, the risk of mortality also increased by per 0.01 or per SD in FI-LAB, demonstrating its ability to predict long-term mortality.
Conclusions
Frailty assessed by routine laboratory data indicates an increase risks of chemotherapy adverse reactions and death among older patients with primary lung cancer receiving their first course of chemotherapy. It is clinically feasible and significant to construct FI-LAB to assess frailty during risk assessments prior to chemotherapy in older patients with lung cancer.
Acknowledgments
The authors thank Kathleen Steeves at McMaster university for the English revision of our manuscript and appreciate all participants and legal proxies for their great contributions. This work was supported by the National Natural Science Foundation of China (No. 81601220), the Project of Science and Technology Bureau of Sichuan Province (2017FZ0051), the Science Foundation for Young Researchers of Sichuan University (2017SCU11044) and the Project of Health and family planning commission of Sichuan Province (16PJ328). The sponsors played no role in the design, methods, data collection, analysis and preparation of this paper.
Author contributions
All authors contributed to data analysis, drafting and revising the article, gave final approval of the version to be published, and agree to be accountable for all aspects of the work.
Disclosure
The authors report no conflicts of interest in this work.
References
1. Prince MJ, Wu F, Guo Y, et al. The burden of disease in older people and implications for health policy and practice. Lancet (London, England). 2015;385(9967):549–562. doi:10.1016/s0140-6736(14)61347-7
2. Chen W, Zheng R, Baade PD, et al. Cancer statistics in China, 2015. CA Cancer J Clin. 2016;66(2):115–132. doi:10.3322/caac.21338
3. Walters S, Maringe C, Coleman MP, et al. Lung cancer survival and stage at diagnosis in Australia, Canada, Denmark, Norway, Sweden and the UK: a population-based study, 2004–2007. Thorax. 2013;68(6):551–564. doi:10.1136/thoraxjnl-2012-202297
4. Eberhardt WE, De Ruysscher D, Weder W, et al. 2nd ESMO consensus conference in lung cancer: locally advanced stage III non-small-cell lung cancer. Ann Oncol. 2015;26(8):1573–1588. doi:10.1093/annonc/mdv187
5. Shrestha S, Shrestha S, Khanal S. Establishment of the first cancer hospital-based pharmacovigilance center in Nepal. Res Social Adm Pharm. 2018;14(11):1088–1089. doi:10.1016/j.sapharm.2018.07.017s
6. Petrelli F, Barni S. Non-cancer-related mortality after cisplatin-based adjuvant chemotherapy for non-small cell lung cancer: a study-level meta-analysis of 16 randomized trials. Med Oncol. 2013;30(3):641. doi:10.1007/s12032-013-0641-5
7. Clegg A, Young J, Iliffe S, Rikkert MO, Rockwood K. Frailty in elderly people. The Lancet. 2013;381(9868):752–762. doi:10.1016/s0140-6736(12)62167-9
8. Ethun CG, Bilen MA, Jani AB, et al. Frailty and cancer: implications for oncology surgery, medical oncology, and radiation oncology. CA Cancer J Clin. 2017;67(5):362–377. doi:10.3322/caac.21406
9. Handforth C, Clegg A, Young C, et al. The prevalence and outcomes of frailty in older cancer patients: a systematic review. Ann Oncol. 2015;26(6):1091–1101. doi:10.1093/annonc/mdu540
10. Cohen HJ, Smith D, Sun CL, et al. Frailty as determined by a comprehensive geriatric assessment-derived deficit-accumulation index in older patients with cancer who receive chemotherapy. Cancer. 2016;122(24):3865–3872. doi:10.1002/cncr.30269
11. Song X, Mitnitski A, Rockwood K. Prevalence and 10-year outcomes of frailty in older adults in relation to deficit accumulation. J Am Geriatr Soc. 2010;58(4):681–687. doi:10.1111/j.1532-5415.2010.02764.x
12. Theou O, Sluggett JK, Bell JS, et al. Frailty, hospitalization, and mortality in residential aged care. J Gerontology Series A, Biol Sci Med Sci. 2018;73(8):1090–1096. doi:10.1093/gerona/glx185
13. Theou O, Campbell S, Malone ML, Rockwood K. Older adults in the emergency department with frailty. Clin Geriatr Med. 2018;34(3):369–386. doi:10.1016/j.cger.2018.04.003
14. Howlett SE, Rockwood MR, Mitnitski A, et al. Standard laboratory tests to identify older adults at increased risk of death. BMC Med. 2014;12:171. doi:10.1186/s12916-014-0171-9
15. Blodgett JM, Theou O, Howlett SE, Rockwood K. A frailty index from common clinical and laboratory tests predicts increased risk of death across the life course. GeroScience. 2017. doi:10.1007/s11357-017-9993-7
16. Rockwood K, McMillan M, Mitnitski A, Howlett SE. A frailty index based on common laboratory tests in comparison with a clinical frailty index for older adults in long-term care facilities. J Am Med Dir Assoc. 2015;16(10):842–847. doi:10.1016/j.jamda.2015.03.027
17. Ritt M, Jager J, Ritt JI, Sieber CC, Gaßmann K-G. Operationalizing a frailty index using routine blood and urine tests. Clin Interv Aging. 2017;12:1029–1040. doi:10.2147/cia.s131987
18. Blodgett JM, Theou O, Howlett SE, Wu FCW, Rockwood K. A frailty index based on laboratory deficits in community-dwelling men predicted their risk of adverse health outcomes. Age Ageing. 2016;45(4):463–468. doi:10.1093/ageing/afw054
19. Searle SD, Mitnitski A, Gahbauer EA, Gill TM, Rockwood K. A standard procedure for creating a frailty index. BMC Geriatr. 2008;8(24). doi:10.1186/1471-2318-8-24
20. Franco I, Chen YH, Chipidza F, et al. Use of frailty to predict survival in elderly patients with early stage non-small-cell lung cancer treated with stereotactic body radiation therapy. J Geriatr Oncol. 2018;9(2):130–137. doi:10.1016/j.jgo.2017.09.002
21. Portal D, Hofstetter L, Eshed I, et al. L3 skeletal muscle index (L3SMI) is a surrogate marker of sarcopenia and frailty in non-small cell lung cancer patients. Cancer Manag Res. 2019;11(2579–2588). doi:10.2147/cmar.S195869
22. Raghavan G, Shaverdian N, Chan S, Chu F-I, Lee P. Comparing outcomes of patients with early-stage non-small-cell lung cancer treated with stereotactic body radiotherapy based on frailty status. Clin Lung Cancer. 2018;19(5):e759–e66. doi:10.1016/j.cllc.2018.05.008
23. Klausen HH, Petersen J, Bandholm T, et al. Association between routine laboratory tests and long-term mortality among acutely admitted older medical patients: a cohort study. BMC Geriatr. 2017;17(1):62. doi:10.1186/s12877-017-0434-3
24. Baijal P, Periyakoil V. Understanding frailty in cancer patients. Cancer J. 2014;20(5):358–366. doi:10.1097/ppo.0000000000000068
25. Clough-Gorr KM, Stuck AE, Thwin SS, Silliman RA. Older breast cancer survivors: geriatric assessment domains are associated with poor tolerance of treatment adverse effects and predict mortality over 7 years of follow-up. J Clin Oncol. 2010;28(3):380–386. doi:10.1200/jco.2009.23.5440
26. Pallis AG, Gridelli C, Wedding U, et al. Management of elderly patients with NSCLC; updated expert’s opinion paper: EORTC elderly task force, lung cancer group and international society for geriatric oncology. Ann Oncol. 2014;25(7):1270–1283. doi:10.1093/annonc/mdu022
27. Ommundsen N, Wyller TB, Nesbakken A, et al. Frailty is an independent predictor of survival in older patients with colorectal cancer. Oncologist. 2014;19(12):1268–1275. doi:10.1634/theoncologist.2014-0237
28. Tegels JJ, de Maat MF, Hulsewe KW, Hoofwijk AGM, Stoot JHMB. Value of geriatric frailty and nutritional status assessment in predicting postoperative mortality in gastric cancer surgery. J Gastrointestinal Surg. 2014:18(3):439–445. discussion 45-6. doi:10.1007/s11605-013-2443-7.
29. Fujisawa Y, Yoshino K, Otsuka A, et al. Fluctuations in routine blood count might signal severe immune-related adverse events in melanoma patients treated with nivolumab. J Dermatol Sci. 2017;88(2):225–231. doi:10.1016/j.jdermsci.2017.07.007
30. Cui MM, Li N, Liu X, et al. Platelet distribution width correlates with prognosis of non-small cell lung cancer. Sci Rep. 2017;7(1):3456. doi:10.1038/s41598-017-03772-z
31. Darvall JN, Gregorevic KJ, Story DA, Hubbard RE, Lim WK. Frailty indexes in perioperative and critical care: A systematic review. Arch Gerontol Geriatr. 2018;79:88–96. doi:10.1016/j.archger.2018.08.006
32. Burn R, Hubbard RE, Scrase RJ, et al. A frailty index derived from a standardized comprehensive geriatric assessment predicts mortality and aged residential care admission. BMC Geriatr. 2018;18(1):319. doi:10.1186/s12877-018-1016-8
Supplementary material
 |
Table S1 Laboratory variables for frailty index |
 © 2019 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2019 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.

