Back to Journals » Drug Design, Development and Therapy » Volume 10
Paracetamol sharpens reflection and spatial memory: a double-blind randomized controlled study in healthy volunteers
Authors Pickering G, Macian N, Dubray C, Pereira B
Received 28 April 2016
Accepted for publication 20 June 2016
Published 5 December 2016 Volume 2016:10 Pages 3969—3976
DOI https://doi.org/10.2147/DDDT.S111590
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 4
Editor who approved publication: Prof. Dr. Wei Duan
Gisèle Pickering,1–3 Nicolas Macian,1,2 Claude Dubray,1–3 Bruno Pereira4
1University Hospital, CHU Clermont-Ferrand, Centre de Pharmacologie Clinique, 2Inserm, CIC 1405, UMR Neurodol 1107, 3Clermont Université, Laboratoire de Pharmacologie, Faculté de médecine, 4CHU de Clermont-Ferrand, Délégation Recherche Clinique Innovation, Clermont-Ferrand, France
Background: Acetaminophen (APAP, paracetamol) mechanism for analgesic and antipyretic outcomes has been largely addressed, but APAP action on cognitive function has not been studied in humans. Animal studies have suggested an improved cognitive performance but the link with analgesic and antipyretic modes of action is incomplete. This study aims at exploring cognitive tests in healthy volunteers in the context of antinociception and temperature regulation. A double-blind randomized controlled study (NCT01390467) was carried out from May 30, 2011 to July 12, 2011.
Methods: Forty healthy volunteers were included and analyzed. Nociceptive thresholds, core temperature (body temperature), and a battery of cognitive tests were recorded before and after oral APAP (2 g) or placebo: Information sampling task for predecisional processing, Stockings of Cambridge for spatial memory, reaction time, delayed matching of sample, and pattern recognition memory tests. Analysis of variance for repeated measures adapted to crossover design was performed and a two-tailed type I error was fixed at 5%.
Results: APAP improved information sampling task (diminution of the number of errors, latency to open boxes, and increased number of opened boxes; all P<0.05). Spatial planning and working memory initial thinking time were decreased (P=0.04). All other tests were not modified by APAP. APAP had an antinociceptive effect (P<0.01) and body temperature did not change.
Conclusion: This study shows for the first time that APAP sharpens decision making and planning strategy in healthy volunteers and that cognitive performance and antinociception are independent of APAP effect on thermogenesis. We suggest that cognitive performance mirrors the analgesic rather than thermic cascade of events, with possibly a central role for serotonergic and cannabinoid systems that need to be explored further in the context of pain and cognition.
Keywords: paracetamol, cognition, spatial memory, decision making, analgesia, therapeutic dose
Introduction
Acetaminophen (APAP, paracetamol) is the most widely used drug for its analgesic and antipyretic properties. APAP has been suggested to be a prodrug with analgesic APAP metabolites and a central mechanism of action that involves a large number of neurotransmitters and serotonergic, opioidergic, vanilloid, and cannabinoid receptors.1–10 A small percentage of APAP is transformed in the liver via deacetylases to p-aminophenol, which is itself metabolized in the brain by fatty acid amide hydrolase to N-(4-hydroxyphenyl)-5Z, 8Z, 11Z, 14Z-eicosatetraenamide (AM404). AM404 has been shown to have analgesic properties and is a potent activator of transient receptor potential vanilloid 1 receptor (TRPV1), which is an essential factor for APAP analgesic effect.7 Subsequent action on cannabinoid receptors type 1 (CB1) and calcium channels, such as Ca(v) 3.2 modulate descending serotonergic pathways, and a number of other factors, including transient receptor potential cation channel member A1 receptors, have been described.9,11 Hypothermic/antipyretic effects have been largely studied and it appears in animals that APAP action on temperature is independent of the cascade of events leading to analgesia.12 Cognitive-affective changes with APAP have been studied in animals and an improved cognitive performance,13 an anxiolytic effect14 involving cannabinoid mechanism, and a potentiation of antidepressant and anticompulsive effect have been described.15 However, exposure to and presence of APAP during a critical period of brain development can induce long-lasting effects on cognitive function and alter the adult response to paracetamol in mice.16 Cognitive function with APAP intake has, however, not been studied so far in humans. In a previous study (NTC01053650), we showed that APAP had a positive effect on spatial memory and decision making but this study had no placebo group and results might have been linked to the placebo effect of APAP. We present here a double-blind randomized controlled study to evaluate the impact of APAP on a battery of cognitive tests in healthy volunteers.
Materials and methods
Study
This randomized, double-blind, crossover, controlled trial in healthy volunteers took place in the Clinical Investigation Centre/Clinical Pharmacology Centre, University Hospital of Clermont-Ferrand, France, from May 30, 2011 to July 12, 2011. The study was reviewed and approved by the French Institutional Review Board and the French Drug Agency. It followed standardized ethical and safety Good Clinical Practice Guidelines and procedures were in accordance with the Declaration of Helsinki. It was registered on ClinialTrials.gov (NCT01390467).
Volunteers
Participants were recruited through the database of the Clinical Pharmacology Centre of the University Hospital of Clermont-Ferrand, France. Male volunteers (to avoid pain threshold variability due to menstrual cycle in females) were eligible if they were ≥18 years old, did not take analgesic or anti-inflammatory treatment in the last 7 days, and were nonsmokers. Exclusion criteria included: a known hypersensitivity to APAP, concomitant pathologies, contraindications to APAP, any abuse consumption of alcohol, addiction to drugs, or use of drugs of abuse. Eligible volunteers were informed about the protocol and provided a signed informed consent.
Study design
The trial consisted of two randomized sessions 1 week apart, with APAP or placebo according to the randomization list established beforehand by a research assistant who was not involved in the trial. On the day of the experiment, at baseline (T0), volunteers in each of the treatment periods, after clinical examination, had body temperature recording, cognitive tests, and pain tests. Subjects were then randomized in APAP or placebo groups. Double-blinding was fully respected with the volunteers and members of staff. A research nurse who was only involved in drug allocation was in charge of drug administration. Volunteers were administered APAP 2 g or placebo. Nociceptive tests, cognitive assessment, and body temperature were repeated at T0 +2 hours, as 120 minutes is the point for maximum APAP pharmacodynamic effect.1,2 A single oral dose of 2 g APAP was chosen considering that with such a dose on the high side compared to typical analgesic doses (1 g), but not toxic, any cognitive impairment or cognitive improvement could be revealed and statistically significant, but an effect-dose of APAP should be done in future studies.13,17
Body temperature
It was measured using the Braun ThermoScan tympanic thermometer (EC, Pro 4000 type 6021; Braun, Kronberg, Germany) with a disposable speculum applied in the auditory canal according to the manufacturer’s instructions.
Cognitive tests
A program of tests had been set up on the Cambridge Neuropsychological Test Automated Battery (Cantab®, Cambridge UK) and included spatial memory, decision making, reaction time (RTI), pattern recognition memory, and delayed matching to sample. The subject taking these tests interacts with the computer system by touching the touch screen under the supervision of a clinical research assistant. The choice of tests resulted from a previous trial experience (NTC01053650).
Choice RTI is an attention test that measures the speed of response and movement in five-choice paradigm. The outcome measures are 1) the total five-choice RTI (in milliseconds, ms), that is, the speed with which the subject releases the press pad button in response to a stimulus in any one of five locations and 2) the movement time latency (RTM), that is, the time taken to touch the stimulus after the press pad button has been released.
Spatial planning and working memory are evaluated with the Stockings of Cambridge (SOC) test. The subject is shown two displays containing colored balls and must use the balls in the lower display to copy the pattern shown in the upper display. Several outcome measures for the SOC test are chosen: 1) the mean initial thinking time (ms) for five moves gives an indication of the time taken to plan the problem solution, 2) the mean subsequent thinking time (ms) for five moves reflects the subject’s speed of movement after the initial move has been made for five-move problems, and 3) the number of problems solved, that is, the number of occasions upon which a subject has successfully completed a test problem in the minimum possible number of moves; higher is better.
Information sampling task (IST) tests impulsivity and decision making. IST is a task designed to measure predecisional processing in which the subject gathers and evaluates information prior to making a decision. Inadequate reflection means that a decision will be made on the basis of less evidence, and, therefore will reduce the accuracy of the eventual decision. The subject is instructed that he/she is playing a game for points, which he/she can win by making a correct decision about which color is mainly displayed under the gray boxes on the screen. The outcome measures are: 1) the number of discrimination errors, that is, the number of trials in which the subject chose a color and made a decision which was not logically based on evidence available to the subject at the time; 2) sampling errors, that is, the number of trials in which the subject chose a color that was not in the overall majority but was in the majority at the point of decision for the specific win condition; 3) mean box opening latency, that is, the time elapsed between the subject opening a box and then opening a subsequent box; 4) mean color decision latency, that is, the time elapsed between the start of the trial and the point at which the subject selects the color he or she believes to be in the overall majority; and 5) mean number of boxes opened per trial. Lower is better for the four first outcomes and higher for the last.
Pattern recognition memory is a test of visual pattern recognition memory in a two-choice forced discrimination paradigm. The outcomes measures were 1) number and percent incorrect responses and 2) latency. It is a good indicator of overall performance on a test of visual short-term recognition memory. It is mostly useful in cognitive impairment. This test was chosen in the context of APAP effect on central structures in order to explore a potential improvement of recognition processes with APAP.18
Delayed matching to sample is a test sensitive to damage in the medial temporal lobe area with some input from the frontal lobes. This test was chosen in the context of APAP effect on central structures looking for potential improvement of matching.18 The chosen outcome was the total number of trials for which the subject selected the correct stimulus on his or her first response when the target stimulus and the three distractors were presented after the stimulus had been hidden with delays of 0, 4,000, and 12,000 ms. A higher score is better.
All the tests were evaluated at baseline (T0) and 2 hours later (T2h). The battery of tests took 40–50 minutes to be completed. The tests were always presented in the same order (RTI, SOC, IST, pattern recognition memory, delayed matching to sample) by the same person. The difference between T2h and T0 (T2h–T0) gave the impact of APAP or placebo. Differences were compared.
Pain tests consisted of evoked mechanical pain stimulation. The electronic von Frey test (Bioseb, Chaville, France) involved applying pressure via the tip of a plastic cone on the dorsal–medial side of the dominant arm at an even rate using a pressure instrument. The von Frey test was determined when the volunteer started to feel pain in response to increasing intensity of the stimulus and pressed the off button; the pressure pain threshold value was registered on a digital screen. Areas under the curve (0–120 minutes) of these pain scores were calculated for both treatments and compared.
Statistical analysis
The sample size estimation for this study was done from the results of a previous APAP trial in healthy volunteers (ClinicalTrials.gov, NTC01053650) with the same methodology and where the Cantab psychometric test was used for cognitive assessment, but this previous study had no placebo group. Estimations were based on SOC test with 90% statistical power, two-sided type I error a=0.05, expected difference 0.8 (paired difference to be detected) for a standard deviation of 1.68 (expected standard deviation of difference), and correlation coefficient due to crossover design r=0. N=40 subjects were analyzed in an intention to treat analysis. Statistical analysis was performed using Stata 13 software (StataCorp LP, College Station, TX, USA). The tests were two-sided, with a type I error set at a=0.05. Subject’s characteristics were presented as mean (±standard deviation) or median (interquartile range), according to statistical distribution (assumption of normality assessed using the Shapiro–Wilk test), for continuous data and as the number of patients and associated percentages for categorical parameters. Considering the design of this study as crossover, statistical analysis was performed using mixed models with unstructured variance–covariance structure. These models used the delta values (ie, value at the end of the intervention period minus value at baseline) as the dependent variables and included period, treatment, sequence, and possible carryover as fixed effects. In these models, we always considered random subject effects (random intercept and slope). The residual normality was checked for all models presented in this article as well as the normality of dependent variables. When appropriate, a log-transformation was proposed to achieve normality.
Results
A total of 40 healthy volunteers (23±2 years old) were included and analyzed (Figure 1) in this double-blind randomized study.
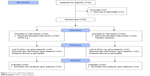  | Figure 1 Consort 2010 flow diagram. |
Temperature did not decrease significantly 2 hours after APAP intake. Temperature was 36.23°C±0.33°C (T0), 36.24°C±0.36°C (T2), and 36.27°C±0.41°C (T0), 36.29°C±0.33°C (T2), respectively, for APAP and for placebo.
Cognitive tests are presented in Table 1 and Figure 2A and B. RTI (P=0.7), RTM (P=0.5), pattern recognition memory (P=0.2), and delayed matching to sample (P=0.2) tests were not statistically modified by APAP at 2 hours compared to baseline. IST was significantly improved (diminution of the number of errors, P=0.05; diminution of the latency to open boxes, P=0.04; and increased number of opened boxes, P=0.03 with APAP) (Figure 2B). Spatial planning and working memory initial thinking time were decreased (P=0.04) with APAP, with no significant decrease of subsequent thinking time but with a tendency for the number of problems solved (P=0.07) (Figure 2A).
  | Table 1 Comparison of cognitive tests differences with paracetamol (APAP) and placebo between baseline T0 and T2h (mean [SEM]) |
Pain tests showed an antinociceptive effect of APAP versus placebo: area under the curve (0–120 minutes) APAP =222±482 kPa × min and area under the curve (0–120 minutes) placebo =23±431 kPa × min (P=0.0047).
Discussion
This study demonstrates for the first time that APAP induces cognitive function improvement and antinociception independently of an effect on thermogenesis, as APAP induces no hypothermia in healthy volunteers. The findings confirm the results of preclinical studies on the effect of APAP on cognitive performance showing that APAP in nontoxic dosage may improve cognitive performance13 and has an effect on the central nervous system.14,15 The findings also confirm unpublished works of our group showing improved cognition with 2 g APAP (NTC01053650). This present study also underlines the dichotomy of APAP antinociception and antipyresis12 and shows that APAP did not induce hypothermia in healthy volunteers at the dose tested.
In this study, APAP improves significantly two domains of cognitive function, decision making and spatial memory. A number of brain regions and cerebral interconnections have been described to be involved in predecisional processing and planning actions: parietal-frontal circuits, anterior cingulate and orbitofrontal cortices, and hippocampus.19,20 These cerebral areas and others are also activated during the pain experience; they belong to the pain matrix and have been shown to be deactivated by APAP as described by a recent functional magnetic resonance imaging study.18,21 Evidence from pharmacological experiments in humans has underlined the complex roles of dopamine and serotonin in decision making.22 Serotonin is a key neurotransmitter for APAP central analgesic mechanism of action: an elegant cascade of events has been described with APAP being metabolized from p-aminophenol to AM404 with an effect on TRPV1 receptors, on the endocannabinoid system, and with stimulation of serotonergic pain inhibitory descending pathways.1–10,23 This reinforcement of action of the descending inhibitory pathways by APAP and the necessary presence of serotonin for APAP analgesic effect have been demonstrated in preclinical and clinical studies.1,2,24,25 Serotonin is associated with decision making; it does influence value representations within the subcortical and striatal brain areas and modulates ubiquitously many mechanisms via multiple receptors.22,26 Dopamine does influence the representation of decision outcomes within cortical regions, modulates serotonin, and the duo probably play complementary rather than oppositional roles in choice behavior.22 Another factor involved in predecisional processing is the endocannabinoid system. The role of cannabinoid signaling in decision-making pathways and frontal cortical circuits of decision making has been recently studied.20 The authors showed in rats that the activation of the cannabinoid system in anterior cingulate and orbitofrontal cortices, two brain regions with a high prevalence of cannabinoid-1-receptors, modulates decision making. These brain areas have also been shown to be involved in social pain, which is often associated with decision-making difficulties and shown to be relieved by APAP.27
Concerning spatial memory, our results show a better memory acquisition (P<0.05) and a tendency to improve problem solving (P=0.07). Cyclooxygenases (COXs; COX-1, COX-2) play a pivotal role in memory acquisition, memory retention, and information processing.13,28,29 COXs are constitutively expressed in several areas of the brain, such as hippocampus, amygdale, and neocortex.30,31 COX-2 activity has been suggested as necessary for the acquisition of spatial memory, possibly via production of prostaglandins acting upon astroglia leading to increased release of glutamate, enhanced neuronal communication, and dendritic spine plasticity; COX-2 inhibitors (but not COX-1 inhibitors) appear to impair memory consolidation.28,29 Unlike nonsteroidal anti-inflammatory drugs, paracetamol has been demonstrated not to reduce tissue inflammation but is also suggested to act by inhibition of COX-1- and −2-mediated production of prostaglandins.30 It may also act on a discrete COX-1 splice variant (initially thought to be a distinct isoenzyme, COX-3).31 Very high doses of APAP have shown impaired memory performance in animals12, probably linked to endogenous COX-2 inhibition, with a sex-dependent effect of COX-2 inhibition on spatial memory13,32 and a more impaired spatial retention in female mice.32 While APAP effect appears deleterious at high doses, normal/low doses of APAP do enhance spatial memory and performance possibly via the central serotonergic system and increased release of endogenous monoamines (serotonin and noradrenaline) in the brain.33,34 The endocannabinoid system also plays a role in spatial memory and in attention, cognition, and mood, via CB1Rs localized to noradrenergic axon terminals, with norepinephrine release, and stimulation of the α2A receptor that improves the attention/cognition performance.35–37 It has been also reported that APAP produces anxiolytic-like effect that could be consequent to the activation of CB1 receptors via endocannabinoid system or via endocannabinoids and serotonergic systems.8,14,38 APAP also potentiates antidepressant-like effect of fluoxetine (a serotonin reuptake inhibitor), suggesting that subthreshold doses of fluoxetine and APAP may enable better management in depression.15 APAP has also been shown to modulate emotion by blunting the effect of social pain and reducing agitation in demented patients.27,39 The anxiolytic-like effect could be its pivotal property to improve concentration for spatial memory test as well as for predecisional processing.
Decision making and spatial memory improvement fit quite well with the recently described analgesic mechanism of action of APAP with indirect or mutual interactions among the serotonergic, cannabinoid, and adrenergic systems, including probably also the opioidergic system, although its role in APAP analgesia appears weaker in humans than in animals.40
Our study showed that APAP does not diminish core temperature in healthy volunteers. This confirms animal studies that suggested independent modes of action of APAP for analgesic and antipyretic effects.12 APAP has been shown to reduce the core temperatures of febrile and nonfebrile animals, but our study is the first in healthy (nonfebrile by definition) volunteers as studies so far had been carried out in nonfebrile stroke patients, known to have a probable degree of inflammation and in whom APAP induced some degree of hypothermia.41–45 APAP has been shown to induce hypothermia independently of cannabinoids and TRPV1, and AM404 does not mediate this response.12 COXs have an effect at high concentration, acting on the COX-2 dependent-induced PGE2 production that occurs in brain endothelial cells upon inflammatory challenge.46,47 However, COX-2, although constitutive, is induced upon inflammation and this is not the case in our group of healthy volunteers who had a C-reactive protein within normal range. APAP probably requires inflammation to be present in the tissues in order to have a hypothermic effect. Several mechanisms have been suggested and a recent publication confirms that the hypothermic activity is independent of TRPV1 and that transient receptor potential cation channel member A1 is important for the hypothermic effects of APAP.3,11
Collectively, our data show for the first time a substantial effect of APAP on decision making and spatial memory in a group of healthy volunteers with 2 g paracetamol. These findings stand in support of preclinical publications claiming beneficial use of APAP at therapeutic dosage on cognitive processes. We also showed that APAP does not reduce body temperature in healthy nonfebrile volunteers demonstrating that hypothermia is not required for APAP effect on cognitive function and antinociception. However, this might be different in patients with a silent low grade and even more hyperthermic syndrome.
Future studies should focus on sex differences that are to be expected considering the preclinical data, on age differences as APAP is widely used in children and in elderly persons, who often have cognitive impairment and osteoarthritis, and where APAP is the first-line pharmacological treatment.48 Pharmacokinetics studies, APAP metabolites measurements, pharmacogenetic evaluations and correlations with memory, decision making, and other cognitive functions could bring information on factors involved in APAP mechanism of action.
Disclosure
The authors report no conflicts of interest in this work.
References
Pickering G, Loriot MA, Libert F, Eschalier A, Beaune P, Dubray C. Acetaminophen: first evidence of a central serotonergic mechanism of action in humans. Clin Pharmacol Ther. 2006;79(4):371–378. | ||
Pickering G, Estève V, Loriot MA, Eschalier A, Dubray C. Acetaminophen reinforces descending inhibitory pain pathways. Clin Pharmacol Ther. 2008;84(1):47–51. | ||
Andersson DA, Gentry C, Alenmyr L, et al. TRPA1 mediates spinal antinociception induced by acetaminophen and the cannabinoid Δ(9)-tetrahydrocannabiorcol. Nat Commun. 2011;2:551. | ||
Högestätt ED, Jönsson BA, Ermund A, et al. Conversion of acetaminophen to the bioactive N-acylphenolamine AM404 via fatty acid amide hydrolase-dependent arachidonic acid conjugation in the nervous system. J Biol Chem. 2005;280(36):31405–31412. | ||
Barrière DA, Mallet C, Blomgren A, et al. Fatty acid amide hydrolase-dependent generation of antinociceptive drug metabolites acting on TRPV1 in the brain. PLoS One. 2013;8(8):e70690. | ||
Bonnefont J, Daulhac L, Etienne M, et al. Paracetamol recruits spinal p42/p44 MAPKs and GH/IGF-1 receptors to produce analgesia via the serotonergic system. Mol Pharmacol. 2007;71(2):407–415. | ||
Mallet C, Barriere DA, Ermund A, et al. TRPV(1) in brain is involved in acetaminophen-induced antinociception. PLoS One. 2010;5(9):e12748. | ||
Mallet C, Daulhac L, Bonnefont J, et al. Endocannabinoid and serotonergic systems are needed for acetaminophen-induced analgesia. Pain. 2008;139(1):190–200. | ||
Kerckhove N, Mallet C, François A, et al. Ca(v)3.2 calcium channels: the key protagonist in the supraspinal effect of paracetamol. Pain. 2014;155(4):764–772. | ||
Sandrini M, Vitale G, Ruggieri V, Pini LA. Effect of acute and repeated administration of paracetamol on opioidergic and serotonergic systems in rats. Inflamm Res. 2007;56(4):139–142. | ||
Gentry C, Andersson DA, Bevan S. TRPA1 mediates the hypothermic action of acetaminophen. Sci Rep. 2015;5:12771. | ||
Ayoub SS, Pryce G, Seed MP, Bolton C, Flower RJ, Baker D. Paracetamol-induced hypothermia is independent of cannabinoids and transient receptor potential vanilloid-1 and is not mediated by AM404. Drug Metab Dispos. 2011;39(9):1689–1695. | ||
Ishida T, Sato T, Irifune M, Tanaka K, Nakamura N, Nishikawa T. Effect of acetaminophen, a cyclooxygenase inhibitor, on Morris water maze task performance in mice. J Psychopharmacol. 2007;21(7):757–767. | ||
Umathe SN, Manna SS, Utturwar KS, Jain NS. Endocannabinoids mediate anxiolytic-like effect of acetaminophen via CB1 receptors. Prog Neuropsychopharmacol Biol Psychiatry. 2009;33(7):1191–1199. | ||
Manna SS, Umathe SN. Paracetamol potentiates the antidepressant-like and anticompulsive-like effects of fluoxetine. Behav Pharmacol. 2015;26(3):268–281. | ||
Viberg H, Eriksson P, Gordh T, Fredriksson A. Paracetamol (acetaminophen) administration during neonatal brain development affects cognitive function and alters its analgesic and anxiolytic response in adult male mice. Toxicol Sci. 2014;138(1):139–147. | ||
Pickering G, Moustafa F, Macian N, Pereira B, Schmidt J, Dubray C. A new transmucosal-buccal formulation of acetaminophen for acute traumatic pain: a non-inferiority randomised double-blind clinical trial. Pain Physician. 2015;18(3):249–257. | ||
Pickering G, Kastler A, Macian N, et al. The brain signature of paracetamol in healthy volunteers: a double-blind randomized trial. Drug Des Devel Ther. 2015;9:3853–3862. | ||
Andersen RA, Cui H. Intention, action planning, and decision making in parietal-frontal circuits. Neuron. 2009;63(5):568–583. | ||
Khani A, Kermani M, Hesam S, Haghparast A, Argandoña EG, Rainer G. Activation of cannabinoid system in anterior cingulate cortex and orbitofrontal cortex modulates cost-benefit decision making. Psychopharmacology. 2015;232(12):2097–2112. | ||
Tracey I, Johns E. The pain matrix: reloaded or reborn as we image tonic pain using arterial spin labelling. Pain. 2010;148(3):359–360. | ||
Rogers RD. The roles of dopamine and serotonin in decision making: evidence from pharmacological experiments in humans. Neuropsychopharmacology. 2011;36(1):114–132. | ||
Graham GG, Scott KF. Mechanism of action of paracetamol. Am J Therap. 2005;12(1):46–55. | ||
Courade JP, Besse D, Delchambre C, et al. Acetaminophen distribution in the rat central nervous system. Life Sci. 2001;69(12):1455–1464. | ||
Pelissier T, Alloui A, Caussade F, et al. Paracetamol exerts spinal antinociceptive effect involving an indirect interaction with 5-hydroxytryptamine 3 receptors: in vivo and in vitro evidence. J Pharmacol Exp Ther. 1996;278(1):8–14. | ||
Nishizawa S, Benkelfat C, Young SN, et al. Differences between males and females in rates of serotonin synthesis in human brain. Proc Natl Acad Sci U S A. 1997;94(10):5308–5313. | ||
Dewall CN, Macdonald G, Webster GD, et al. Acetaminophen reduces social pain: behavioral and neural evidence. Psychol Sci. 2010;21(7):931–937. | ||
Rall JM, Mach SA, Dash PK. Intrahippocampal infusion of a cyclooxygenase-2 inhibitor attenuates memory acquisition in rats. Brain Res. 2003;968(2):273–276. | ||
Teather LA, Packard MG, Bazan NG. Post-training cyclooxygenase-2 (COX-2) inhibition impairs memory consolidation. Learn Mem. 2002;9(1):41–47. | ||
Hinz B, Cheremina O, Brune K. Acetaminophen (paracetamol) is a selective cyclooxygenase-2 inhibitor in man. FASEB J. 2008;22(2):383–390. | ||
Chandrasekharan NV, Dai H, Roos KL, et al. COX-3, a cyclooxygenase-1 variant inhibited by acetaminophen and other analgesic/antipyretic drugs: cloning, structure, and expression. Proc Natl Acad Sci U S A. 2002;99(21):13926–13931. | ||
Guzmán CB, Graham KA, Grace LA, Moore AH. Sex-dependent effect of cyclooxygenase-2 inhibition on mouse spatial memory. Behav Brain Res. 2009;199(2):355–359. | ||
Markus CR, Olivier B, de Haan EH. Whey protein rich in alpha lactalbumin increases the ratio of plasma tryptophan to the serum of the other large neutral amino acids and improves cognitive performance instress-vulnerable subjects. Am J Clin Nutr. 2002;75(6):1051–1056. | ||
Blecharz-Klin K, Piechal A, Pyrzanowska J, Joniec-Maciejak I, Kiliszek P, Widy-Tyszkiewicz E. Paracetamol – the outcome on neurotransmission and spatial learning in rats. Behav Brain Res. 2013;253:157–164. | ||
Cathel AM, Reyes BA, Wang Q, et al. Cannabinoid modulation of alpha2 adrenergic receptor function in rodent medial prefrontal cortex. Eur J Neurosci. 2014;40(8):3202–3214. | ||
Kawaura K, Karasawa J, Chaki S, Hikichi H. Stimulation of postsynapse adrenergic α2A receptor improves attention/cognition performance in an animal model of attention deficit hyperactivity disorder. Behav Brain Res. 2014;270:349–356. | ||
Mendiguren A, Pineda J. Cannabinoids enhance N-methyl-D-aspartate-induced excitation of locus coeruleus neurons by CB1 receptors in rat brain slices. Neurosci Lett. 2004;363(1):1–5. | ||
Deshpande LS, DeLorenzo RJ. Acetaminophen inhibits status epilepticus in cultured hippocampal neurons. Neuroreport. 2011;22(1):15–18. | ||
Husebo BS, Ballard C, Sandvik R, Nilsen OB, Aarsland D. Efficacy of treating pain to reduce behavioural disturbances in residents of nursing homes with dementia: cluster randomised clinical trial. BMJ. 2011;343:d4065. | ||
Pickering G, Moustafa F, Desbrandes S, Cardot JM, Roux D, Dubray C. Paracetamol and opioid pathways: a pilot randomized clinical trial. Fundam Clin Pharmacol. 2013;27(3):339–345. | ||
Ayoub SS, Botting RM, Goorha S, Colville-Nash PR, Willoughby DA, Ballou LR. Acetaminophen-induced hypothermia in mice is mediated by a prostaglandin endoperoxide synthase 2 gene-derived protein. Proc Nat Acad Sci U S A. 2007;101(30):11165. | ||
Massey TE, Walker RM, McElligott TF, Racz WJ. Acetaminophen-induced hypothermia in mice: evidence for a central action of the parent compound. Toxicology. 1982;25(2–3):187. | ||
Walker RM, Massey TE, McElligott TF, Racz WJ. Acetaminophen-induced hypothermia, hepatic congestion, and modification by N-acetylcysteine in mice. Toxicol Appl Pharmacol. 1981;59(3):500. | ||
Kasner SE, Wein T, Piriyawat P, et al. Acetaminophen for altering body temperature in acute stroke: a randomized clinical trial. Stroke. 2002;33(1):130–134. | ||
Dippel DWJ, van Breda EJ, van der Worp HB, et al. Effect of paracetamol (acetaminophen) and ibuprofen on body temperature in acute ischemic stroke PISA, a phase II double-blind, randomized, placebo-controlled trial [ISRCTN98608690]. BMC Cardiovasc Dis. 2003;3:2. | ||
Wong T, Stang AS, Ganshorn H, et al. Combined and alternating paracetamol and ibuprofen therapy for febrile children. Cochrane Database Syst Rev. 2013;10:CD009572. | ||
Engström Ruud L, Wilhelms DB, Eskilsson A, et al. Acetaminophen reduces lipopolysaccharide-induced fever by inhibiting cyclooxygenase-2. Neuropharmacology. 2013;71:124–129. | ||
Legler A, Monory K, Lutz B. Age differences in the role of the cannabinoid type 1 receptor on glutamatergic neurons in habituation and spatial memory acquisition. Life Sci. 2015;138:57–63. |
 © 2016 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2016 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.

