Back to Archived Journals » Gastrointestinal Cancer: Targets and Therapy » Volume 7
Pancreatic cancer pain: impact and management challenges
Received 29 September 2016
Accepted for publication 2 March 2017
Published 24 May 2017 Volume 2017:7 Pages 13—17
DOI https://doi.org/10.2147/GICTT.S95532
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 3
Editor who approved publication: Dr Eileen O'Reilly
Wesley B Jones,1 Allyson L Hale2
1University of South Carolina School of Medicine Greenville, Greenville, SC, USA; 2Department of Surgery, Greenville Health System, Greenville, SC, USA
Abstract: The majority of patients with pancreatic cancer experience a pain that will significantly alter their quality of life. Based on the low survival rates associated with pancreatic cancer, management of pain is an important component of palliation. Current management options include medication and intervention, specifically celiac plexus neurolysis (CPN) and bilateral thoracoscopic splanchnicectomy (BTS). The purpose of this paper is to outline the current state of interventional palliation of pain associated with malignant neural involvement in patients with pancreatic cancer. At present, CPN and BTS are not typically used until after failure of narcotic medications, even though narcotics have numerous side effects. Multiple studies have evaluated CPN or BTS and shown excellent outcomes for pain control, with 60%–90% successful palliation. Moreover, few complications have been reported in the literature. Because of the side effects commonly experienced with narcotics, as well as the high success and low complication rates of intervention, most authors recommend early intervention by way of BTS or CPN for patients with pancreatic cancer in significant pain.
Keywords: splanchnicectomy, celiac neurolysis, pancreatic cancer
Introduction
The diagnosis of pancreatic cancer is dismal. A majority of patients are incurable when diagnosed or have disease progression during treatment. Most intervention is therefore directed toward palliation. Pain control is an important aspect of palliative management. Up to 80% of patients with incurable pancreatic cancer will report increased abdominal and/or back pain. The impact of pain is difficult to objectively quantify but it adversely affects patient quality of life and possibly survival.1
Pancreatic cancer can cause pain by obstruction of the biliary and/or pancreatic ducts, but it is predominantly from malignant visceral afferent neural invasion of the celiac plexus in the retroperitoneal epigastrium. Narcotics are often the first line of treatment. Their use, however, comes with significant side effects, such as depression, fatigue, constipation, tolerance, and dependence. Other than narcotics, pain may also be treated by celiac plexus neurolysis (CPN) or bilateral thoracoscopic splanchnicectomy (BTS), two procedures theoretically believed to decrease reliance on pain medications. Despite this, there may be a delay of offering neurolysis/neurectomy for the control of pain associated with pancreatic cancer.
Many clinicians have the misconception that surgical or endoscopic treatment is more invasive and therefore riskier to the patient. So, currently, therapeutic intervention is usually offered only after failure of narcotic treatment. The purpose of this paper is to outline the current state of interventional palliation of pain associated with malignant neural involvement in patients with pancreatic cancer, examining approach, timing, and efficacy of intervention.
Anatomy
The celiac plexus lies immediately bilateral to the aorta at the level of celiac artery. It is made up of visceral afferent and sympathetic/parasympathetic efferent fibers. The plexus may consist of 2–5 ganglia lying between T12 and L2.2 Efferent sympathetic fibers synapse in these ganglia. From this location, neural signals, via visceral afferent fibers, leave and travel retrograde through the greater, lesser, and least splanchnic nerves before being registered by the central nervous system. This stimulus is usually interpreted as pain in the abdomen with radiation to the back.
The splanchnic nerves are easily identified grossly as they lie inferior and ventral to the sympathetic trunk, obliquely oriented toward the diaphragmatic hiatus. They may be located between T5 and T9 and range in number from 3 to 5 nerves on each side of the spinal column in the posterior thorax.
Techniques
Celiac plexus neurolysis
CPN is usually performed using radiologic or endosonographic guidance, but may also be done laparoscopically or at the time of laparotomy. Regardless of the approach, CPN is performed using alcohol, which is injected bilaterally into the peri-aortic fat pad at the level of celiac artery and diaphragmatic hiatus. Percutaneous CPN is usually performed by interventional radiologists or pain management specialists by way of image guidance with the patient in the prone position. Surgical neurolysis was originally performed during staging laparotomy, but has been replaced by laparoscopic approaches.1,3
Endosonographic guidance has become a more common approach and usually takes less than an hour to perform. This technique allows enhanced needle precision, the ability to inject the neurolytic agent into a larger area, and the ability to perform CPN at the time of tumor biopsy and staging.4 Endoscopic ultrasound (EUS) may be performed using linear array endosonographic imaging by way of a GF-UC30P (Olympus Corporation, Center Valley, PA, USA), GF UC140P-AL5, or GF UC 160 PAT8 (Pentax Precision Instruments, Orangeburg, NY, USA). Visualization of the celiac plexus is best seen from the posterior lesser curve of the stomach. The aorta is seen longitudinally and the first arterial branch below the diaphragm is identified. With experience, the celiac plexus and ganglia can be readily found. Traditionally, a 22- gauge needle is advanced through the scope after being purged of air in anticipation of injection. The needle is advanced near the lateral anterior aorta, flushed, and aspirated. For CPN in pancreatic cancer patients, 10 mL (0.25%) of bupivacaine (Hospira, Inc., Lake Forest, IL, USA) is injected, followed by 10 mL of dehydrated (98%) alcohol. The needle is then flushed and directed to the contralateral side of the aorta where the injection sequence is repeated. Sakamoto et al showed improved outcomes with broad plexus neurolysis, injecting around the superior and inferior mesenteric arteries as well as the celiac.4 Complications of CPN occur in ~1.5%–2% of patients. Hypotension, retroperitoneal abscess, and severe self-limited postprocedural pain have all been reported.5 Postprocedural diarrhea and hypotension due to sympathetic blockade may occur but are usually temporary. Permanent, unremitting diarrhea has been reported in very rare cases.6 Spinal complications, including extremity weakness, rarely, paresthesias, and paraplegia have also been described, particularly with the posterior approach.7,8
Bilateral thoracoscopic splanchnicectomy
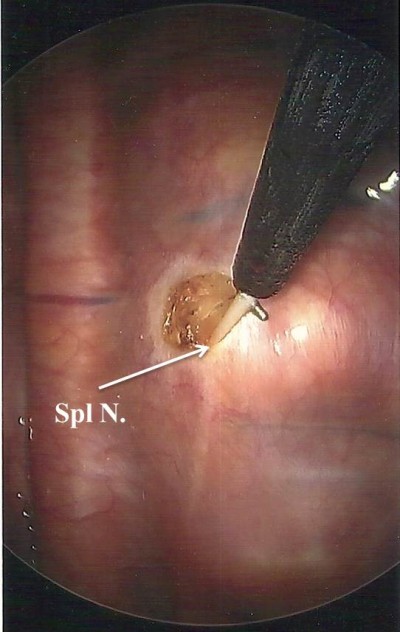
Reports of thoracic splanchnicectomy date back to 1969. The first description of bilateral splanchnicectomy for pain secondary to pancreatic cancer, however, was described by Sadar and Cooperman in 1974. This was performed by way of thoracotomy and included concomitant sympathectomy.9,10 The thoracoscopic approach was first described in 1993 in the British Journal of Surgery.11 At our institution, we perform BTS. BTS can be performed with a standard, single-lumen endotracheal tube with minimal monitoring and the patient in the prone position. The first trochar (5 mm) is placed at the inferior apex of the scapula and carbon dioxide insufflation is instilled at a pressure of 12 mmHg. A 5 mm, 30° angled scope is used. A second 5 mm trochar is placed two intercostal spaces inferior to the first trochar and ~2 cm medially. A third trochar can be used if needed. The surgeon then turns his or her attention to the posterior thorax, identifying the sympathetic trunk. The splanchnic nerves are seen running in an inferior and ventral position. Once the splanchnics are identified, a small opening is made in the pleura on either side of the nerve with a right angle cautery. We recommend lifting the nerve with the right angle cautery, so division is obvious once the nerve recedes into the pleura (Figure 1). Typically there are 2–5 nerves easily found on each side. After searching for and dividing all of the nerves, the insufflation is released and a rubber catheter is placed into the hemithorax, with the exterior end placed under water, creating a water seal. The lung is re-inflated with large tidal volumes. The procedure is then repeated on the right side.12
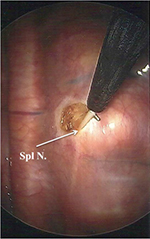  | Figure 1 Thoracoscopic view of the right hemithorax with a Spl N. lifted with right angle cautery prior to neurectomy. Abbreviation: Spl N., splanchnic nerve. |
Complications of splanchnicectomy occur in <2% of patients. They include pneumothorax, chylothorax, hemothorax, need for thoracotomy, persistent pain, transient hypotension, and diarrhea.13 Pneumothorax may be the most common with up to 2% of patients requiring an unplanned thoracostomy tube postoperatively.
Timing
The question of when to intervene has been a source of debate. The predominant clinical approach seems to be to avoid invasive procedures, reserving CPN/BTS for those who fail medical management with narcotics. Medical palliation is often guided by the “analgesic ladder” as described by the World Health Organization, an approach that can delay intervention to the later stages of disease.14 Trials of chemotherapy, and/or treatment with radiotherapy, along with the lack of an objective measurement to assess worsening of pain, may also lead to later use of CPN/BTS.
In the literature, most authors recommend early treatment when possible. In a prospective randomized trial by Lillemoe et al, they performed alcohol injection into the retroperitoneum at the time of staging laparotomy. This was often performed early in the treatment paradigm of their study patients. This added only a small amount of time to the operation and they found a significant decrease in pain. They also demonstrated an improved survival rate in patients who had decreased pain postoperatively.1 Some patients required re-intervention on average 6 months later. A study by Seicean et al included only patients naïve to chemotherapy and radiation, emphasizing the early timing of intervention. They found that EUS-guided CPN significantly decreased pain and improved quality of life.15 Iwata et al found that, in those patients with favorable characteristics to successful outcomes, early intervention was preferable.16 Wyse wrote in a randomized control trial of patients undergoing staging EUS early on in the course of their disease that “CPN is currently used late in the disease evolution as a salvage therapy for morphine-resistant pancreatic cancer pain. Because EUS is used at the beginning of the diagnostic algorithm for pancreatic cancer, it gives an opportunity to perform EUS-CPN early”. Therefore, they recommend CPN at the time of staging EUS, where it may be performed along with fine needle biopsy as needed. They also found that early EUS CPN resulted in less morphine consumption overall. Pietrabissa et al recommended that palliative splanchnicectomy “…should be indicated earlier in the course of pancreatic cancer”. They based this on the fact that their study demonstrated significant reduction in pain and improvement in quality of life.17 Finally, in a meta-analysis, Eisenberg et al wrote that
[…]most authors believe that this procedure (CPN) should not be reserved for a “last resort” when “nothing else works,” but should be applied earlier in the course of illness.18
Efficacy
There have been multiple studies to assess efficacy of pain control in the palliation of pancreatic cancer. Bilateral thoracoscopic splanchnicectomy and CPN have similar outcomes overall, but head-to-head comparisons have not been reported. Measured outcomes are heterogeneous between studies and include patient-reported pain scores, durability of pain relief, quality of life, survival rates, and narcotic use/side effects. Results of these studies are summarized in Table 1.
Overall, 70%–80% of patients undergoing CPN and BTS report decreased pain for 1–6 months.1,15,19 Puli et al, Lillemoe et al, and Iwata et al, all, found that bilateral CPN was associated with improved pain control compared with unilateral neurolysis.1,16,19 This likely holds true despite specific approach. In a randomized controlled trial, Wong et al demonstrated decreased pain with quality of life improvement throughout the duration of the study period (12 weeks).20 In another randomized control trial, Wyse et al showed pain relief at 3 months, with a trend toward lower morphine usage, but no change in quality of life.21 In a prospective nonrandomized trial, Pietrabissa et al showed pain relief and improved quality of life at 3 months postintervention in all 24 patients enrolled in the study.17 Seicean et al reported successful decrease in pain for 75% of patients undergoing CPN at their institution.15 In a meta-analysis of 21 studies by Eisenberg et al, 89% of patients had pain relief in the initial 2 weeks. Some degree of pain relief continued in nearly 90% of patients at 3 months and 70%–90% of patients until death.18 They were unable to demonstrate a change in opioid usage, narcotic side effects, or survival. In another meta-analysis by Yan and Myers, they found decreased pain scores, opioid usage, and constipation.22 They were unable to find an improvement in survival for those who underwent CPN. In a third meta-analysis, Puli et al reported pain relief in 80% of patients who underwent CPN.19 Lillemoe et al study of operative CPN performed during staging laparotomy was the only one to report an increase in survival.1 It was a prospective randomized trial with comparable control and experimental groups, but it has not been replicated.
Given these results, it seems obvious that CPN results in a decrease in pain for the majority of patients who undergo the procedure. However, there is only one study showing improved survival, minimal data that shows improved quality of life, and conflicting data on narcotic usage. Expectations should be discussed with patients during preoperative counseling, emphasizing that pain will likely improve but that there are multiple factors at play when it comes to how much this will affect the quality of their lives after intervention.
Summary
Pancreatic cancer is often incurable; but due to the proximity of the pancreas to the celiac plexus, pain is a common complaint. Therefore, palliation of symptoms plays an important role in the treatment of these patients. Narcotics are considered by many to be the first line of treatment. Their use, however, comes with significant side effects. Other available palliative interventions include CPN and BTS. Despite multiple recommendations for early intervention, CPN and BTS are not typically explored until after failure of narcotic treatment. Based on the increasing literature, early palliative intervention should be considered, as multiple studies have shown that patients who undergo CPN/BTS will experience some degree of pain control for varying lengths of time.
Disclosure
The authors report no conflicts of interest in this work.
References
Lillemoe KD, Cameron JL, Kaufman HS, Yeo CJ, Pitt HA, Sauter PK. Chemical splanchnicectomy in patients with unresectable pancreatic cancer. A prospective randomized trial. Ann Surg. 1993;217(5):447–455. | ||
Ward EM, Rorie DK, Nauss LA, Bahn RC. The celiac ganglia in man: normal anatomic variations. Anesth Analg. 1979;58(6):461–465. | ||
Strong VE, Dalal KM, Malhotra VT, et al. Initial report of laparoscopic celiac plexus block for pain relief in patients with unresectable pancreatic cancer. J Am Coll Surg. 2006;203(1):129–131. | ||
Sakamoto H, Kitano M, Kamata K, et al. EUS-guided broad plexus neurolysis over the superior mesenteric artery using a 25-gauge needle. Am J Gastroenterol. 2010;105(12):2599–2606. | ||
ASGE Standards of Practice Committee, Early DS, Acosta RD, Chandrasekhara V, et al. Adverse events associated with EUS and EUS with FNA. Gastrointest Endosc. 2013;77(6):839–843. | ||
Toukhy ME, Campkin NT. Severe diarrhea following neurolytic coeliac plexus block: case report and literature. Am J Hosp Palliat Care. 2011;28(7):511–514. | ||
Hayakawa J, Kobayashi O, Murayama H. Paraplegia after intraoperative celiac plexus block. Anesth Analg. 1997;84(2):447–448. | ||
De Conno F, Caraceni A, Aldrighetti L, et al. Paraplegia following coeliac plexus block. Pain. 1993;55(3):383–385. | ||
Copping J, Willix R, Kraft R. Palliative chemical splanchnicectomy. Arch Surg. 1969;98(4):418–420. | ||
Sadar ES, Cooperman AM. Bilateral thoracic sympathectomy-splanchnicectomy in the treatment of intractable pain due to pancreatic carcinoma. Clev Clin Q. 1974;41(4):185–188. | ||
Worsey J, Ferson PF, Keenan RJ, et al. Thoracoscopic pancreatic denervation for pain control in irresectable pancreatic cancer. Br J Surg. 1993;80(8):1051–1052. | ||
Jones WB, Jordan P, Pudi M. Pain management of pancreatic head adenocarcinomas that are unresectable: celiac plexus neurolysis and splanchnicectomy. J Gastrointest Oncol. 2015;6(4):445–451. | ||
Krishna S, Chang VT, Shoukas JA, Donahoo J. Video-assisted sympathectomy-splanchnicectomy for pancreatic cancer pain. J PainSymptom Manage. 2001;22(1):610–616. | ||
World Health Organization. Cancer Pain Relief. 2nd ed. Geneva, Switzerland: World Health Organization; 1996. | ||
Seicean A, Cainap C, Gulei I, Tantau M, Seicean R. Pain palliation by endoscopic ultrasound-guided celiac plexus neurolysis in patients with unresectable pancreatic cancer. J Gastorintest Liver Dis. 2013;22(1):59–64. | ||
Iwata K, Yasuda I, Enya M, et al. Predictive factors for pain relief after endoscopic ultrasound-guided celiac plexus neurolysis. Dig Endosc. 2011;23(2):140–145. | ||
Pietrabissa A, Vistoli F, Carobbi A, Boggi U, Bisa M, Mosca F. Thoracoscopic splanchnicectomy for pain relief in unresectable pancreatic cancer. Arch Surg. 2000;135(3):332–335. | ||
Eisenberg E, Carr DB, Chalmers TC. Neurolytic celiac plexus block for treatment of cancer pain: a meta-analysis. Anesth Analg. 1995;80(2):290–295. | ||
Puli SR, Reddy JB, Bechtold ML, Antillon MR, Brugge WR. EUS-guided celiac plexus neurolysis for pain due to chronic pancreatitis or pancreatic cancer pain: a meta-analysis and systematic review. Dig Dis Sci. 2009;54(11):2330–2337. | ||
Wong GY, Schroeder DR, Carns PE, et al. Effect of neurolytic celiac plexus block on pain relief, quality of life, and survival in patients with unresectable pancreatic cancer: a randomized controlled trial. JAMA. 2004;291(9):1092–1099. | ||
Wyse JM, Carone M, Paquin SC, Usatii M, Sahai AV. Randomized, double-blind, controlled trial of early endoscopic ultrasound-guided celiac plexus neurolysis to prevent pain progression in patients with newly diagnosed, painful, inoperable pancreatic cancer. J Clin Oncol. 2011;29(26):3541–3546. | ||
Yan BM, Myers RP. Neurolytic celiac plexus block for pain control in unresectable pancreatic cancer. Am J Gastroenterol. 2007;102(2):430–438. |
 © 2017 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2017 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.

