Back to Journals » Drug Design, Development and Therapy » Volume 9
Paeonia lactiflora Pall. protects against ANIT-induced cholestasis by activating Nrf2 via PI3K/Akt signaling pathway
Authors Ma X, Zhao Y, Zhu Y, Chen Z, Wang J, Li R, Chen C, Wei S, Li J, Liu B, Wang R, Li Y, Wang L, Xiao X
Received 7 June 2015
Accepted for publication 28 July 2015
Published 2 September 2015 Volume 2015:9 Pages 5061—5074
DOI https://doi.org/10.2147/DDDT.S90030
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 3
Editor who approved publication: Prof. Dr. Wei Duan
Xiao Ma,1,2 Yan-ling Zhao,2 Yun Zhu,3 Zhe Chen,1,2 Jia-bo Wang,4 Rui-yu Li,1,4 Chang Chen,1,2 Shi-zhang Wei,1,2 Jian-yu Li,3 Bing Liu,5 Rui-lin Wang,3 Yong-gang Li,3 Li-fu Wang,3 Xiao-he Xiao4
1Pharmacy College, Chengdu University of Traditional Chinese Medicine, Chengdu, People’s Republic of China; 2Department of Pharmacy, 302 Military Hospital of People’s Liberation Army, Beijing, People’s Republic of China; 3Department of Integrative Medical Center, 302 Military Hospital of People’s Liberation Army, Beijing, People’s Republic of China; 4China Military Institute of Chinese Medicine, 302 Military Hospital of People’s Liberation Army, Beijing, People’s Republic of China; 5School of Chinese Medicine, The University of Hong Kong, Hong Kong
Background: Paeonia lactiflora Pall. (PLP), a traditional Chinese herbal medicine, has been used for hepatic disease treatment over thousands of years. In our previous study, PLP was shown to demonstrate therapeutic effect on hepatitis with severe cholestasis. The aim of this study was to evaluate the antioxidative effect of PLP on alpha-naphthylisothiocyanate (ANIT)-induced cholestasis by activating NF-E2-related factor 2 (Nrf2) via phosphatidylinositol 3-kinase (PI3K)/Akt signaling pathway.
Materials and methods: Liquid chromatography-mass spectrometry (LC-MS) was performed to identify the main compounds present in PLP. The mechanism of action of PLP and its therapeutic effect on cholestasis, induced by ANIT, were further investigated. Serum indices such as total bilirubin (TBIL), direct bilirubin (DBIL), aspartate aminotransferase (AST), alanine aminotransferase (ALT), alkaline phosphatase (ALP), γ-glutamyl transpeptidase (γ-GT), and total bile acid (TBA) were measured, and histopathology of liver was also performed to determine the efficacy of treatment with PLP. Moreover, in order to illustrate the underlying signaling pathway, liver glutathione (GSH) content and mRNA or protein levels of glutamate-cysteine ligase catalytic subunit (GCLc), glutamate-cysteine ligase modulatory subunit (GCLm), Akt, heme oxygenase-1 (HO-1), NAD(P)H/quinone oxidoreductase 1 (Nqo1), and Nrf2 were further analyzed. In addition, validation of PLP putative target network was also performed in silico.
Results: Four major compounds including paeoniflorin, albiflorin, oxypaeoniflorin, and benzoylpaeoniflorin were identified by LC-MS analysis in water extract of PLP. Moreover, PLP could remarkably downregulate serum levels of TBIL, DBIL, AST, ALT, ALP, γ-GT, and TBA, and alleviate the histological damage of liver tissue caused by ANIT. It enhanced antioxidative system by activating PI3K/Akt/Nrf2 pathway through increasing Akt, Nrf2, HO-1, Nqo1, GCLc, and GCLm expression. The putative targets network validation also confirmed the important role of PLP in activating Akt expression.
Conclusion: The potential mechanism of PLP in alleviating ANIT-induced cholestasis could to be related to the induction of GSH synthesis by activating Nrf2 through PI3K/Akt-dependent pathway. This indicates that PLP might be a potential therapeutic agent for cholestasis.
Keywords: Paeonia lactiflora Pall., cholestatic hepatitis, antioxidation, Nrf2, Akt
Introduction
Cholestasis, characterized by decreased bile flow and accumulation of bile acids, is one of the most common and devastating manifestations associated with a variety of hereditary and acquired liver diseases.1,2 This condition will ultimately lead to hepatic failure and cirrhosis without proper treatment.3 Due to the serious outcome, it always brings heavy burden to patients and society all over the world.4–6 Cholestasis is observed in various dysfunctions, but current studies mainly concentrate on dysregulation of bile acid transporters, oxidative stress, and inflammation in hepatocytes. Among the three aspects, cholestasis associated with liver diseases is most commonly featured by the enhancement of reactive oxygen species (ROS) and reduction of antioxidant systems such as glutathione (GSH), which finally results in oxidative stress and directly induces the apoptosis of hepatocytes.
Recently, NF-E2-related factor 2 (Nrf2) has been proved to play a central role in defense against oxidative stress. Expression of many antioxidant proteins and detoxifying enzymes such as GSH, glutamate-cysteine ligase catalytic subunit (GCLc), and GCL modulatory subunit (GCLm) are primarily controlled by Nrf2.7 It has been indicated that the translocation of Nrf2 can ultimately inhibit the oxidative stress via mediating the positive regulatory signal of GSH, GCLc, and GCLm.8 Once the intracellular concentrations of GCLc and GCLm increase, GSH acting as the downstream signal, correspondingly reduces ROS and provides protection against oxidative stress. Given the importance of GSH in maintaining normal cell function, dysregulation in its synthesis generally worsens the disease process.9,10 Phosphatidylinositol 3-kinase (PI3K)/Akt pathway has been recently proved to be one of the potential upstream signaling regulators of Nrf2.8 Accumulated evidence has shown that nuclear translocation of Nrf2 and GSH synthesis might be induced by phosphorylation of Akt.11 Therefore, there seems to be a close relationship between the activation of GSH and PI3K/Akt/Nrf2 pathway in cholestasis.
Complementary and alternative medicine displays its unique role in the treatment of cholestasis along with the development of medical science. In the traditional Chinese medicine, the dried root of Paeonia lactiflora Pall. (PLP, Chishao in Chinese) has been used for the treatment of hepatic diseases for over thousands of years.12 In our previous meta-analysis, PLP was shown to demonstrate excellent therapeutic effect on hepatitis associated with severe cholestasis.6 However, the protective mechanism of PLP on cholestasis seems to be inadequate compared with clinical application. Current studies indicate that PLP might exhibit protective effect against liver diseases through antioxidation and free radicals scavenging mechanisms,13 but the potential pathway still remains to be clarified. Therefore, in the current study, we aimed to investigate the role of PLP in alleviating alpha-naphthylisothiocyanate (ANIT)-induced cholestasis. To further explore its mechanism of antioxidative action in depth, we specifically investigated the PI3K/Akt and Nrf2 signaling pathways, which might provide a novel insight into improving the treatment for cholestatic liver injury (Figure 1).
Materials and methods
Chemicals and reagents
In this study, the dried root of PLP was used. PLP was purchased from Beijing Lvye Medicinal Materials Company (Beijing, People’s Republic of China). ANIT was purchased from Sigma-Aldrich Co. (St Louis, MO, USA). Total bilirubin (TBIL), direct bilirubin (DBIL), aspartate transaminase (AST), alanine transaminase (ALT), alkaline phosphatase (ALP), γ-glutamyltranspeptidase (γ-GT), total bile acid (TBA), and GSH assay kits were obtained from Nanjing Jiancheng Bioengineering Institute (Nanjing, People’s Republic of China). All the other chemicals of analytical grade were purchased from commercial sources.
Identification of compounds present in PLP by ultra high performance liquid chromatography-quadruple-time of flight
The dried root of PLP was cut into small pieces, mixed, and soaked in water (1/10 w/v) for 0.5 hours; then the mixture was boiled and reflux extraction was performed two times (2 hours for the first time, 1.5 hours for the second time). The aqueous extract of PLP was decanted, filtered through six-layer gauze, and evaporated to dryness under reduced pressure. The weight ratio (w/w) of powder to raw herb was 25.8%. The powder was dissolved in purified water to obtain a final concentration of 0.02 g/mL, which was used as the injection sample.
Ultra high performance liquid chromatography-quadruple-time of flight (UHPLC-Q-TOF) was performed with the following conditions: column: 2.1×100 mm, 1.8 μm (Zorbax Eclipse Plus C18, Agilent, Santa Clara, CA, USA); injection volume: 2 μL; flow rate: 0.3 mL/min; solvents: A, H2O with 0.1% H3PO4 and B, 100% acetonitrile; gradient: 0–2 minutes, 95% A; 2–5 minutes, 85% A; 5–10 minutes, 75% A; 10–12 minutes, 70% A; 12–14 minutes, 62% A; 14–16 minutes, 20% A; 16–20 minutes, 95% A.
For operation in MS/MS mode, a mass spectrometer (MS) fitted with an orthogonal Z-spray ion interface was used for all the analyses. Ionization was achieved using electrospray technique. For analysis, the electrospray source parameters were fixed as follows: electrospray capillary voltage was 3.0 kV in negative ionization mode and 4.0 kV in positive ionization mode; the mass range was set from 80 m/z to 1,000 m/z; gas temperature was 200°C in negative ionization mode and 225°C in positive ionization mode; gas flow was 11 L/min; nebulizer was set to 35 pisg (negative) and 45 pisg (positive); sheath gas temperature was 350°C and sheath gas flow was 12 L/min; nozzle voltage was 500 V in both negative and positive modes.
Animals and treatments
All animal procedures were approved by the Animal Experiment Committee of 302 Military Hospital of People’s Liberation Army. Male Sprague Dawley rats weighing 200±20 g were obtained from the laboratory animal center of The Military Medical Science Academy of the PLA (Permission No SCXK-(A) 2012-0004). All the animals were acclimated for 1 week prior to the experiment and were kept under the same temperature (25°C±2°C) and lighting (12:12-hour light:dark cycle) conditions. Water and food were available for rats ad libitum. All the studies were performed in accordance with the guidelines of the Council on Animal Care of Academia Sinica. The animals were randomly divided into six groups with ten rats in each group. The aqueous extract of PLP was dissolved in normal saline (PLP group) and was given to rats intragastrically 80 g/kg, 40 g/kg and 20 g/kg doses respectively for 5 days. Rats in PLP groups were intragastrically treated with 60 mg/kg ANIT (dissolved in olive oil) (ANIT group) on the third day. PLP doses adopted in this study were based on preliminary experiments, and were proved to have no toxic effects.12,14 ANIT group was administrated with normal saline for five days and on the third day was treated with 60 mg/kg ANIT. On the third day, ANIT group was also treated with 60 mg/kg ANIT. The rats serving as negative control were administrated normal saline every day and intragastrical treatment with the vehicle (olive oil) alone on the third day. Ursodeoxycholic acid (UDCA), the positive control, was given to rats at a dosage of 60 mg/kg daily for 5 days and was given ANIT 60 mg/kg on the third day. Sample collection and liver functional assays.
Rats were sacrificed after the last administration. Blood samples were collected and centrifuged at 3,000× g for 10 minutes and serum was separated. The serum samples thus obtained were sterile, hemolysis-free, and stored at −70°C before determining the biochemical parameters. The serum levels of TBIL, DBIL, AST, ALT, ALP, γ-GT, and TBA were measured by Synergy Hybrid Reader (Biotek, Winooski, VT, USA) using assay kits, wherein assays were performed according to the manufacturer’s protocol.15 Histological assessment of liver damage.
Liver tissues were excised and fixed in 10% phosphate-buffered saline (PBS)-buffered formalin. Three or four paraffin-embedded sections (4–5 μm thick) per specimen were prepared and stained with hematoxylin-eosin (HE). The stained sections were examined under Nikon microscope and analyzed by NIS-Elements (version F 4.0) software.
Measurement of GSH content in liver tissue
A portion of the liver tissue was homogenized with a buffer containing 0.15 M KCl to obtain a 1:10 (w/v) solution. The homogenates were then centrifuged at 12,000× g (4°C) for 20 minutes and the supernatants were collected. The supernatant was measured using the GSH assay kit (Nanjing Jiancheng Bioengineering Institute, Nanjing, People’s Republic of China). All the steps were performed according to the manufacturer’s instructions for the determination of GSH content.16
Western blot for detecting GLCc, GCLm, pAkt, and Nrf2 in liver tissue
Rat liver tissue (0.1 g) was homogenized and subsequently lysed in ice-cold lysis buffer containing 1 mM phenylmethylsulfonyl fluoride and a protease inhibitor mixture. The sample was subjected to centrifugation at 8,000× g for 10 minutes at 4°C to remove any debris. After centrifugation, the supernatant was aliquoted and stored at −80°C. These samples were used to perform Western blot assay to detect the presence of GLCc, GCLm, and pAkt. The nuclear and cytoplasmic extractions were performed using the nuclear and cytoplasmic extraction kits (Biosynthesis Biotechnology Company, Beijing, People’s Republic of China) following manufacturer’s instructions. Then, nuclear and cytoplasmic proteins were assayed by Western blot analysis for the presence of Nrf2. Fifty micrograms of protein was separated by 12% SDS–polyacrylamide gel electrophoresis and transferred onto a nitrocellulose membrane. Immunodetection was performed using a rabbit anti-GCLc antibody (1:5,000), anti-GCLm antibody (1:5,000), anti-Akt antibody (1:2,000), anti-Nrf2 antibody (1:2,000), and mouse anti-GAPDH antibody (1:1,000) in a solution of 5% milk, Tris-buffered saline (TBS), and 0.05% Tween-20 to detect GCLc, GCLm, Akt, Nrf2, and GAPDH, respectively. After incubation with the appropriate peroxidase-conjugated secondary antibody, the membrane was washed in TBST (TBS with Tween 20) for 60 minutes, and the immunoreactive bands were visualized with chemiluminescence.
Quantitative RT-PCR analyses for GCLc, GCLm, Akt, Nrf2, Nqo1, and HO-1 in liver tissue
The effect of PLP on mRNA expressions of GCLc, GCLm, Akt, Nrf2, NAD(P)H/quinone oxidoreductase 1 (Nqo1), and heme oxygenase-1 (HO-1) in the liver tissue of ANIT-induced cholestasis rats were determined by quantitative reverse transcription polymerase chain reaction (RT-PCR). Total RNA was extracted from the liver tissues of each group using Trizol reagent following the manufacturer’s protocols. RNA concentration was determined by optical density measurement at 260 nm on a spectrophotometer. RNA (2 μg) was reverse-transcribed using a PrimeScript™ RT reagent kit, and 2 μL cDNA was used for the PCR reaction. List of primers used in our study are listed in Table 1. The subsequent PCR amplification was carried out by ABI Step One Plus (Applied Biosystems Inc, Carlsbad, CA, USA) PCR machine, running 40 cycles at 95°C for 5 seconds and 60°C for 60 seconds. Data were calculated for comparison through 2−ΔΔCT method.
  | Table 1 Primers sequences for RT-PCR |
PLP putative target network validation
The ingredients of PLP were collected from TCM Database@Taiwan (http://tcm.cmu.edu.tw/). Data about putative targets of PLP’s main ingredients were collected from Herbal Ingredients’ Targets Database (http://lifecenter.sgst.cn/hit/) and ChEMBL (https://www.ebi.ac.uk/chembl/), two well-known databases including information about the targets of herb ingredients. The targets were saved as Uniprot ID and uploaded into the David 6.7 (http://david.abcc.ncifcrf.gov/home.jsp) information tool to get the gene oncology and pathway information. The relationship among herb, ingredients, and targets were visualized by using Navigator software.
Statistical analysis
The data were expressed as the mean ± standard deviation (SD) and analyzed with the SPSS software program (version 20.0; SPSS Inc., Chicago, IL, USA). The differences between the group means were calculated by one-way analysis of variance. The differences were considered to be statistically significant when P<0.05 and highly significant when P<0.01. Principal component analysis (PCA) was also performed to analyze the comprehensive effect of seven liver function parameters.
Results
Identification of compounds present in PLP by UHPLC-Q-TOF
Both negative and positive electron spray (ES) modes were applied to analyze and identify the chemical components in the aqueous extract of PLP. The total current chromatogram at the positive ES mode is shown in Figure 2. Four compounds including paeoniflorin, albiflorin, oxypaeoniflorin, and benzoylpaeoniflorin were detected and identified by comparing the MS fragments, characteristics of the compounds, with previous reference records and database (provided by Agilent) connected to Agilent MassHunter software (Figure S1).17–19 The analyzed and identified compounds are listed in Table 2.
Effects of PLP on serum indices of liver function
The serum levels of ALT and AST were measured as the sensitive indices of liver damage. Rats administered with only ANIT displayed remarkable increase in the levels of ALT and AST (Figure 3A and B). The administration of PLP at both 80 g/kg and 40 g/kg significantly reduced the serum levels of ALT and AST, respectively, which is almost equal to the results obtained by administering UDCA. In addition, ALT, but not AST, levels were also altered by PLP at a dosage of 20 g/kg. As shown in Figure 3C–G, the serum levels of TBIL, DBIL, ALP, TBA, and γ-GT levels, which are crucial indices of cholestasis, were significantly higher in the ANIT-treated rats than in the control rats. Furthermore, the levels of ALP, TBIL, γ-GT, and TBA were remarkably decreased in rats treated with 80 g/kg, 40 g/kg, and 20 g/kg of PLP. Moreover, the serum level of DBIL decreased significantly in 80 g/kg and 40 g/kg PLP groups.
PCA was performed to analyze the comprehensive effect of seven liver function parameters. As shown in Figure 3H, there was a large Euclidean distance from control to ANIT group, which demonstrated the distinguished liver status and indicated the successful establishment of cholestatic hepatitis. Furthermore, the Euclidean distance from control group to other groups displayed as UDCA≈PLP 80 g/kg < PLP 40 g/kg < PLP 20 g/kg ≈ANIT. It indicated that PLP at a dosage of 80 g/kg presented a regulation effect of the comprehensive liver function similar to that shown by UDCA. In addition, PLP demonstrated weaker effect at 20 g/kg and 40 g/kg doses when compared with 80 g/kg dose.
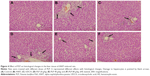
Histological assessment of liver damage
Histological evaluations provided the direct evidence for protective effect of PLP on ANIT-induced cholestasis. The liver tissue of control group exhibited normal structure with no abnormal morphological changes (Figure 4A). The specimens in the ANIT group showed acute infiltration with polymorphonuclear neutrophils, edema, sinusoid congestion, severe demolition or loss of the interlobular ducts, and hepatic necrosis (Figure 4B). Administration of UDCA and 80 g/kg PLP both exhibited a mild degree of bile duct epithelial damage and hepatocyte hydropic degeneration with less neutrophil infiltration (Figure 4C and F), which was almost similar to the control group. The specimens treated with 40 g/kg PLP displayed a moderately reduced severity of inflammatory cell infiltration and histological damage (Figure 4E). The liver damage in the specimens treated with 20 g/kg PLP was similar to that observed in ANIT-treated rats. PLP at 20 g/kg did not attenuate any portal tract edema, cholangitis, and bile duct epithelial damage (Figure 4D).
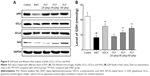
Regulation of PLP on antioxidation and PI3K/Akt/Nrf2 pathway
To study the role of PLP in cholestasis, the oxidative stress related to PI3K/Akt/Nrf2 pathway was explored. Results indicate that ANIT decreased the protein expressions of GCLm, GCLc, pAkt, and Nrf2, whereas PLP at 80 g/kg and 40 g/kg doses could promote the protein levels of GCLm, GCLc, p-Akt, and Nrf2 (Figure 5A). Furthermore, exposure to ANIT markedly decreased GSH content compared with control, suggesting the reduction of antioxidation in hepatocytes. PLP at 80 g/kg and 40 g/kg could significantly restore GSH content compared with ANIT-treated rats (Figure 5B). However, PLP at 20 g/kg presented no obvious change in ANIT-induced depletion of GSH.
Expression of mRNAs involved in PI3K/Akt/Nrf2 signaling pathway
As shown in Figure 6A and B, we examined the effect of PLP on ANIT-reduced mRNA expressions of GCLc and GCLm. The results showed that PLP increased the mRNA expressions of GCLc and GCLm in a concentration-dependent manner. The downstream signaling of Nrf2, such as mRNA expressions of Nqo1 and HO-1, was not significantly altered by ANIT, but was upregulated by PLP at doses of 80 g/kg and 40 g/kg (Figure 6C and D). This indicated that PLP possibly affects Nqo1 and HO-1 upstream gene expression such as Nrf2. To elucidate the regulation of PI3K/Akt pathway and Nrf2, we further examined the effect of PLP on mRNA expressions of Akt and Nrf2 (Figure 6E and F). We observed that PLP could increase the mRNA expression of Nrf2 at all doses, and there was a significant increase in regulation of Akt in the group treated with PLP at doses of 80 g/kg and 40 g/kg.
PLP putative targets network validation
Based on Herbal Ingredients’ Targets Database and ChEMBL, five main ingredients of PLP and 33 known targets of these similar ingredients as putative targets for PLP were identified (Table S1, Figure 7A). As shown in Figure 7B and D, the putative targets were analyzed by David 6.7 information tool to obtain the gene annotation. Figure 7B demonstrates the top 20 gene functions of the putative targets. The top gene function was related to cell death or apoptosis, which occupied approximately 42.4% of the total genes. Figure 7D displays the top 20 pathways of the putative targets. The results indicated that cancer (27.3%), Toll-like receptor (24.2%), neurotrophin (21.2%), T-cell receptor (21.2%), and prostate cancer (21.2%) signaling pathways were relatively important of these 33 putative targets. Moreover, according to the annotation of KEGG, Akt was regulated in all the top five pathways, which indicated that Akt played an important role in PLP’s putative targets (Figure 7C).
Discussion
Cholestasis is characterized by intrahepatic accumulation of potentially toxic bile acids that are associated with several liver diseases as an effect of obstruction or destruction of bile ducts.20 Cholestasis is one of the main features in chronically progressive hepatic disorders such as biliary atresia, primary biliary cirrhosis, and primary sclerosing cholangitis. The primary event of cholestasis has been implicated in the later development of hepatocellular injury, progressive hepatic fibrogenesis, cirrhosis, and death due to liver failure.21 Accumulated evidence indicates that free radicals, oxidative stress, and lipid peroxidation are observed in cholestatic damage. Several studies further demonstrate the pro-oxidant potential of hydrophobic bile acids in hepatocytes.22 It is therefore believed that oxidative stress is a decisive generating factor in the pathogenesis of cholestatic damage, and antioxidant therapy will be the recommended therapeutic strategy. ANIT is a hepatotoxicant commonly used in rodents to mimic human intrahepatic cholestasis, which acts by interrupting the bile flow and accumulating bile acids or other bile components in the liver. In ANIT-induced liver injury, the change of hepatic antioxidant defense system with enhancement of hepatic lipid peroxidation is suggested to be the primary contributor to the progress of liver damage.23,24 Therefore, ANIT-induced liver injury model was applied in this study to mimic the characteristics and mechanism of cholestatic hepatitis.
PLP is one of the most well-known herbs in People’s Republic of China, Korea, and Japan for more than 1,200 years. PLP consistently exhibits wide pharmacological effects such as vasodilatation of thoracic aorta;25 liver protection;1 antiallergic,26 anti-inflammation, and immunoregulation effects.27 Current studies further indicate that PLP and its active components demonstrate multiple effects mainly through the function of antioxidation.28,29 Paeoniflorin, one of the active compounds in PLP, is proved to attenuate apoptosis in PC12 cells via suppressing ROS-mediated PKC/NF-κB pathway.28 Kim also reported that the aqueous extracts of PLP was able to protect primary cultures of rat cortical cells exposed to oxidative stress induced by H2O2.29 PLP and its components show the antioxidant effect not only in neurocytes, but also in other cells such as hepatocytes. The aqueous extract of PLP can protect against CCl4-induced liver injury with oxidative damage in rats, and the hepatoprotective effects might be correlated with its antioxidation and free radical scavenger effect.12 This result has also been validated by our previous research. In our former study, we first proved that the prescriptions consisting of PLP are effective against cholestatic hepatitis in clinical application.6 The efficacy of paeoniflorin on cholestasis is then validated in the ANIT-induced liver injury rats, where it acts as free radical scavenger.1 These studies indicate that PLP is efficient in treating cholestatic hepatitis, which might be due to its antioxidant effect. Therefore, we further carried out this study in order to illustrate the mechanism of action of PLP on cholestatic hepatitis based on our previous result.
Our study showed that the pretreatment of PLP significantly and dose-dependently decreased ANIT-induced elevation of serum ALT, AST, ALP, TBIL, DBIL, TBA, and γ-GT levels. Furthermore, histological injuries were remarkably relieved after PLP pretreatment at the dose of 80 g/kg and 40 g/kg but with weaker effect at the dose of 20 g/kg. These results indicated that PLP was effective on ANIT-induced liver injury associated with cholestasis. GSH, involved in the detoxification of chemical substances conjugated by the catalytic action of GST and extracellular transport of conjugated compounds, is one of the major cellular molecules against ROS.8 GSH synthesis occurs in the cytosol of all mammalian cells via two enzymatic steps: the formation of γ-glutamylcysteine from glutamate and cysteine catalyzed by glutamate cysteine ligase (GCL) and the formation of GSH from γ-glutamylcysteine and glycine catalyzed by GSH synthase. GCLc and GCLm, the constituents of GCL, are therefore believed to play crucial roles against oxidative stress. Previously, Mitsuyoshi indicated that UDCA, the only medication approved by the FDA for the treatment of cholestasis, increased the cellular content of GSH and induction of GCL to protect the cell from oxidative damage.30 According to the important role of GSH in antioxidation, we examined the effect of PLP on the expression of GSH and its synthetic enzymes. From our experiment, it was observed that PLP significantly increased GSH levels and upregulated the mRNA/protein expressions of GCLc and GCLm in a dose-dependent manner. The result was also in accordance with our former study and Wang’s reports,1,31 which suggested that PLP might partly contribute to protecting ANIT-induced cholestasis from oxidative damage.
Nrf2, a key transcription factor, serves as a sensor for regulating the expression of GCL and other antioxidative stress genes in response to oxidative stimulations.32 Under normal conditions, Nrf2 is repressed in the cytoplasm by the actin-binding protein Keap1. Upon oxidative stress, Nrf2 dissociates from Keap1 and translocates to the nucleus to bind to antioxidant response element, which is an enhancer element that initiates the transcription of a battery of genes encoding phase-II enzymes and antioxidant enzymes.33 Eminent evidence has shown that Nrf2 activation protects against intrahepatic cholestasis by regulating expression of several detoxifying and antioxidant enzymes in both BDL and ANIT models.34,35 Further research proves that Nrf2, which serves as the endogenous therapeutic agent, is able to positively regulate the expression of GCL subunits and glutamine synthetase in cholestasis mice. In the present study, the expression of Nrf2 in ANIT-treated rats decreased significantly compared with control rats. This result was similar to Tanaka’s former report.34 In order to further investigate the downstream regulation of Nrf2, we also detected the mRNA expressions of Nqo1 and HO-1, which are the prototypical Nrf2 target genes and crucial antioxidation elements. The results demonstrated that ANIT did not significantly alter the Nqo1 and HO-1 mRNA expressions. It suggested that the cholestasis induced by ANIT was not remarkably related to dysfunction of these two genes. However, PLP could significantly promote these target genes, suggesting the potent antioxidation effect of PLP through Nrf2. In addition, our results also showed that the expression of Nrf2 increased remarkably after pretreatment with PLP. All the results indicated that Nrf2 was the important target of GCLm and GCLc in cholestasis. It also proved the positive function of PLP in increasing Nrf2 expression in cholestasis treatment.
Akt is the primary mediator of PI3K-initiated signaling pathway. It commonly acts as an antiapoptotic signaling molecule in many different cell death paradigms when it is phosphorylated and activated at residue Ser473. In recent years, it is believed that the PI3K/Akt pathway creates a survival signal against oxidative stress-induced injuries by regulating Nrf2 expression in numerous cells.11,36 Even in HepG2 cells, the activity of GCL subunits is proved to be stimulated by the activation of PI3K/Akt signaling accompanied with nuclear translocation of Nrf2.8 These results indicate that PI3K/Akt plays an important role in regulation of Nrf2 and oxidative stress. Therefore, we further investigated the relationship between Nrf2 and PI3K/Akt signaling pathway. As shown in our result, PLP could enhance the mRNA expression of Akt and protein expression of pAkt. Moreover, the result of PLP putative targets network validation also demonstrated that Akt was the crucial target of PLP. The results revealed the involvement of PI3K/Akt pathway in regulating GSH level and Nrf2 translocation (Table S1, Figure 7C).
A phytochemical analysis was performed to determine the main active components of PLP. Four representative compounds including paeoniflorin, albiflorin, oxypaeoniflorin, and benzoylpaeoniflorin were identified by UPLC-MS. Paeoniflorin is the most bioactive component among the four compounds, and its liver protection effects have been demonstrated by many studies.37–39 Apart from paeoniflorin, oxypaeoniflorin also shows antioxidative and anti-inflammatory activities to a little extent.40 Therefore, from the aspect of chemical composition, we might speculate that the protective effect of PLP on ANIT-induced cholestasis was due to the presence of active compounds such as paeoniflorin, and oxypaeoniflorin.
All the evidences mentioned earlier indicate that PLP is able to alleviate ANIT-induced cholestasis by downregulating oxidative stress via PI3K/Akt/Nrf2 signaling pathway.
Conclusion
In summary, various biochemical tests and histological assessments have revealed that PLP could provide protection against ANIT-induced cholestasis. The potential mechanism seems to be related to the induction of GSH synthesis by activating Nrf2 through PI3K/Akt-dependent pathway. The results indicate that PLP might be a potential therapeutic agent for cholestasis.
Acknowledgments
This work was financially supported by grants from National Natural Science Foundation of China (81173571 and 81303120), The “Twelfth Five Year Plan” Foundation of China People’s Liberation Army (CWS11C164), and the major projects of the National Science and Technology (2012ZX10005010-002-002).
Disclosure
The authors report no conflicts of interest in this work.
References
Zhao Y, Zhou G, Wang J, et al. Paeoniflorin protects against ANIT-induced cholestasis by ameliorating oxidative stress in rats. Food Chem Toxicol. 2013;58:242–248. | ||
Anwer MS. Role of protein kinase C isoforms in bile formation and cholestasis. Hepatology. 2014;60(3):1090–1097. | ||
Padda MS, Sanchez M, Akhtar AJ, Boyer JL. Drug-induced cholestasis. Hepatology. 2011;53(4):1377–1387. | ||
Yang H, Ramani K, Xia M, et al. Dysregulation of glutathione synthesis during cholestasis in mice: molecular mechanisms and therapeutic implications. Hepatology. 2009;49(6):1982–1991. | ||
Weerachayaphorn J, Luo Y, Mennone A, Soroka CJ, Harry K, Boyer JL. Deleterious effect of oltipraz on extrahepatic cholestasis in bile duct-ligated mice. J Hepatol. 2014;60(1):160–166. | ||
Ma X, Wang J, He X, et al. Large dosage of chishao in formulae for cholestatic hepatitis: a systematic review and meta-analysis. Evid Based Complement Alternat Med. 2014;2014:328152. | ||
Xu W, Hellerbrand C, Köhler UA, et al. The Nrf2 transcription factor protects from toxin-induced liver injury and fibrosis. Lab Invest. 2008;88(10):1068–1078. | ||
Arisawa S, Ishida K, Kameyama N, et al. Ursodeoxycholic acid induces glutathione synthesis through activation of PI3K/Akt pathway in HepG2 cells. Biochem Pharmacol. 2009;77(5):858–866. | ||
Lu SC. Regulation of glutathione synthesis. Mol Aspects Med. 2009;30(1–2):42–59. | ||
Ko K, Yang H, Noureddin M, et al. Changes in S-adenosylmethionine and glutathione homeostasis during endotoxemia in mice. Lab Invest. 2008;88(10):1121–1129. | ||
Li C, Pan Z, Xu T, Zhang C, Wu Q, Niu Y. Puerarin induces the upregulation of glutathione levels and nuclear translocation of Nrf2 through PI3K/Akt/GSK-3β signaling events in PC12 cells exposed to lead. Neurotoxicol Teratol. 2014;46:1–9. | ||
Zhao Y, Ma X, Wang J, et al. Large dose means significant effect – dose and effect relationship of Chi-Dan-Tui-Huang decoction on alpha-naphthylisothiocyanate-induced cholestatic hepatitis in rats. BMC Complement Altern Med. 2015;15:104. | ||
Li R, Guo W, Fu Z, Ding G, Zou Y, Wang Z. Hepatoprotective action of Radix Paeoniae Rubra aqueous extract against CCl4-induced hepatic damage. Molecules. 2011;16(10):8684–8694. | ||
Wei S. ‘Dose-Effect-Response’ Relationships of Chi-Dan-Tui-Huang decoction on Acute Cholestatic Hepatitis [dissertation]. Chengdu: Chengdu University of Traditional Chinese Medicine; 2012. | ||
Wu Q, Zhang D, Tao N, et al. Induction of Nrf2 and metallothionein as a common mechanism of hepatoprotective medicinal herbs. Am J Chin Med. 2014;42(1):207–221. | ||
Li J, Peng L, Du H, et al. The Protective Effect of Beraprost Sodium on Diabetic Cardiomyopathy through the Inhibition of the p38 MAPK Signaling Pathway in High-Fat-Induced SD Rats. Int J Endocrinol. 2014;2014:901437. | ||
Jiang M, Zhou M, Han Y, et al. Identification of NF-κB Inhibitors in Xuebijing injection for sepsis treatment based on bioactivity-integrated UPLC-Q/TOF. J Ethnopharmacol. 2013;147(2):426–433. | ||
Zhang HJ, Cheng YY. An HPLC/MS method for identifying major constituents in the hypocholesterolemic extracts of Chinese medicine formula ‘Xue-Fu-Zhu-Yu decoction’. Biomed Chromatogr. 2006;20(8):821–826. | ||
Zhang Y, Qian D, Pan Y, et al. Comparisons of the pharmacokinetic profile of four bioactive components after oral administration of gan-sui-ban-xia decoction plus-minus gansui and gancao drug combination in normal rats. Molecules. 2015;20(5):9295–9308. | ||
Gonzalez-Sanchez E, Firrincieli D, Housset C, Chignard N. Nuclear receptors in acute and chronic cholestasis. Dig Dis. 2015;33(3):357–366. | ||
Oguz S, Kanter M, Erboga M, Erenoglu C. Protective effects of thymoquinone against cholestatic oxidative stress and hepatic damage after biliary obstruction in rats. J Mol Histol. 2012;43(2):151–159. | ||
Fuentes-Broto L, Martínez-Ballarín E, Miana-Mena J, et al. Lipid and protein oxidation in hepatic homogenates and cell membranes exposed to bile acids. Free Radic Res. 2009;43(11):1080–1089. | ||
Cui YJ, Aleksunes LM, Tanaka Y, Goedken MJ, Klaassen CD. Compensatory induction of liver efflux transporters in response to ANIT-induced liver injury is impaired in FXR-null mice. Toxicol Sci. 2009;110(1):47–60. | ||
Ohta Y, Kongo M, Sasaki E, Harada N. Change in hepatic antioxidant defense system with liver injury development in rats with a single alpha-naphthylisothiocyanate intoxication. Toxicology. 1999;139(3):265–275. | ||
Jin SN, Wen JF, Wang TT, Kang DG, Lee HS, Cho KW. Vasodilatory effects of ethanol extract of Radix Paeoniae Rubra and its mechanism of action in the rat aorta. J Ethnopharmacol. 2012;142(1):188–193. | ||
Lee B, Shin YW, Bae EA, et al. Antiallergic effect of the root of Paeonia lactiflora and its constituents paeoniflorin and paeonol. Arch Pharm Res. 2008;31(4):445–450. | ||
He DY, Dai SM. Anti-inflammatory and immunomodulatory effects of paeonia lactiflora pall: a traditional chinese herbal medicine. Front Pharmacol. 2011;25:2–10. | ||
Dong H, Li R, Yu C, Xu T, Zhang X, Dong M. Paeoniflorin inhibition of 6-hydroxydopamine-induced apoptosis in PC12 cells via suppressing reactive oxygen species-mediated PKCδ/NF-κB pathway. Neuroscience. 2015;285:70–80. | ||
Kim SH, Lee MK, Lee KY, Sung SH, Kim J, Kim YC. Chemical constituents isolated from Paeonia lactiflora roots and their neuroprotective activity against oxidative stress in vitro. J Enzyme Inhib Med Chem. 2009;24(5):1138–1140. | ||
Mitsuyoshi H, Nakashima T, Sumida Y, et al. Ursodeoxycholic acid protects hepatocytes against oxidative injury via induction of antioxidants. Biochem Biophys Res Commun. 1999;263(2):537–542. | ||
Wang T, Zhou ZX, Sun LX, et al. Resveratrol effectively attenuates α-naphthyl-isothiocyanate-induced acute cholestasis and liver injury through choleretic and anti-inflammatory mechanisms. Acta Pharmacol Sin. 2014;35(12):1527–1536. | ||
Okada K, Shoda J, Taguchi K, et al. Nrf2 counteracts cholestatic liver injury via stimulation of hepatic defense systems. Biochem Biophys Res Commun. 2009;389(3):431–436. | ||
Wang G, Xiu P, Li F, Xin C, Li K. Vitamin A supplementation alleviates extrahepatic cholestasis liver injury through Nrf2 activation. Oxid Med Cell Longev. 2014;2014:273692. | ||
Tanaka Y, Aleksunes LM, Cui YJ, Klaassen CD. ANIT-induced intrahepatic cholestasis alters hepatobiliary transporter expression via Nrf2-dependent and independent signaling. Toxicol Sci. 2009;108(2):247–257. | ||
Aleksunes LM, Slitt AL, Maher JM, et al. Nuclear factor-E2-related factor 2 expression in liver is critical for induction of NAD(P)H: quinone oxidoreductase 1 during cholestasis. Cell Stress Chaperones. 2006;11(4):356–363. | ||
Yeh YH, Kuo CT, Chang GJ, et al. Rosuvastatin suppresses atrial tachycardia-induced cellular remodeling via Akt/Nrf2/heme oxygenase-1 pathway. J Mol Cell Cardiol. 2015;82:84–92. | ||
Zhao Y, Ma X, Wang J, et al. Paeoniflorin alleviates liver fibrosis by inhibiting HIF-1α through mTOR-dependent pathway. Fitoterapia. 2014;99:318–327. | ||
Chen M, Cao L, Luo Y, et al. Paeoniflorin protects against concanavalin A-induced hepatitis in mice. Int Immunopharmacol. 2015;24(1):42–49. | ||
Chen X, Liu C, Lu Y, et al. Paeoniflorin regulates macrophage activation in dimethylnitrosamine-induced liver fibrosis in rats. BMC Complement Altern Med. 2012;12:254. | ||
Zhang MH, Feng L, Zhu MM, Gu JF, Wu C, Jia XB. Antioxidative and anti-inflammatory activities of paeoniflorin and oxypaeoniflora on AGEs-induced mesangial cell damage. Planta Med. 2013;79(14):1319–323. |
Supplementary materials
  | Table S1 PLP putative targets |
References
Chen CY. TCM Database@Taiwan: the world's largest traditional Chinese medicine database for drug screening in silico. PLoS One. 2011; 6(1):e15939. | ||
Hao Ye, Li Ye, Hong Kang, et al. HIT: linking herbal active ingredients to targets. Nucleic Acids Res. 2011;39:D1055–D1059. | ||
Kruger FA, Rostom R, Overington JP. Mapping small molecule binding data to structural domains. BMC Bioinformatics. 2012;13:S11. |
 © 2015 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2015 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.