Back to Journals » Clinical Interventions in Aging » Volume 10
Oral treatment with herbal formula B307 alleviates cardiac failure in aging R6/2 mice with Huntington’s disease via suppressing oxidative stress, inflammation, and apoptosis
Authors Lin C , Wang S, Hsu C , Sheu S, Wu C
Received 12 April 2015
Accepted for publication 12 May 2015
Published 20 July 2015 Volume 2015:10 Pages 1173—1187
DOI https://doi.org/10.2147/CIA.S86493
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 4
Editor who approved publication: Dr Zhi-Ying Wu
Video abstract presented by Chung-Hsin Wu.
Views: 167
Ching-Lung Lin,1 Sheue-Er Wang,2 Chih-Hsiang Hsu,1 Shuenn-Jyi Sheu,3 Chung-Hsin Wu1
1Department of Life Science, National Taiwan Normal University, Taipei, 2Department of Pathological Inspection, Soeurs de Saint Paul de Chartres Medical Corporate Body, Taoyuan City, 3Brion Research Institute of Taiwan, New Taipei City, Taiwan
Abstract: Cardiac failure is often observed in aging patients with Huntington’s disease (HD). However, conventional pharmacological treatments for cardiac failure in HD patients have rarely been studied. Chinese herbal medicines, especially combined herbal formulas, have been widely used to treat cardiac dysfunctions over the centuries. Thus, we assess whether oral treatment with herbal formula B307 can alleviate cardiac failure in transgenic mice with HD. After oral B307 or vehicle treatment for 2 weeks, cardiac function and cardiomyocytes in 12-week-old male R6/2 HD mice and their wild-type littermate controls (WT) were examined and then compared via echocardiography, immunohistochemistry, and Western blotting. We found that cardiac performance in aging R6/2 HD mice had significantly deteriorated in comparison with their WT (P<0.01). Cardiac expressions of superoxide dismutase 2 (SOD2) and B-cell lymphoma 2 (Bcl-2) in aging R6/2 HD mice were significantly lower than their WT (P<0.01), but cardiac expressions of tumor necrosis factor alpha (TNF-α), neurotrophin-3 (3-NT), 4-hydroxynonenal (4-HNE), Bcl-2-associated X protein (Bax), calpain, caspase 12, caspase 9, and caspase 3 of aging R6/2 HD mice were significantly higher than their WT (P<0.05). Furthermore, we found that cardiac performance in aging R6/2 HD mice had significantly improved under oral B307 treatment (P<0.05). Cardiac expressions of SOD2 and Bcl-2 of aging R6/2 HD mice were significantly higher under oral B307 treatment (P<0.01), but cardiac expressions of TNF-α, 3-NT, 4-HNE, Bax, calpain, caspase 12, caspase 9, and caspase 3 of aging R6/2 HD mice were significantly reduced under oral B307 treatment (P<0.05). Oral B307 treatment may briefly alleviate cardiac failure in aging HD R6/2 mice via suppressing cardiac oxidative stress, inflammation, and apoptosis. We suggested that the herbal formula B307 may be further developed as a potential health supplement for ameliorating cardiac failure associated with aging.
Keywords: Chinese herbal medicines, cardiomyocytes, echocardiography, aging, transgenic mouse model
Introduction
Chinese herbal medicines, especially combined herbal formulas, have been widely used to treat cardiac dysfunctions over the centuries.1 Many herbal formulas have been shown to relieve cardiovascular diseases by stimulating blood circulation and supplementing vital energy.2 It is possible that Chinese herbs may be used as a potential therapeutic strategy for alleviating cardiac failure. Huntington’s disease (HD) has been considered primarily a neurodegenerative disease, but cardiac failure is also a cause of death in HD patients.3–5 In addition to the brain, mutant huntingtin protein and poly-Q aggregates have also been found in cardiomyocytes of HD patients.6 Huntingtin protein and poly-Q aggregates in cardiomyocytes may cause cardiovascular diseases such as cardiac dilatation, hypertrophy, fibrosis, and heart failure.7 However, the connection of mutant huntingtin protein with the cardiac pathogenesis of HD patients remains obscure. The predicament of conventional pharmacological treatment in HD patients allows us to search for alternative treatments. One such possible alternative therapy for cardiovascular diseases in HD patients is using traditional herbal medicine.
In Taiwan, the Brion Research Institute of Taiwan has developed the herbal formula B307 as a health supplement. This herbal formula is widely used to enhance cardiovascular function. The main herbal ingredients of the herbal formula B307 are Ginseng (extracts from Panax ginseng Radix) and Danshen (extracts from Salvia miltiorrhiza Radix). Ginseng has been a widely used traditional herbal medicine for its multifunctional activities such as anti-excitotoxicity, antioxidation, and anti-inflammatory actions.8–11 As for Danshen, it has been widely used to treat heart disease and ameliorate an atherosclerosis effect in humans and rodents.12–15 Our previous study has shown that subcutaneous microcirculation of R6/2 HD mice was enhanced under oral B307 treatment. Furthermore, cardiac expressions of angiogenesis-related vascular endothelial growth factor and endothelial nitric oxide synthase in R6/2 HD mice were also enhanced under oral B307 treatment.16 It is possible that effects of the herbal formula B307 in ameliorating cardiac failure in R6/2 HD mice may be facilitated through other protective mechanisms.
In this study, R6/2 HD mice served as the HD model for studying cardioprotection with oral B307 treatment in cardiac failure associated with HD. We examined and compared cardiac functions, expressions of inflammation, oxidative stress, and apoptosis-related proteins in the heart tissue between R6/2 HD mice under oral B307 and sham treatments. Our experimental results had demonstrated that the aqueous extract from the herbal formula B307 exhibited protective effects in the heart tissue of R6/2 HD mice. The cardioprotection of B307 is probably mediated by reducing inflammation, oxidative stress, and apoptosis. This study has proven our hypothesis and serves as an example by showing that botanical extracts have great potential in being developed as cardioprotective agents against cardiac failure in HD patients.
Methods
Chromatographic fingerprint analysis of the Chinese herbal formula B307
The herbal formula B307 (supplied by Sun-Ten Pharmaceutical Company, Taipei, Taiwan) contains ingredients such as ginsenosides Rb1 from P. ginseng, rosmarinic acid, salvianolic acid B, tanshinone IIA from Salvia miltiorrhiza, schizandrin and gomisin A from Schisandra chinensis, and methylophiopogonanone B from Liriope spicata. All chemicals were solubilized in distilled H2O/MeOH and then analyzed. The chromatographic fingerprint analysis was done using high-performance liquid chromatography-grade acetonitrile (Burdick & Jackson, Gyeonggi-do, Korea) and methanol (Avantor, Center Valley, PA, USA). Moreover, a Milli-Q water purification system (EMD Millipore, Billerica, MA, USA) provided purified water to dissolve ingredients of the herbal formula B307. The half maximal inhibitory concentration (IC50) of the herbal formula B307 was evaluated by the (3-(4,5-dimethylthiazol-2-yl)-2,5-diphenyltetrazolium bromide) MTT assay.
MTT assay of the herbal formula B307
Human neuroblastoma SH-SY5Y cells were plated in 96-well culture plates at a density of 3.0×104 cells/well for complete attachment at 37°C with 5% CO2 for 24 hours, and then treated with the herbal formula B307 at doses of 5 mg/mL, 10 mg/mL, 20 mg/mL, 30 mg/mL, and 50 mg/mL for 24 hours to determine the IC50 of cytotoxicity. The culture medium was then removed, followed by incubation with 100 mL of MTT solution (0.5 mg/mL) at 37°C for 3 hours, and then, 100 μL of 10% sodium dodecyl sulfate −0.01 N HCl solution was added into each well and incubated at 37°C overnight to dissolve the formazan. The absorbance was measured at 570 nm with an enzyme-linked immunosorbent assay reader (uQuant, BioTek Inc., Winooski, VT, USA), and the results were expressed as the relative cell viability of treated cells against those of the controls.
R6/2 transgenic mouse model of HD
Male R6/2 (B6CBA-Tg(HDexon1)62Gpb/1J; HD exon 1 of the human huntingtin gene with an expanded CAG repeat) mice and their wild-type littermate controls (WT) were bred in an animal facility of National Taiwan Normal University (NTNU). Breeder pairs of R6/2 HD mice were obtained from Jackson Laboratories (Bar Harbor, ME, USA). The R6/2 HD mice and their WT were housed and maintained on a 12-hour:12-hour light–dark cycle with water and food being given ad libitum. The genotyping of R6/2 HD mice was conducted by polymerase chain reaction at 4 weeks of age. All animals in the experiment were maintained according to the international guidelines for care and use of laboratory animals. All animal procedures have been approved by the Committee on Animal Research of NTNU and implemented under the guidelines of the Committee (Protocol number: NTNU Animal Use No 13017/November 26, 2013).
Oral B307 treatments in R6/2 HD mice
Ten-week-old R6/2 HD mice and their WT were orally treated with either B307 extract (30 mg/mL, the pH value was closed to the 7.0) or their vehicle (dimethyl sulfoxide) twice daily for 2 consecutive weeks. We used gavage to orally treat the mice with B307 extract or their vehicle in this study. The dosage of B307 was 50 mg/kg, and the pH value of B307 extract was close to 7.0. All doses were adjusted according to the individual weight of each mouse. The dosage and administration of B307 extract in R6/2 HD mice was much lower than the dosage of IC50. Then, we compared cardiovascular performance, cardiac immunohistochemistry, and Western blotting among R6/2 HD mice and their WT under sham or oral B307 treatment at 12 weeks of age.
Life span, weight (body, heart, and brain), and motor analysis in R6/2 HD mice
Life span and body weight of the R6/2 HD mice and their WT were monitored daily. The heart and brain tissues removed from some of these R6/2 HD mice and their WT were also weighed. R6/2 HD mice euthanasia was judged under the circumstance of moribund, including lack of movement even after prodding and lying on the side with lack of righting reflex. For determining motor function and coordination, a rotarod testing was done in R6/2 HD mice and their WT by using a standard rotarod (Ugo Basile North America Inc., Schwenksville, Pennsylvania, USA). As described previously, mice were acclimatized to the apparatus at the minimum speed of 4 rpm for 20 seconds during each trial.16 Once the mice were acclimatized, the rod was progressively accelerated from 4 rpm to 40 rpm over a duration of 5 minutes. Latency to fall from the rod was recorded and then analyzed.
Cardiovascular performance
Cardiovascular performance was assessed in R6/2 HD mice and their WT by using echocardiography (S-Sharp Corporation, Taipei, Taiwan, ROC). Echocardiography is a routine clinical procedure to diagnose cardiac functions of R6/2 HD mice and their WT. The mice were anesthetized, placed in a sealed box, and exposed to a 2% isoflurane gas (Baxter Healthcare, New Providence, RI, USA) at a flow rate of 2 L/min. Then, the mice were placed on a heated working platform for monitoring electrocardiogram and respiration gating. The anterior and left lateral thoracic regions of the mice were shaved, and US gel (Home Care Technology Co., Ltd, Tainan, Taiwan) was smeared as a coupling agent on their skin. The transducer (PB406; its center frequency and frame rate were 40 MHz and 30 Hz individually) was pasted to get left ventricular M-mode images. From pulsed-wave Doppler images, mitral and aortic blood flow velocities were measured from an apical view. By M-mode images, changes in left ventricular fractional shortening, ejection fraction, stroke volume, cardiac output, maximal mitral valve blood flow velocity, and maximal aortic valve blood flow velocity could be obtained from the R6/2 HD mice with and without oral B307 treatment, and of their WT. At least three stable consecutive cardiac cycles were selected and averaged for each mouse.
Cardiac immunohistochemistry analysis
Anesthetized R6/2 HD mice and their WT were cardiac perfused first with phosphate-buffered saline containing 4% formaldehyde. Then, cardiac tissue was removed and fixed with 4% formaldehyde (EM grade). The cardiac specimens were embedded in paraffin and cut into tissue sections with a thickness of 5 μm. The tissue sections were mounted on slides for histological and immunohistochemical (IHC) analysis. By using the heat-induced epitope retrieval method, the cardiac tissue sections were separately stained at room temperature for 1 hour with antibodies of mutant huntingtin (Cat ab45169, Abcam Inc., Cambridge, MA, USA), tumor necrosis factor alpha (TNF-α) (Cat #11948, Cell Signaling Technology Inc., Danvers, MA, USA), superoxide dismutase 2 (SOD2) (Cat #13141, Cell Signaling Technology Inc.), neurotrophin-3 (3-NT) (Cat ab61392, Abcam Inc.), 4-hydroxynonenal (4-HNE) (Cat ab46545, Abcam Inc.), B-cell lymphoma 2 (Bcl-2) (Cat sc-7382, Santa Cruz Biotechnology Inc., Santa Cruz, CA, USA), Bcl-2-associated X protein (Bax) (Cat #MS-714, Thermo Fisher Scientific Inc., Waltham, MA, USA), cytochrome c (Cyto-C) (Cat #1896-1, Abcam Inc.), calpain (Cat #2539, Cell Signaling Technology Inc.), caspase 12 (Cat #2202, Cell Signaling Technology Inc.), caspase 9 (Cat #9504, Cell Signaling Technology Inc.), and caspase 3 (Cat #9662, Cell Signaling Technology Inc.). As immunostaining controls for each antibody, serial 5 μm cross-sections were treated with the unanimous staining protocol. Immunostaining detection was executed by incubation with biotinylated secondary antibodies (Novolink™ polymer detection system l) at room temperature for 30 minutes, and then by incubation with avidin–biotin–horseradish peroxidase complex (Novolink™ polymer detection system l) for additional 30 minutes. Immunostaining visualization was performed with 3,3′-Diaminobenzidine (DAB) Chromogen (Novolink™ polymer detection system l) and counterstained with hematoxylin (Novolink™ polymer detection system l) following the supplier’s protocol.
Cardiac Western blotting analysis
The removed cardiac tissue was kept in a buffer solution to maintain pH till the preparation of Western blotting analysis. Thus, brain protein was subjected to sodium dodecyl sulfate–polyacrylamide gel electrophoresis, and transferred to a polyvinylidene difluoride membrane. This study used antibodies such as b-actin (Thermo Fisher Scientific Inc.), TNF-α (Cat #11948, Cell Signaling Technology Inc.), SOD2 (Cat #13141, Cell Signaling Technology Inc.), 3-NT (Cat ab61392, Abcam Inc.), 4-HNE (Cat ab46545, Abcam Inc.), Bcl-2 (Cat sc-7382, Santa Cruz Biotechnology Inc.), Bax (Cat #MS-714, Thermo Fisher Scientific Inc.), Cyto-C (Cat #1896-1, Abcam Inc.), calpain (Cat #2539, Cell Signaling Technology Inc.), caspase 12 (Cat #2202, Cell Signaling Technology Inc.), caspase 9 (Cat #9504, Cell Signaling Technology Inc.), and caspase 3 (Cat #9662, Cell Signaling Technology Inc.) to identify expression levels of these proteins in cardiac tissue by means of a horseradish peroxidase-linked secondary antibody. In addition, Enhanced chemiluminescence (ECL) Western blotting detection reagents (GE Healthcare Life Sciences, Piscataway, NJ, USA) were utilized to make immunoreactive bands perceivable. An ImageQuant LAS-4000 biomolecular imager (GE Healthcare Life Sciences) was used to detect the chemiluminescence. Image J software (version 1.48t, Wayne Rasnabd, Washington, DC, USA) was used to perform densitometric assessments of the bands.
Statistical analysis
The data of cardiac performance, subcutaneous microcirculation, and Western blotting analysis were obtained from at least six independent experiments. Values for the data were expressed as the means ± standard error of the mean. Differences among R6/2 HD mice groups with oral B307 and sham treatments and their WT were evaluated by two-way analysis of variance. If a significant F-value was observed, the Student–Newman–Keuls multiple comparisons posttest was performed to determine where differences existed. The P-values of at least <0.05 were considered significant.
Results
Chromatographic fingerprint and IC50 of the herbal formula B307
Chromatographic fingerprint analysis using high-performance liquid chromatography analysis for ingredients of the herbal formula B307 is shown in Figure 1A. Bioactive marker substances for Ginseng Radix were ginsenoside Rb1; marker substances for Schizandrae Fructus were schizandrin and gomisin A; marker substance for Ophiopogonis Tuber were methylophiopogonanone B; marker substances for Salviae Miltiorrhizae Radix were rosmarinic acid, salvianolic acid B, and tanshinone IIA. Figure 1B shows cell viabilities of SH-SY5Y cancer cells under B307 treatment using several dosage levels. Our data had shown that viability of SH-SY5Y cells was not significant under B307 treatment at 5–50 mg/mL (P>0.05). Our results had also shown that the herbal formula B307 caused very little cytotoxicity to SH-SY5Y cells under treatment at a dose of less than 50 mg/mL (IC50 of B307 >50 mg/mL).
Effect of oral B307 treatment on survival, body weight, motor performance, and heart weight of R6/2 HD mice
Life span of R6/2 HD mice with oral B307 and sham treatments and their WT is shown in Figure 2A. Life span of R6/2 HD mice was 14–17 weeks for the sham treatment group, 17–21 weeks for the B307 treatment group, and >22 weeks for the WT mice. Our results had shown that the average survival durations of the R6/2 HD mice were significantly longer under oral B307 treatment. Figure 2B shows the measured body weights of R6/2 HD mice with oral B307 and sham treatments, and of their WT. R6/2 HD mice and their WT under oral B307 and sham treatment exhibited normal growth before 8 weeks of age. At 10 weeks of age, R6/2 HD mice with sham treatment began to lose body weight, but R6/2 HD mice under oral B307 treatment and their WT still exhibited normal growth in their body weight. The average body weight of R6/2 HD mice under sham treatment had been significantly reduced in comparison with their WT from 10 weeks of age and thereafter (P<0.01). Furthermore, R6/2 HD mice under sham treatment exhibited significantly reduced body weight as compared with R6/2 HD mice under oral B307 treatment from 12 weeks of age and thereafter (P<0.05). Furthermore, brain (cerebrum plus cerebellum) weight of R6/2 HD mice was significantly reduced in comparison with their WT from 10 weeks of age and thereafter. However, it had significantly increased under oral B307 from 10 weeks of age and thereafter. Figure 2C shows the effect of oral B307 treatment on motor function of R6/2 HD mice under oral B307 and sham treatments and their WT by rotarod testing. We found that latencies of R6/2 HD mice with sham treatment were significantly reduced in comparison with those of their WT (P<0.01). We also found that latencies of R6/2 HD mice under oral B307 treatment were significantly improved in comparison with the R6/2 HD mice under sham treatment from 10 weeks of age and thereafter (P<0.01). Figure 2D shows the heart weight of R6/2 HD mice under oral B307 and sham treatments, and of their WT. At 10 weeks of age, the R6/2 HD mice under sham treatment began to lose heart weight, but R6/2 HD mice under oral B307 treatment and their WT still exhibited normal heart weight. We found that the average heart weight of the R6/2 HD mice under sham treatment had been significantly lower than those of their WT and those of R6/2 HD mice under oral B307 treatment from 10 weeks of age and thereafter (P<0.05). As shown in Figure 2D, the cardiac expressions of myocarditis and mutant huntingtin aggregation in the R6/2 HD mice under sham treatment were obviously elevated in comparison with those of their WT. Furthermore, the cardiac expressions of myocarditis and mutant huntingtin aggregation in the R6/2 HD mice under oral B307 treatment were weaker than those of the R6/2 HD mice under sham treatment.
Effect of oral B307 treatment on cardiac function of R6/2 HD mice
Figure 3A shows echocardiographic evidence in 12-week-old R6/2 HD mice under sham and oral B307 treatments, and of their WT. By analysis of M-mode images in the hearts of R6/2 HD mice and their WT, it can be seen that the left ventricular fractional shortening, the ejection fraction, the maximal mitral valve and aortic valve blood flow velocity, and the stroke volume of the R6/2 HD mice under sham treatment were significantly lowered in comparison to those of their WT (Figure 3B, HD(sham) vs WT, P<0.01). The echocardiographic evidence of the R6/2 HD mice under oral B307 treatment was significantly enhanced in comparison to those of the R6/2 HD mice under sham treatment (Figure 3B, HD(B307) vs HD(sham), P<0.01). In this study, we also observed that echocardiographic evidence in both R6/2 HD mice under sham and oral B307 treatments had been significantly lowered in comparison to those of their WT (Figure 3B, HD(sham) vs WT, P<0.01; HD(B307) vs WT, P<0.05).
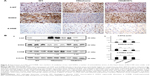
Effect of oral B307 treatment on cardiac oxidative stress of R6/2 HD mice
Effects of oral B307 treatment on cardiac oxidative stress in aging R6/2 HD mice are reported in Figure 4. Figure 4A shows IHC staining of cardiac expressions of 3-NT, SOD2, and 4-HNE in 12-week-old R6/2 HD mice under sham and oral B307 treatments, and of their WT. As observed in the IHC staining, cardiac expressions of 3-NT and 4-HNE in the R6/2 HD mice were more obvious than those of their WT, but expressions of SOD2 in the R6/2 HD mice were weaker than those of their WT. Furthermore, oral B307 treatment reduced the cardiac expressions of 3-NT and 4-HNE but enhanced the cardiac expressions of SOD2 in R6/2 HD mice. By Western blotting analysis, Figure 4B shows that the relative expressions of 3-NT and 4-HNE in the heart tissue of R6/2 HD mice under sham treatment were significantly increased in comparison to those of their WT (Figure 4Bb, HD(sham) vs WT: 3-NT, P<0.01; 4-HNE, P<0.05), yet the relative expressions of 3-NT and 4-HNE in the heart tissue of R6/2 HD mice under oral B307 treatment had been significantly reduced in comparison with those of the R6/2 HD mice under sham treatment (Figure 4Bb, HD(B307) vs HD(sham): 3-NT, P<0.01; 4-HNE, P<0.05). Furthermore, the expression of SOD2 in the heart tissue of R6/2 HD mice under sham treatment was significantly lower than those of their WT (Figure 4Bb, HD(sham) vs WT, P<0.01), but the relative expression of SOD2 in the heart tissue of R6/2 HD mice under oral B307 treatment had been significantly reduced in comparison with those of the R6/2 HD mice under sham treatment (Figure 4Bb, HD(B307) vs HD(sham), P<0.01).
Effect of oral B307 treatment on cardiac inflammation of R6/2 HD mice
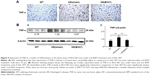
Figure 5A shows IHC staining of cardiac expressions of TNF-α in 12-week-old R6/2 HD mice under sham and oral B307 treatments, and of their WT. As observed in the IHC staining, cardiac expressions of TNF-α in R6/2 HD mice were more obvious than those of their WT. Furthermore, oral B307 treatment reduced the cardiac expressions of TNF-α in R6/2 HD mice. By Western blotting analysis, Figure 5B shows that the relative expression of TNF-α in the heart tissue of R6/2 HD mice under sham treatment was significantly greater in comparison to those of their WT (Figure 5Bb, HD(sham) vs WT, P<0.01), but the relative expression of TNF-α in the heart tissue of R6/2 HD mice under oral B307 treatment had been significantly reduced in comparison to those of the R6/2 HD mice under sham treatment (Figure 5Bb, HD(B307) vs HD(sham), P<0.01).
Effect of oral B307 treatment on cardiac apoptosis of R6/2 HD mice
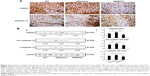
Cardiac apoptosis in aging R6/2 HD mice under oral B307 and sham treatments is compared in Figures 6–8. Figure 6A shows IHC staining of cardiac expressions of Bcl-2, Bax, and Cyto-C in 12-week-old R6/2 HD mice under sham and oral B307 treatments, and of their WT. As observed in the IHC staining, cardiac expressions of Bcl-2 in R6/2 HD mice were weaker in comparison to those of their WT, but expressions of Bax and Cyto-C in R6/2 HD mice were more obvious than those in their WT. Furthermore, oral B307 treatment enhanced the cardiac expressions of Bcl-2, while it reduced the cardiac expressions of Bax and Cyto-C in R6/2 HD mice. By Western blotting analysis, Figure 4B shows that the ratio of Bcl-2/Bax in the heart tissue of R6/2 HD mice was significantly reduced when compared with those of their WT (Figure 6Bb, HD(sham) vs WT, P<0.01), while the ratio of Bcl-2/Bax in the heart tissue of R6/2 HD mice under oral B307 treatment had been significantly reduced in comparison with those of the R6/2 HD mice under sham treatment (Figure 6Bb, HD(B307) vs HD(sham), P<0.01). Furthermore, expression of Cyto-C in the heart tissue of R6/2 HD mice was significantly greater than in their WT (Figure 6Bb, HD(sham) vs WT, P<0.01), while the relative expression of Cyto-C in the heart tissue of R6/2 HD mice under oral B307 treatment had been significantly reduced in comparison with those of the R6/2 HD mice under sham treatment (Figure 6Bb, HD(B307) vs HD(sham), P<0.05).
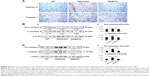
Figure 7A shows IHC staining of cardiac expressions of calpain and caspase 12 in 12-week-old R6/2 HD mice under sham and oral B307 treatments, and of their WT. As observed in the IHC staining, cardiac expressions of calpain and caspase 12 in R6/2 HD mice were more obvious than those of their WT. Furthermore, oral B307 treatment reduced the cardiac expressions of calpain and caspase 12 in R6/2 HD mice. By Western blotting analysis, Figure 7B shows that the relative expression of calpain, pro-caspase 12 (55 kDa), and cleaved (c-) caspase 12 (42 kDa) in the heart tissue of R6/2 HD mice was significantly higher in comparison with those of their WT (Figure 7Bb, HD(sham) vs WT: calpain, P<0.05; pro-caspase 12, P<0.05; c-caspase 12, P<0.05), while the relative expression of calpain, pro-caspase 12, and c-caspase 12 in the heart tissue of R6/2 HD mice under oral B307 treatment was significantly reduced in comparison with those of the R6/2 HD mice under sham treatment (Figure 6Bb, HD(B307) vs HD(sham): calpain, P<0.01; pro-caspase 12, P<0.05; c-caspase 12, P<0.05). Figure 8A shows IHC staining of cardiac expressions of caspase 9 and caspase 3 in 12-week-old R6/2 HD mice under sham and oral B307 treatments, and of their WT. As observed in the IHC staining, cardiac expressions of caspase 9 and caspase 3 in the R6/2 HD mice were more obvious than those of their WT. Furthermore, oral B307 treatment reduced the cardiac expressions of pro-caspase 9, c-caspase 9, pro-caspase 3, and c-caspase 3 in R6/2 HD mice. By Western blotting analysis, Figure 8B shows that the relative expression of pro-caspase 9 (49 kDa) and c-caspase 9 (39 kDa) in the heart tissue of R6/2 HD mice was significantly higher than those of their WT (Figure 8Bb, HD(sham) vs WT: pro-caspase 9, P<0.05; c-caspase 9, P<0.05), while the relative expression of pro-caspase 9 and c-caspase 9 in the heart tissue of R6/2 HD mice under oral B307 treatment was significantly reduced in comparison with those of R6/2 HD mice under sham treatment (Figure 8Bb, HD(B307) vs HD(sham): pro-caspase 9, P<0.05; c-caspase 9, P<0.05). Figure 8C shows that the relative expression of pro-caspase 3 (35 kDa) and c-caspase 3 (17–19 kDa) in the heart tissue of R6/2 HD mice was significantly higher than those of their WT (Figure 8Cb, HD(sham) vs WT: pro-caspase 3, P<0.01; c-caspase 3, P<0.01), while the relative expression of pro-caspase 3 and c-caspase 3 in the heart tissue of R6/2 HD mice under oral B307 treatment was significantly reduced in comparison with those of the R6/2 HD mice under sham treatment (Figure 8Cb, HD(B307) vs HD(sham): pro-caspase 3, P<0.01; c-caspase 3, P<0.01).
Discussion
In this study, clinical implications of our findings show that oral B307 treatment can benefit aging HD mice by promoting increased longevity, improved movement, and weight maintenance (Figure 2). Furthermore, we found that oral B307 treatment may alleviate cardiac failure in aging HD mice via suppressing cardiac oxidative stress, inflammation, and apoptosis. Now the herbal formula B307 has been developed as a potential health supplement for ameliorating cardiac failure in aging patients. However, it is worth mentioning that the herbal formula B307 may have side effects for those patients taking anticoagulants.
It has been suggested that mutant huntingtin aggregation might have significant cardiotoxic effects in the heart tissue of R6/2 HD mice.17 As observed in the heart tissue of R6/2 HD mice, we found that myocarditis and mutant huntingtin aggregation was more prevalent than in their WT, yet it was alleviated under oral B307 treatment (Figure 2D). Our data provided evidence that oral B307 treatment may ameliorate cardiotoxicity and cardiac failure via reducing mutant huntingtin in heart tissue. Furthermore, a review paper suggested that mutant huntingtin arises from altered interactions with mitochondria-related proteins, thereby causing oxidative stress and inflammation.18,19 Oxidative damage and inflammation are believed to be intimately involved in the pathogenesis and progression in the brain of HD patients.19–21 Previous studies have suggested that 3-NT and 4-HNE are two key markers of oxidative stress, while SOD2 is a key marker of antioxidative stress in cardiac mitochondrial injury.22 We observed that expressions of 3-NT and 4-HNE in the heart tissue of R6/2 HD mice were significantly higher than in their WT, yet they were significantly suppressed under oral B307 treatment. Furthermore, expressions of SOD2 in the heart tissue of R6/2 HD mice were significantly weaker than their WT, while they were significantly enhanced under oral B307 treatment (Figure 4). Evidence from our study supported that oxidative damage may have been implicated in the cardiac pathogenesis of the R6/2 HD mice, while oxidative damage in cardiac tissue of the mice can be ameliorated under oral B307 treatment. Inflammation is related to the induction of intracellular stresses such as oxidative stress and mitochondrial endoplasmic reticulum (ER) stress.23 We observed that TNF-α expression in the heart tissue of R6/2 HD mice was significantly higher than in their WT, while being significantly lower when they were treated with oral B307 (Figure 5). TNF-α is a marker protein of inflammation and widely found in inflammatory conditions.24 Thus, evidence from our study supported that inflammation was also involved in cardiac pathogenesis in the R6/2 HD mice, while inflammatory damage in the cardiac tissue of the mice was ameliorated under oral B307 treatment.
It has been described that apoptosis is dominant in the pathogenesis of myocardial dysfunction.25 In addition, mitochondria play a key role in apoptotic signal transduction.26 Bcl-2 and Bax are members of the Bcl-2 family, and they regulate apoptosis by controlling mitochondrial integrity. Bcl-2 acts to inhibit apoptosis, whereas Bax counteracts this effect. The ratio of Bcl-2/Bax indicated the occurrence of apoptosis. Our data reported that the ratio of Bcl-2/Bax in the heart tissue of R6/2 HD mice was significantly lower than in their WT, while it was significantly enhanced under oral B307 treatment (Figure 6B). In mitochondrial dysfunction-related apoptosis, the disruption of the mitochondria leads to the release of Cyto-C into the cytosol. Here, we observed that expressions of Cyto-C in the heart tissue of R6/2 HD mice were significantly higher than in their WT, while they were significantly suppressed under oral B307 treatment (Figure 6). Moreover, ER stress has been reported to play a key role in apoptotic signal transduction.26 In addition, calpain and caspase 12 are two key markers of ER stress-related apoptosis myocardial dysfunction.26 Here, we found that both expressions of calpain and caspase 12 in the heart tissue of R6/2 HD mice were also higher than in their WT, while they were decreased in the R6/2 HD mice under oral B307 treatment (Figure 7). It is well known that caspases 9 and 3 also play key roles in cardiac apoptosis.26 Caspase 9 has been linked to the intrinsic mitochondrial death pathway. The release of Cyto-C from the mitochondria may trigger the activation of pro-caspase 9, and c-caspase 9 can activate the downstream effector caspases, such as caspase 3, which eventually lead to apoptosis. Here, we observed that expressions of pro-caspase 9 and c-caspase 9 in R6/2 HD mice were significantly higher than in their WT, while they were significantly reduced under oral B307 treatment (Figure 8B). Furthermore, pro-caspase 3 and c-caspase 3 (19 kDa) in R6/2 HD mice were significantly elevated in comparison to their WT, but they were significantly reduced under oral B307 treatment (Figure 8C). Based on the above discussion, we suggested that both mitochondrial dysfunction and ER stress-related apoptosis can be involved in cardiac pathogenesis of R6/2 HD mice, while apoptotic damage in the cardiac tissue of R6/2 HD mice can be ameliorated under oral B307 treatment. Furthermore, we observed higher quantified relative expressions of cardiac c-caspase 9 over c-caspase 3. It is possible that more mitochondrial dysfunction apoptosis than ER stress-related apoptosis was involved in cardiac pathogenesis of the R6/2 HD mice.
As summarized in Figure 9, our studies demonstrated that mutant huntingtin aggregation (Figure 2D), oxidative stress (Figure 4), inflammation (Figure 5), mitochondrial dysfunction-related apoptosis (Figures 6 and 8), and ER stress-related apoptosis (Figures 7 and 8) are involved in cardiotoxicity in aging R6/2 HD mice, while oral B307 treatment can attenuate cardiotoxicity in aging R6/2 HD mice via suppressing huntingtin aggregation (Figure 2D), oxidative stress (Figure 4), inflammation (Figure 5), mitochondrial dysfunction-related apoptosis (Figures 6 and 8), and ER stress-related apoptosis (Figures 7 and 8). These results provide evidence for the possible cardioprotective effects of the herbal formula B307 in aging patients.
Acknowledgments
We thank Prof Chiung-Mei Chen (Department of Neurology, College of Medicine, Chang-Gung University, Taoyuan, Taiwan) for providing R6/2 HD mice. We acknowledge Sun-Ten Pharmaceutical Company for the technical assistance. This work was supported by the Industry-University Cooperative Grant of Brion Research Institute of Taiwan, Top University Project of NTNU, and Transnational Research Centers Grant (103T3040B04) from NTNU. The funding agency had no role in the study design, data collection/analysis, and the decision to publish the manuscript.
Disclosure
The authors report no conflicts of interest in this work.
References
Liu Q, Li J, Wang J, Li J, Janicki JS, Fan D. Effects and mechanisms of Chinese herbal medicine in ameliorating myocardial ischemia-reperfusion injury. Evid Based Complement Alternat Med. 2013;2013:925625. | ||
Liang D, Zhang M. The thinking on TCM differential treatment of congestive heart failure. J Tradit Chin Med. 2000;20(1):44–47. | ||
Chiu E, Alexander L. Causes of death in Huntington’s disease. Med J Aust. 1982;1(4):153. | ||
Lanska DJ, Lavine L, Lanska MJ, Schoenberg BS. Huntington’s disease mortality in the United States. Neurology. 1988;38(5):769–772. | ||
Sorensen SA, Fenger K. Causes of death in patients with Huntington’s disease and in unaffected first degree relatives. J Med Genet. 1992;29(12):911–914. | ||
Strong TV, Tagle DA, Valdes JM, et al. Widespread expression of the human and rat Huntington’s disease gene in brain and nonneural tissues. Nat Genet. 1993;5(3):259–265. | ||
Pattison JS, Sanbe A, Maloyan A, Osinska H, Klevitsky R, Robbins J. Cardiomyocyte expression of a polyglutamine preamyloid oligomer causes heart failure. Circulation. 2008;117(21):2743–2751. | ||
Gu B, Nakamichi N, Zhang WS, et al. Possible protection by notoginsenoside R1 against glutamate neurotoxicity mediated by N-methyl-daspartate receptors composed of an NR1/NR2B subunit assembly. J Neurosci Res. 2009;87(9):2145–2156. | ||
Chen XC, Zhu YG, Zhu LA, et al. Ginsenoside Rg1 attenuates dopamine-induced apoptosis in PC12 cells by suppressing oxidative stress. Eur J Pharmacol. 2003;473(1):1–7. | ||
Lee JS, Song JH, Sohn NW, Shin JW. Inhibitory effects of ginsenoside Rb1 on neuroinflammation following systemic lipopolysaccharide treatment in mice. Phytother Res. 2012;27(9):1270–1276. | ||
Lin WM, Zhang YM, Moldzio R, Rausch WD. Ginsenoside Rd attenuates neuroinflammation of dopaminergic cells in culture. J Neural Transm Suppl. 2007;72:105–112. | ||
Xu YY, Wan RZ, Lin YP, Yang L, Chen Y, Liu CX. Recent advance on research and application of Salvia miltiorrhiza. Asian J Pharmacodyn Pharmacokinet. 2007;7(2):99–130. | ||
Sieveking DP, Woo KS, Fung KP, Lundman P, Nakhla S, Celermajer DS. Chinese herbs Danshen and Gegen modulate key early atherogenic events in vitro. Int J Cardiol. 2005;105(1):40–45. | ||
Tam WY, Chook P, Qiao M, et al. The efficacy and tolerability of adjunctive alternative herbal medicine (Salvia miltiorrhiza and Pueraria lobata) on vascular function and structure in coronary patients. J Altern Complement Med. 2009;15(4):415–421. | ||
Wu L, Qiao H, Li Y, Li L. Protective roles of puerarin and Danshensu on acute ischemic myocardial injury in rats. Phytomedicine. 2007;14(10):652–658. | ||
Lin CL, Hsu CH, Wang SE, et al. Cardiac protection of the oral herbal formula B307 in a R6/2 mouse model of Huntington’s disease via angiogenesis. Jokull. 2015;65(2):29–47. | ||
Mihm MJ, Amann DM, Schanbacher BL, Altschuld RA, Bauer JA, Hoyt KR. Cardiac dysfunction in the R6/2 mouse model of Huntington’s disease. Neurobiol Dis. 2007;25(2):297–308. | ||
Crotti A, Benner C, Kerman BE, et al. Mutant Huntingtin promotes autonomous microglia activation via myeloid lineage-determining factors. Nat Neurosci. 2014;17(4):513–521. | ||
Browne SE, Ferrante RJ, Beal MF. Oxidative stress in Huntington’s disease. Brain Pathol. 1999;9(1):147–163. | ||
Calabrese V, Bates TE, Stella AM. NO synthase and NO-dependent signal pathways in brain aging and neurodegenerative disorders: the role oxidant/antioxidant balance. Neurochem Res. 2000;25(9–10):1315–1341. | ||
McCoy MK, Tansey MG. TNF signaling inhibition in the CNS: implications for normal brain function and neurodegenerative disease. J Neuroinflammation. 2008;5:45. | ||
Chaiswing L, Cole MP, St Clair DK, Ittarat W, Szweda LI, Oberley TD. Oxidative damage precedes nitrative damage in adriamycin-induced cardiac mitochondrial injury. Toxicol Pathol. 2004;32(5):536–547. | ||
Cai D, Liu T. Inflammatory cause of metabolic syndrome via brain stress and NF-κB. Aging (Albany NY). 2012;4(2):98–115. | ||
Popa C, Netea MG, van Riel PL, van der Meer JW, Stalenhoef AF. The role of TNF-alpha in chronic inflammatory conditions, intermediary metabolism, and cardiovascular risk. J Lipid Res. 2007;48(4):751–762. | ||
Buja LM. Myocardial ischemia and reperfusion injury. Cardiovasc Pathol. 2005;14(4):170–175. | ||
Machado NG, Alves MG, Carvalho RA, Oliveira PJ. Mitochondrial involvement in cardiac apoptosis during ischemia and reperfusion: can we close the box? Cardiovasc Toxicol. 2009;9(4):211–227. |
 © 2015 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2015 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.