Back to Journals » Drug Design, Development and Therapy » Volume 10
Oncolytic adenovirus expressing interleukin-18 improves antitumor activity of dacarbazine for malignant melanoma
Authors Yang C, Cao H, Liu N, Xu K, Ding M, Mao LJ
Received 16 June 2016
Accepted for publication 3 August 2016
Published 15 November 2016 Volume 2016:10 Pages 3755—3761
DOI https://doi.org/10.2147/DDDT.S115121
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 3
Editor who approved publication: Dr Georgios Panos
Chunhua Yang,1,2 Hang Cao,1 Ning Liu,1 Kai Xu,1 Meng Ding,1 Li-jun Mao1
1Department of Urinary Surgery, The Affiliated Hospital of Xuzhou Medical University, 2Jiangsu Key Laboratory of Biological Cancer Therapy, Xuzhou Medical College, Xuzhou, People’s Republic of China
Abstract: Conditionally replicating adenoviruses have emerged as novel therapeutic agents for cancer. This study aimed to evaluate synergistic antitumor activity of replication-competent adenovirus armed with interleukin (IL)-18 (ZD55-IL-18) and dacarbazine (DTIC) against melanoma. Melanoma A375 cells or nude mouse tumor xenografts were treated with ZD55-IL-18 alone or together with DTIC. The results showed that ZD55-IL-18 competently replicated in A375 cells and expressed IL-18, and these were not affected by DTIC. ZD55-IL-18 enhanced the cytotoxicity of DTIC accompanied by increased apoptosis. Moreover, ZD55-IL-18 and DTIC synergistically inhibited the growth but promoted the apoptosis of A375 xenografts and inhibited vascular endothelial growth factor expression and lung metastasis in xenografts of nude mice. In conclusion, this is the first study to show synergistic anticancer activity of ZD55-IL-18 and DTIC for malignant melanoma. Our results provide evidence that chemo-gene-viro therapeutic approach has greater potential for malignant cancers than conventional chemotherapy or gene therapy.
Keywords: melanoma, IL-18, dacarbazine, oncolytic adenovirus
Introduction
Approximately 76,000 cases of melanoma were diagnosed and 9,700 people died of melanoma in 2014.1 The prognosis of melanoma is very poor, and the median survival is less than 1 year due to the metastasis of the brain, the liver, and the lungs. Dacarbazine (DTIC) and interleukin (IL)-2 are commonly used for the treatment of metastatic melanoma, but they could not improve the median survival of patients.2,3
The administration of the proinflammatory cytokine IL-18 led to melanoma regression in syngeneic mice through the activation of CD4+ T and/or natural killer cell-mediated response.4,5 IL-18 inhibits tumor angiogenesis via the induction of the anti-angiogenic chemokines CXCL9 and CXCL10 and the downregulation of angiogenin expression.6,7 IL-18 also demonstrated potent adjuvant activity in combination with a variety of cell-based or molecularly defined cancer vaccines.8
Conditionally replicating adenoviruses have emerged as novel therapeutic agents for cancer.9 In a previous study, we found that oncolytic adenovirus harboring IL-18 (ZD55-IL-18) showed strong antitumor activity against melanoma.10 In this study, we aimed to evaluate the synergistic antitumor activities of ZD55-IL-18 and DTIC against melanoma. We found that DTIC did not interfere with the replication of ZD55-IL-18 in melanoma, but it augmented antitumor activity of ZD55-IL-18 in xenografts of nude mice via the induction of apoptosis and the inhibition of angiogenesis and metastasis.
Materials and methods
Cell culture and vector
The melanoma cell line A375 was purchased from Shanghai Cell Bank (Shanghai, People’s Republic of China) and grown in Roswell Park Memorial Institute-1640 supplemented with 10% fetal bovine serum (Thermo Fisher Scientific, Waltham, MA, USA), 100 U/mL penicillin, and 100 mg/L streptomycin (Thermo Fisher Scientific) at 37°C in humidified atmosphere with 5% CO2. DTIC was purchased from Nanjing Pharmaceutical Factory (Nanjing, People’s Republic of China). Adenovirus ZD55-IL-18 was described previously.10
Cell viability assay
A375 cells were seeded in 96-well plates and treated with different agents for 4 days. The cell viability was determined by using MTT assay. The experiments were conducted in triplicates.
Western blot analysis
Cells were collected and lysed, and lysates were separated on a 15% sodium dodecyl sulfate-polyacrylamide gel and transferred to nitrocellulose membranes. The membranes were incubated with antibody for Ad2 E1A (Santa Cruz Biotechnology Inc., Dallas, TX, USA) or IL-18 (Abcam, Cambridge, MA, USA) at 4°C overnight. The membranes were then washed and incubated with alkaline phosphatase-conjugated secondary antibodies at room temperature for 1 hour. The membranes were developed using NBT/BCIP color substrate (Promega Corporation, Fitchburg, WI, USA), and the bands were scanned and analyzed using an image analyzer.
Apoptosis assay
Cells were washed with ice-cold phosphate-buffered saline (PBS) and incubated with annexin V-FITC and propidium iodide (BioVision, Milpitas, CA, USA) at room temperature for 10 minutes in the dark. Then, the stained cells were immediately subjected to flow cytometry, and the data were analyzed by Cell Quest software.
Xenograft tumor model
Male BALB/c nude mice aged 4–5 weeks were purchased from Shanghai Experimental Animal Center of Chinese Academy of Sciences. All animal experiments were approved by Animal Use and Care Committee of Xuzhou Medical College and followed the institutes guidelines for animal care and welfare. The xenografts tumor models were established by subcutaneous injection of A375 cells (2×106) into the right flank. When tumors grew to ~100 mm3, the mice were randomly divided into four groups (n=8) and received three consecutive daily intratumoral injection of 10 MOI ZD55-IL-18 plus 100.0 μg/mL DTIC, 10 MOI ZD55-IL-18, 100.0 μg/mL DTIC, and saline control, respectively. Four days after the last injection, tumor samples were dissected from three mice randomly selected from each group. For other five mice in each group, tumor size was measured every week for 6 weeks using the formula: V (mm3) = length × width2/2. All mice were killed at the end of the experiments.
Terminal deoxynucleotidyl transferase dUTP nick end labeling assay
Tumor samples dissected from the mice were fixed in formalin, embedded in paraffin, and cut as sections. Apoptotic cells in the sections were detected by using In Situ Apoptosis Detection Kit based on fluorescein-2′-deoxyuridine-5′-triphosphate (dUTP; Hoffman-La Roche Ltd., Basel, Switzerland) following the manufacturer’s protocols.
Immunohistochemical staining
Tumor sections were deparaffinized, and endogenous peroxidase was blocked after exposure to 3% H2O2. Sections were preincubated with goat serum and then incubated with appropriate primary and secondary antibodies. Finally, the sections were exposed to diaminobenzidine, counterstained with hematoxylin, and observed under microscope. For negative control, PBS was used instead of primary antibody. Under microscopy, six fields were randomly selected from every sample, and relative vascular endothelial growth factor (VEGF) expression was calculated as (staining intensity of treated samples/control samples) ×100%.
Statistical analysis
Values are presented as mean ± standard deviation and analyzed by one-way analysis of the variance followed by Duncan or Newman–Keuls test. P-value <0.05 was considered to be significant.
Results
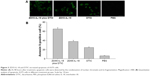
Combined treatment with ZD55-IL-18 and DTIC decreased the viability of A375 cells
The survival rate of A375 cells after 4 days of treatment with 10 MOI ZD55-IL-18 plus 100.0 μg/mL DTIC (29.9%±2.2%) was significantly lower than the 10 MOI ZD55-IL-18 group (40.6%±2.01%) or the 100.0 μg/mL DTIC group (63.3%±4.23%) (P<0.01) (Figure 1). Thus, ZD55-IL-18 increased the sensitivity of A375 cells to DTIC.
DTIC had no effects on E1A and IL-18 expression in A375 cells
By Western blot analysis, we found that ZD55-IL-18 infection led to higher protein levels of IL-18 and E1A in A375 cells, but DTIC had no significant effects on IL-18 and E1A protein levels. A375 cells treated with PBS or DTIC exhibited no E1A expression and little endogenous IL-18 expression (Figure 2).
ZD55-IL-18 and DTIC increased the apoptosis of A375 cells
Flow cytometry analysis showed that annexin V-positive cells in the ZD55- IL-18 plus DTIC group was 65.10%±2.96%, significantly higher than in the ZD55-IL-18 group (37.60%±2.65%), DTIC group (24.63%±1.33%), and PBS group (6.43%±0.60%) (P<0.01) (Figure 3).
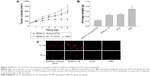
Synergistic antitumor effect of ZD55-IL-18 and DTIC in melanoma xenografts
Next, we evaluated the antitumor effects of ZD55-IL-18 and/or DTIC treatment in mouse A375 tumor xenografts. In the PBS control group, tumors grew rapidly with a mean tumor size of 792.3±92.3 mm3. By sharp contrast, the mean tumor size in the ZD55-IL-18 plus DTIC group was 279.9±43.2 mm3, significantly smaller than in the ZD55-IL-18 group (558.9±72.4 mm3) and the DTIC group (589.4±81.4 mm3) (P<0.05, Figure 4A). In addition, tumor weight was only 0.232±0.0312 mg in the ZD55-IL-18 plus DTIC group, significantly less than in the ZD55-IL-18 group (0.451±0.0421 mg), the DTIC group (0.476±0.0442 mg), and the PBS group (0.696±0.0912 mg) (P<0.05, Figure 4B).
Furthermore, we detected the expression of IL-18 and E1A in xenografts. Compared to the PBS or DTIC group, IL-18 staining was much stronger in the ZD55-IL-18 plus DTIC or ZD55-IL-18 group. Notably, E1A staining was only observed in the ZD55-IL-18 plus DTIC and ZD55-IL-18 groups but not in the PBS or DTIC group (Figure 4C).
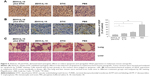
ZD55-IL-18 and DTIC induced apoptosis and upregulated VEGF expression in melanoma xenografts
Then, we performed terminal deoxynucleotidyl transferase dUTP nick end labeling assay of melanoma xenografts to confirm that ZD55-IL-18 and DTIC induced the apoptosis of melanoma cells in vivo. The apoptosis was significantly increased in the ZD55-IL-18 plus DTIC group (67.4%±6.2%) compared to the control groups with PBS (7.3%±0.9%), ZD55-IL-18 (44.9%±5.4%), or DTIC (29.4%±2.4%) (Figure 5A).
In addition, we performed immunohistochemical analysis of VEGF in melanoma xenografts to assess the effects of ZD55-IL-18 and DTIC on tumor angiogenesis. We found that VEGF staining was significantly weaker in the ZD55-IL-18 plus DTIC group (15.1±1.4) than in the ZD55-IL-18 group (24.8±2.5), the DTIC group (36.9±3.3), and the PBS group (Figure 5B).
Finally, we performed histological analysis of the lung and liver tissues from nude mice. Hematoxylin and eosin staining showed that tumor metastasis was found in the lungs of six nude mice in the PBS group, two mice in the ZD55-IL-18 group, one mouse in the DTIC group, and none in the ZD55-IL-18 plus DTIC group (Figure 5C). We found no abnormal liver morphology in any of the groups. These data indicate that ZD55-IL-18 plus DTIC inhibits distant metastasis of melanoma but do not harm liver function.
Discussion
Malignant melanoma is one of the most common tumors and its incidence is increasing worldwide.11 According to data from the National Cancer Database, 91% of melanomas are cutaneous, 5.3% are localized in the eyes, 1.3% are mucosal melanomas, and 2.2% are melanomas of unknown origin.12 Classical treatment for melanoma includes surgery, chemotherapy, and immunotherapy, but the efficacy is very limited. Currently, DTIC is the only agent approved by the US Food and Drug Administration for melanoma ad has shown some promising outcomes for advanced melanoma.13–15 Nevertheless, the response rate to DTIC is very low, ranging from 10% to 25%, and clinical trials have shown that combination regimens achieved no better outcomes than DTIC monotherapy in patients with advanced melanoma.16 However, in the present study, we combined ZD55-IL-18 gene virotherapy with DTIC chemotherapy to show that combination regimens exhibited superior antitumor activity for melanoma both in vitro and in vivo, compared to the use of these agents alone.
In this study, we found that DTIC did not interfere with the expression of IL-18 and the replication of ZD55 virus in melanoma A375 cells. Moreover, the cytotoxicity of ZD55-IL-18 plus DTIC was greater than ZD55-IL-18 or DTIC alone. Flow cytometry analysis demonstrated that the increased cytotoxicity of ZD55-IL-18 plus DTIC for melanoma cells was at least partially due to the induction of apoptosis. Terminal deoxynucleotidyl transferase dUTP nick end labeling staining of tumor sections further confirmed more apoptotic cells in the ZD55-IL-18 plus DTIC group than in the control treatment group.
Advanced melanoma is characterized by aggressive angiogenesis and hypervascularity.17,18 VEGF is an important proangiogenesis factor in melanoma.19,20 By contrast, IL-18 showed anti-angiogenic activity and inhibited melanoma angiogenesis.21 Consistent with these previous studies, here we showed that ZD55-IL-18 plus DTIC treatment led to significantly decreased VEGF expression in melanoma xenografts compared to treatment with ZD55-IL-18 or DTIC alone. Furthermore, distant metastasis of melanoma to the lungs was significantly inhibited after treatment with ZD55-IL-18 plus DTIC compared to treatment with ZD55-IL-18 or DTIC alone.
To the best of our knowledge, for the first time herein we reported synergistic anticancer activity of ZD55-IL-18 plus DTIC for human malignant melanoma. Our results provide evidence that chemo-gene-viro therapeutic approach has greater potential for malignant cancers than conventional chemotherapy or gene therapy.
Acknowledgment
This study was financially supported by the grant from Jiangsu Province Science Foundation of China (No BK2007032).
Disclosure
The authors report no conflicts of interest in this work.
References
Siegel R, Ma J, Zou Z, Jemal A. Cancer statistics, 2014. CA Cancer J Clin. 2014;64(1):9–29. | ||
Tsao H, Atkins MB, Sober AJ. Management of cutaneous melanoma. N Engl J Med. 2004;351(10):998–1012. | ||
Middleton MR, Grob JJ, Aaronson N, et al. Randomized phase III study of temozolomide versus dacarbazine in the treatment of patients with advanced metastatic malignant melanoma. J Clin Oncol. 2000;18(1):158–166. | ||
Nishio S, Yamada N, Ohyama H, et al. Enhanced suppression of pulmonary metastasis of malignant melanoma cells by combined administration of alpha-galactosylceramide and interleukin-18. Cancer Sci. 2008;99:113–120. | ||
Osaki T, Peron JM, Cai Q, et al. IFN-gamma-inducing factor/IL-18 administration mediates IFN-gamma- and IL-12-independent antitumor effects. J Immunol. 1998;160:1742–1749. | ||
Coughlin CM, Salhany KE, Wysocka M, et al. Interleukin-12 and interleukin-18 synergistically induce murine tumor regression which involves inhibition of angiogenesis. J Clin Invest. 1998;101(6):1441–1452. | ||
Nagai H, Hara I, Horikawa T, Oka M, Kamidono S, Ichihashi M. Gene transfer of secreted-type modified interleukin-18 gene to B16F10 melanoma cells suppresses in vivo tumor growth through inhibition of tumor vessel formation. J Invest Dermatol. 2000;119(3):541–548. | ||
Tian H, Shi G, Yang G, et al. Cellular immunotherapy using irradiated lung cancer cell vaccine co-expressing GM-CSF and IL-18 can induce significant antitumor effects. BMC Cancer. 2014;14:48. | ||
Dorer DE, Holtrup F, Fellenberg K, et al. Replication and virus induced transcriptome of HAdV-5 in normal host cells versus cancer cells: differences of relevance for adenoviral oncolysis. PLoS ONE. 2011;6(11):e27934. | ||
Zheng JN, Pei DS, Mao LJ, et al. Oncolytic adenovirus expressing interleukin-18 induces significant antitumor effects against melanoma in mice through inhibition of angiogenesis. Cancer Gene Ther. 2010;17(1):28–36. | ||
Mayer JE, Swetter SM, Fu T, Geller AC. Screening, early detection, education, and trends for melanoma: current status (2007–2013) and future directions: part I. Epidemiology, high-risk groups, clinical strategies, and diagnostic technology. J Am Acad Dermatol. 2014;71(4):599.e1–599.e12. | ||
Chang AE, Karnell LH, Menck HR. The National Cancer Data Base report on cutaneous and noncutaneous melanoma: a summary of 84,836 cases from the past decade. The American College of Surgeons Commission on Cancer and the American Cancer Society. Cancer. 1998;83(8):1664–1678. | ||
Quirin C, Mainka A, Hesse A, Nettelbeck DM. Combining adenoviral oncolysis with temozolomide improves cell killing of melanoma cells. Int J Cancer. 2007;121(12):2801–2807. | ||
Lillehammer T, Engesaeter BO, Prasmickaite L, Maelandsmo GM, Fodstad O, Engebraaten O. Combined treatment with Ad-hTRAIL and DTIC or SAHA is associated with increased mitochondrial-mediated apoptosis in human melanoma cell lines. J Gene Med. 2007;9(6):440–451. | ||
Loo TL, Housholder GE, Gerulath AH, Saunders PH, Farquhar D. Mechanism of action and pharmacology studies with DTIC (NSC-45388). Cancer Treat Rep. 1976;60(2):149–152. | ||
Eggermont AM, Kirkwood JM. Re-evaluating the role of dacarbazine in metastatic melanoma: what have we learned in 30 years? Eur J Cancer. 2004;40(12):1825–1836. | ||
Redondo P, Sánchez-Carpintero I, Bauzá A, Idoate M, Solano T, Mihm MC Jr. Immunologic escape and angiogenesis in human malignant melanoma. J Am Acad Dermatol. 2003;49(2):255–263. | ||
Folkman J. What is the evidence that tumors are angiogenesis dependent? J Natl Cancer Inst. 1990;82(1):4–6. | ||
Rofstad EK, Halsør EF. Vascular endothelial growth factor, interleukin 8, platelet-derived endothelial cell growth factor, and basic fibroblast growth factor promote angiogenesis and metastasis in human melanoma xenografts. Cancer Res. 2000;60(17):4932–4938. | ||
Drevs J, Hofmann I, Hugenschmidt H, et al. Effects of PTK787/ZK 222584, a specific inhibitor of vascular endothelial growth factor receptor tyrosine kinases, on primary tumor, metastasis, vessel density, and blood flow in a murine renal cell carcinoma model. Cancer Res. 2000;60(17):4819–4824. | ||
Cao R, Farnebo J, Kurimoto M, Cao Y. Interleukin-18 acts as an angiogenesis and tumor suppressor. FASEB J. 1999;13(15):2195–2202. |
 © 2016 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2016 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.