Back to Journals » Clinical Interventions in Aging » Volume 10
Obinutuzumab treatment in the elderly patient with chronic lymphocytic leukemia
Authors Seiter K, Mamorska-Dyga A
Received 16 April 2015
Accepted for publication 9 May 2015
Published 12 June 2015 Volume 2015:10 Pages 951—961
DOI https://doi.org/10.2147/CIA.S69278
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 4
Editor who approved publication: Dr Richard Walker
Karen Seiter, Aleksandra Mamorska-Dyga
Department of Medicine, Division of Hematology/Oncology, New York Medical College, Valhalla, NY, USA
Abstract: Chronic lymphocytic leukemia (CLL) is the most common leukemia in adults in Western countries. Fludarabine-based regimens demonstrate higher response rates in younger patients but have a significant risk of infection and are thus poorly tolerated by older, frail patients. Anti-CD20 monoclonal antibodies have added to the efficacy of chemotherapy in CLL. Obinutuzumab is a potent Type II anti-CD20 monoclonal antibody with enhanced antibody-dependent cellular toxicity and direct cell death compared with rituximab. In Phase I studies, infusion reactions and neutropenia were the predominant toxicities. Phase II studies demonstrated efficacy both as a single agent and in combination with chemotherapy in patients with CLL. The CLL11 trial was a Phase III randomized trial of chlorambucil alone or with either obinutuzumab or rituximab in elderly, unfit patients. Progression-free survival (the primary end point) was 26.7 months for patients receiving obinutuzumab plus chlorambucil versus 16.3 months for those receiving rituximab plus chlorambucil and 11.1 months for those receiving chlorambucil alone (P<0.001). Overall survival was improved for patients receiving obinutuzumab plus chlorambucil versus chlorambucil alone (P=0.002). This trial led to the US Food and Drug Administration (FDA) approval of obinutuzumab in this patient population.
Keywords: chronic lymphocytic leukemia, obinutuzumab, chlorambucil, elderly
Introduction
Therapeutic targeting of the B-lymphocyte surface antigen CD20 has revolutionized the management of B-cell malignancies. Rituximab, the first anti-CD20 monoclonal antibody, was approved by the US Food and Drug Administration (FDA) in 1997. With the incorporation of rituximab into a number of chemotherapeutic regimens, response rates, progression-free survival (PFS), and overall survival rates have improved significantly in a range of B-cell malignancies. However, some patients fail to respond to rituximab upfront, and more commonly, others become resistant to ongoing therapy with this monoclonal antibody. This has led to the development of a newer generation of antibodies targeting CD20.
Anti-CD20 monoclonal antibodies
The CD20 antigen is a transmembrane phosphoprotein expressed on B-cells in all stages of their development from the pre-B-cell, and is present on most B-cell-derived neoplastic cells.1,2 The biological function of CD20 is not clear, and there is no known ligand of the phosphoprotein.3 There are several mechanisms by which targeting of CD20 results in cell death. Complement-dependent cytotoxicity (CDC) occurs after antibody binding shifts CD20 into lipid rafts (large transmembrane domains), which then activate the membrane complement cascade and membrane attack complex.4 Cell death occurs due to the resultant pores in the cell membrane. Antibody-dependent cellular cytotoxicity (ADCC) occurs when the Fc portion of the therapeutic antibody interacts with the Fc receptor on natural killer (NK) cells and macrophages, triggering activation of these cells.5 Direct cell death (DCD) is mediated by the lysosome-dependent homotypic adhesion pathway that, after linking of two cells via adhesion molecules or by cross-linking of anti-CD20 monoclonal antibodies, leads to activation of intracellular kinases.6 Finally, a vaccination effect can occur after presentation of CD20 to T-cells induces a long-term cellular response.7
Anti-CD20 monoclonal antibodies are designated as Type I or Type II based on their binding to CD20 and on the mechanisms of cell death that they activate (Table 1).8–10 Type I antibodies induce potent complement activation following redistribution of CD20 into membrane rafts. Type II antibodies are less effective at triggering the complement cascade but strongly evoke direct programmed cell death. Both Type I and Type II antibodies activate immune effector cells via interaction with Fc receptors (Figure 1).
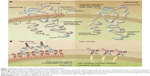  | Figure 1 Binding of Type I and Type II anti-CD20 monoclonal antibodies. |
  | Table 1 Mechanisms of cell death by anti-CD20 monoclonal antibodies |
Rituximab is a Type I, first-generation chimeric anti-CD20 antibody. Rituximab demonstrates specific binding between the Fab region of the antibody and the target antigen CD20 as well as nonspecific binding between the antibody Fc region and Fc receptors, leading to activation of immune cells, ADCC, and phagocytosis.11 Ofatumumab is a second-generation Type I human IgG1 antibody that binds to CD20 at a different epitope than rituximab. Ofatumumab demonstrates a higher CDC effectiveness but a similar ADCC response to rituximab.12
Obinutuzumab, a third-generation humanized IgG1 antibody, is the first glycoengineered Type II anti-CD20 monoclonal antibody.13 Glycoengineering results in decreased fucosylation of the Fc region of the antibody, which significantly enhances its affinity for the Fc receptor on effector cells, including NK cells and macrophages.14–16 Glycoengineering also alters antibody binding and prevents segregation of the CD20 molecule complexes into lipid rafts.17 Instead, Type II antibodies activate lysosomal-dependent apoptosis through homotypic adhesion, which leads to increased DCD rather than ADCC.18,19 Decreased fucosylation also results in an increased ability of obinutuzumab to activate polymorphonuclear neutrophils and to induce phagocytosis through binding to subtypes of Fc receptors more efficiently than Type I monoclonal anti-CD20 antibodies.20 The end result is that obinutuzumab has significantly enhanced ADCC activity, decreased CDC, and superior direct killing compared to Type I antibodies.21
Preclinical studies
Several in vitro studies demonstrated the increased potency of obinutuzumab over other anti-CD20 monoclonal antibodies. Reslan et al studied anti-CD20 antibody-induced cell death in 32 freshly isolated chronic lymphocytic leukemia (CLL) samples.22 Obinutuzumab significantly increased apoptotic cell death compared to the degree of spontaneous apoptosis. Induction of apoptosis by obinutuzumab was accompanied by loss of mitochondrial membrane potential as well as activation and increased expression of the pro-apoptosis BCL2 family members Bax and Bak. This effect was not seen with rituximab indicating that obinutuzumab has a greater ability to induce programmed cell death than rituximab and that obinutuzumab was able to induce programmed cell death and eliminate malignant B-cells without a requirement for immune effector cells. In lymphoma xenograft models, Herting et al demonstrated the dose-dependent efficacy of obinutuzumab and its superiority over rituximab as a single agent and in combination with several chemotherapeutic agents.23 In other studies, obinutuzumab demonstrated superior depletion of normal B-cells (measure as CD19+ depletion) from the blood of healthy volunteers as well as of malignant B-cells from the blood of patients with B-CLL compared with rituximab.13
Chronic lymphocytic leukemia
CLL is the most common leukemia in adults in Western countries. In 2015, an estimated 14,620 people in the United States will be diagnosed with CLL and approximately 4,650 will die from the disease.24 The median age at diagnosis is 72 years with 70% of the cases presenting above the age of 65 years. The diagnosis requires the presence of at least 5×109/L monoclonal B-cells in the peripheral blood with a typical immunophenotype of CD5+, CD10−, CD19−, CD20 (dim), surface immunoglobulin (dim), CD23+, CD43+/−, and cyclin D1−. Prognostic factors include stage of the disease (based on presence or absence of lymphadenopathy, hepatosplenomegaly, anemia and/or thrombocytopenia), β2-microglobulin levels, IgVH mutation status, CD38 expression, and cytogenetic and fluorescence in situ hybridization studies (Table 2).25 Patients with a deletion of chromosome 17p and/or TP53 mutation have a particularly poor prognosis with a typical survival of only a few years. Most of other CLL patients can live for many years. Wierda et al categorized patients into low-, intermediate-, and high-risk groups (Table 3). The median survival had not been reached for the low-risk group and was 10 years and 5 years, respectively, for the intermediate- and high-risk groups.26
  | Table 2 CLL prognostic scoring system |
  | Table 3 CLL adverse prognostic features |
Chemotherapy of CLL
A variety of treatment options are available for patients with CLL. The first decision is whether the patient requires therapy or not. Indications for treatment include progressive and/or symptomatic lymphadenopathy, hepatosplenomegaly, anemia or thrombocytopenia, or systemic symptoms such as fatigue, night sweats, and/or weight loss. Cytogenetic risk group (especially presence or absence of the TP53 mutation), age, and comorbidities are the most important factors when choosing therapy for a particular patient.
Chlorambucil monotherapy
Chlorambucil has been a mainstay of therapy in CLL for more than 40 years. Many consider it to be the standard treatment for elderly, unfit patients. Chlorambucil is a bifunctional alkylating agent of the nitrogen mustard type that cross-links DNA, thus preventing replication and inducing apoptosis. Chlorambucil was first considered a potential treatment for CLL when early work demonstrated that lymphopenia was a prominent toxicity of the drug. In 1956, Ultmann et al administered chlorambucil to 30 patients with various lymphoid malignancies, 18 of whom had CLL. Chlorambucil was given at a dose of 0.1–0.2 mg/kg with a typical course lasting 5–7 weeks.27 Responses were based on changes in physical examination and CBC and were classified as excellent in three patients, good in eight, and slight in nine.
Subsequent trials compared chlorambucil with other alkylating-based multidrug chemotherapy regimens in patients with CLL. In a randomized trial comparing chlorambucil plus prednisone versus cyclophosphamide, melphalan, and prednisone in patients with a median age of 63 years, the overall response rate was 75% for patients receiving chlorambucil and prednisone compared to 54.5% for patients receiving cyclophosphamide, melphalan, and prednisone (P=0.054).28 Complete responses (CRs) were seen in 27% and 12.5% of patients, respectively. In a study of CHOP versus prednisolone plus chlorambucil in patients less than 76 years of age and without comorbidities, patients treated with CHOP had a higher CR rate (63% versus 29%, P<0.005); however, no difference in survival was demonstrated between the two regimens.29 The ECOG compared chlorambucil and prednisone versus cyclophosphamide, vincristine, and prednisone as initial treatment for CLL.30 After a median follow-up of 7 years, there were no significant differences in survival (4.8 years versus 3.9 years, P=0.12), complete remission rate (25% versus 23%, P=0.83), or duration of response (2.0 years versus 1.9 years, P=0.78) between chlorambucil plus prednisone and cyclophosphamide, vincristine, and prednisone.
Fludarabine and bendamustine
Chlorambucil usage declined after studies in 1988 reported that the purine analog fludarabine was highly active in patients with CLL (Table 4). In an early trial of fludarabine as a single agent in previously treated patients, 11 of 33 patients (33%) obtained a complete remission, 13 (39%) a nodular partial remission, and two (6%) a partial response (PR) for an overall response rate of 79%.31 The major morbidity was infection with febrile episodes in 13% of the courses. Fludarabine activity was enhanced by the addition of rituximab. In the CALGB 9712 trial, the overall response rate was 90% (47% CR) for previously untreated patients receiving concurrent fludarabine and rituximab compared with 77% (28% CR) for patients receiving sequential fludarabine and rituximab.32 Patients receiving the concurrent regimen experienced more grade 3 or 4 neutropenia (74% versus 41%) and grade 3 or 4 infusion-related toxicity (20% versus 0%) as compared with the sequential arm.
Response rates increased further with the addition of cyclophosphamide (fludarabine, cyclophosphamide, rituximab [FCR]). In a trial of FCR as initial therapy in 224 patients with progressive or advanced CLL, the CR rate was 70%, the nodular partial remission rate was 10%, and the partial remission rate was 15%, for an overall response rate of 95%.33 Grade 3–4 neutropenia occurred during 52% of courses. With longer follow-up, the 6-year overall survival was 77% and the median time to progression was 80 months.34 The median age was only 58 years in this trial, and age greater than 70 years was associated with a lower CR rate (odds ratio 2.8, P=0.02) and a shortened survival (hazard ratio 0.35, P=0.001). Thus, the improved results seen with this regimen did not apply to the elderly patient population.
Further evidence that fludarabine-based regimens are most beneficial for younger patients without comorbidities comes from the German CLL 5 study.35 Here 193 previously untreated patients with a median age of 70 years received fludarabine (25 mg/m2 for 5 days) or chlorambucil (0.4 mg/kg body weight with an increase to 0.8 mg/kg, every 15 days, for 12 months). Although fludarabine resulted in a significantly higher overall response (72% versus 51%, P=0.003) and CR rate (7% versus 0%, P=0.011), there was no difference in PFS or overall survival of patients treated with fludarabine compared with chlorambucil.
Bendamustine has also been compared directly with chlorambucil. In a trial of patients less than 75 years of age (mean age 63 years) with a good performance status and absence of significant organ dysfunction, the CR rate (21.0% versus 10.8%), median PFS (21.28 months versus 8.8 months, P<0.0001, hazard ratio 2.3) and time-to-next treatment (31 months versus 10.1 months, P<0.0001) were all improved for bendamustine over chlorambucil.36 However, the overall survival rate was not different between the groups, even for younger patients.
Effect of comorbidities
Recently, a number of a scoring systems have been devised in order to predict a patient’s tolerability of treatment. A commonly used system is the Cumulative Illness Rating Scale (CIRS).37 This system looks at many organ systems and assigns points depending on whether the degree of impairment is none, mild, moderate, severe, or extremely severe (Table 5). Patients with higher scores are more physically compromised and are more likely to have poor tolerance of intensive therapies.
Goede et al investigated the impact of comorbidities on treatment outcomes in 555 patients with previously untreated CLL enrolled in two trials of the German CLL study group (CLL4 and CLL5).38 Patients received fludarabine plus cyclophosphamide, fludarabine, or chlorambucil. Overall survival was shorter in patients with two or more comorbidities compared with patients with less than two comorbidities (71.7 months versus 90.2 months, respectively, P<0.001). Differences in survival were significant in both younger (CLL4) and older (CLL5) patients. There was also a difference in PFS (21.0 months versus 31.5 months, P<0.01) of patients with more comorbidities. After adjustment of other prognostic factors and treatment, the presence of comorbidities maintained its independent prognostic value.
Therapy of younger, fit patients
Currently, younger patients without significant comorbidities are most commonly treated with chemoimmunotherapy with either FCR or bendamustine and rituximab (BR). Patients with deletion of chromosome 17p have an inferior response to these regimens, and the optimum treatment for this subgroup remains to be determined. The CLL10 trial was an international Phase III study that evaluated the efficacy and tolerance of BR in comparison to FCR in the frontline treatment of fit patients without deletion of chromosome 17p.39 Five hundred sixty-four patients with a CIRS score less than or equal to 6, creatinine clearance greater than 70 mL/min, and without deletion of chromosome17p were randomized to receive six courses of FCR or BR. The median CIRS score was 2, and the median age was 61.6 years. The overall response rate was 97.8% in both arms with a CR rate of 40.7% for FCR versus 31.5% for BR (P=0.026). The median PFS was 53.7 months in the FCR arm and 43.2 months in the BR arm (P=0.001). At the time of the initial report, there was no difference in the overall survival; however, the median follow-up was short. Severe neutropenia was more common with FCR, as were severe infections (39.8% versus 25.4%, P=0.0010). Although this study demonstrates that both FCR and BR are highly active regimens, significant toxicity occurs even in these younger fit patients, making both regimens difficult to administer to the older, unfit patient population.
Obinutuzumab Phase I/II studies
Owing to the activity of rituximab in B-cell malignances and the increased potency of obinutuzumab in preclinical studies, obinutuzumab is being studied in a range of B-cell malignancies. Phase I studies demonstrated the safety and efficacy of obinutuzumab. In an early study, Sehn et al administered obinutuzumab as an induction followed by 2 years of maintenance to patients with relapsed CD20 positive B-cell malignancies.40 Twenty-two patients received obinutuzumab in cohorts of 200–2,000 mg IV weekly for 4 weeks. Patients with a complete or PR or stable disease and clinical benefit continued to receive obinutuzumab every 3 months for a maximum of eight doses. The median number of prior regimens was 4, and 86% of patients had prior rituximab. Infusion reactions occurred in 73% of patients and were grade 3/4 in 18%. Other adverse events included infections (32%), pyrexia (23%), and neutropenia (23%). At the end of induction, 23% had achieved a PR and 54% had stable disease. Eight patients received maintenance. In the second Phase I study in patients with heavily pretreated, relapsed, or refractory CD20 positive indolent lymphoma, obinutuzumab was given in dose-escalating cohorts with the drug given on days 1 and 8 of the first cycle and on day 1 of subsequent cycles for a total of nine infusions.41 Patients received a 50% dose reduction on the first infusion. Doses ranged from 50/100 to 1,200/2,000. The median age was 64 years (range: 39–83), and the median number of prior treatments was 5. Infusion-related reactions were the most common adverse event, occurring in 18 of 21 patients. The majority (98%) were grade 1 or 2. Symptoms included hypotension, pyrexia, nausea, vomiting, chills, asthenia, flushing, headache, and larynx irritation. The response rate at the end of treatment was 33% with four CRs and three PRs.
The GAUGUIN study evaluated obinutuzumab in cohorts of patients with relapsed, refractory B-cell malignancies, including patients with relapsed/refractory indolent non-Hodgkin lymphoma,42 patients with diffuse large B-cell lymphoma or mantle cell lymphoma,43 and patients with CLL.44 In the cohort with indolent non-Hodgkin lymphoma, patients were randomly assigned to receive obinutuzumab at a flat dose of 400 mg on days 1 and 8 of cycle 1 and on day 1 of cycles 2–8 (400/400 mg) or a dose of 1,600 mg on days 1 and 8 of cycle 1 and 800 mg on day 1 of cycles 2–8 (1,600/800 mg). Forty patients were enrolled. Diagnoses included follicular lymphoma in 34 and other indolent lymphomas in six. The median age was 61 years (42–79). In all, 38 of 40 patients had received prior rituximab and 22 of 40 patients were rituximab refractory. The overall response rate at the end of treatment was 55% for patients receiving 1,600/800 mg and 17% for patients received 400/400 mg. Median PFS was 11.9 months in the 1,600/800 mg group and 6.0 months in the 400/400 mg group. Infusion reactions were seen in 73% of patients but were only grade 3/4 in two patients (both of whom were treated at the higher dose). In the diffuse large B-cell study, patients were randomized to the same two dosage arms. A total of 40 patients were accrued, 25 with diffuse large B-cell lymphoma and 15 with mantle cell lymphoma. The end of treatment response was 30% for patients with diffuse large B-cell lymphoma in the 400/400 mg arm and 27% for the 1,600/800 mg arm. The end of treatment response was 18% for patients with mantle cell lymphoma in the 400/400 mg arm and 50% in the 1,600/800 mg arm. Infusion reactions occurred in 75% of patients and were grade 3/4 in 8% of patients. Other grade 3/4 toxicities included anemia (10%), lymphopenia (15%), thrombocytopenia (3%), and tumor lysis syndrome (2%).
Patients with CLL were also studied in this trial. In the Phase I section, 13 patients received obinutuzumab 400–1,200 mg on days 1 and 8 of cycle 1 and on day 1 of cycles 2–8. In the Phase II study, 20 patients received a fixed dose of 1,000 mg on days 1, 8, and 15 of cycle 1 and on day 1 of cycles 2–8. In the Phase I cohort, the median number of prior treatments was 3 (range: 1–8), and eight (62%) patients had received prior rituximab but none were refractory. In the Phase II trial, the median number of prior treatments was 3 (range: 1–7), ten (50%) patients had received prior rituximab, and three (15%) were rituximab refractory. The median age was 64.0 years (range: 46–81) for the Phase I cohort, with 31% of patients being above the age of 70 years, and 62.5 years (range: 36–81) for the Phase II cohort. In the Phase I cohort, all patients experienced an infusion reaction manifested as hypotension (77%), pyrexia (62%), chills (54%), and vomiting (46%). Infusion reactions were grade 3 in two patients (15%). Other toxicities included neutropenia (54%, all were grade 3/4), thrombocytopenia (31%, grade 3 in 15%), and lymphopenia (31%, grade 3 in 15%). Neutropenic fever occurred in one patient (8%). Toxicities were similar in the patients treated on the Phase II trial.
Both end of treatment response and best overall response to obinutuzumab during the Phase I section were 62%. All responses were PRs. Three additional patients had stable disease. For the best overall response, eight (62%) patients had a PR and five had a stable disease. There appeared to be a dose–response relationship with PRs in one of three patients in each of the 400/800 mg and 1,000/1,000 mg cohorts, two of three patients in the 800/1,200 mg cohort, and all four patients in the 1,200/2,000 mg cohort. With a median follow-up of 38.7 months (range: 14.4–44.5 months), the median duration of response was 10.5 months (range: 8.5–37.0 months).
In the Phase II trial, the end of treatment response was 15% with three PRs. Five additional patients had stable disease. The best overall response was 30%, including one CR and five PRs. Five patients had stable disease. With a median follow-up of 28.8 months, the median PFS was 10.7 months (95% confidence interval, 7.1–11.7). The median duration of response for the six patients with CR or PR at any point during treatment was 8.9 months (range: 0.8–26.1 months). In patients who had received prior rituximab, the end of treatment response and the best overall response were both 62.5% in Phase I (n=5 responders, all PR). In Phase II, there were no responders at the end of treatment, but the best overall response was 20% (n=2, both PR). This study demonstrated that obinutuzumab monotherapy is active in patients with heavily pretreated relapsed/refractory CLL.
German CLL11 Phase III trial
Owing to the potency of obinutuzumab and the tolerability of chlorambucil in the older patient population, the German CLL11 trial evaluated chlorambucil with or without anti-CD20 monoclonal antibody therapy in previously untreated elderly, unfit patients with CD20 positive CLL.45 Based on the results of the trial, in November 2013, the FDA approved obinutuzumab as a first line therapy in this group of patients. Patients had either Binet Stage C and/or symptomatic disease and significant coexisting conditions as reflected by a CIRS score higher than 6, or a creatinine clearance of 30–69 mL/min. Patients were randomized on a 1:2:2 basis to chlorambucil alone, chlorambucil plus rituximab, or chlorambucil plus obinutuzumab. After 118 patients had been assigned to chlorambucil alone, this arm was closed and enrollment continued on the other arms. Patients on the chlorambucil alone arm were allowed to crossover to the obinutuzumab plus chlorambucil group if they had progressive disease during their initial treatment or within 6 months of its completion. Patients received six 28-day cycles of therapy. Chlorambucil was given at a dose of 0.5 mg/kg on days 1 and 15 of each cycle. Obinutuzumab was given intravenously at a dose of 1,000 mg on days 1, 8, and 15 of cycle 1 and on day 1 of cycles 2–6. After it was noted that infusion reactions were common with the first dose, the protocol was amended to give the first infusion of obinutuzumab over a 2-day period. Rituximab was given intravenously at a dose of 375 mg/m squared on day 1 of cycle 1 and 500 mg/m squared on day 1 of cycles 2–6. Patients received premedications (intravenous hydration, allopurinol, acetaminophen, antihistamine, and glucocorticoids) for prevention of infusion reactions and tumor lysis.
The primary end point of the trial was PFS. Secondary end points included response rates, the rate of minimal residual disease (MRD) negative status at the end of treatment, event-free survival, time to new treatment, overall survival, adverse events, and patient-reported outcomes.
A total of 781 patients were enrolled. The baseline characteristics of patients are summarized in Table 6. The median age was 72 years for chlorambucil alone (range: 43–87), 74 years for obinutuzumab plus chlorambucil (range: 39–89), and 73 years for rituximab plus chlorambucil (range: 40–90). The median CIRS score was 8 for the three groups of patients. The median creatinine clearance was 63.8 mg/min for chlorambucil, 62.5 mg/min for obinutuzumab plus chlorambucil, and 62.6 mg/min for rituximab plus chlorambucil.
  | Table 6 CLL11 trial baseline characteristics |
Adverse events occurred more frequently with rituximab plus chlorambucil and obinutuzumab plus chlorambucil compared with chlorambucil alone (Table 7). Grade 3/4 infusion reactions occurred in 67 (20%) of obinutuzumab plus chlorambucil patients compared with 12 (4%) of rituximab plus chlorambucil patients. Grade 3/4 neutropenia occurred in 111 (33%) of obinutuzumab plus chlorambucil patients, 91 (28%) of rituximab plus chlorambucil patients, and 18 (16%) of chlorambucil patients. Grade 3/4 infections occurred in 40 (12%) of obinutuzumab plus chlorambucil patients, 44 (14%) of rituximab plus chlorambucil patients, and 16 (14%) of chlorambucil patients. Other less common toxicities included thrombocytopenia, anemia, and leucopenia.
  | Table 7 CLL11 serious adverse events (grade 3 or greater) |
The primary endpoint, PFS, was significantly improved in patients receiving obinutuzumab plus chlorambucil or rituximab chlorambucil versus chlorambucil alone (Table 8 and Figure 2). PFS was 26.7 months with obinutuzumab plus chlorambucil versus 11.1 months with chlorambucil alone (hazard ratio 0.18, P<0.001) and 16.3 months with rituximab plus chlorambucil versus 11.1 months with chlorambucil alone (hazard ratio 0.44, P<0.001). The benefit was seen in all groups except in patients with deletion of chromosome17p. Response rates were also improved in patients who had received either rituximab or obinutuzumab in addition to chlorambucil. The CR plus PR rate was 77.3% (22.3% CR) for patients receiving obinutuzumab plus chlorambucil, 65.7% (7.3% CR) for rituximab plus chlorambucil compared with 31.4% (no CRs) for chlorambucil alone (P<0.001 for obinutuzumab plus chlorambucil versus chlorambucil and rituximab plus chlorambucil versus chlorambucil). The overall survival was also improved in patients receiving obinutuzumab plus chlorambucil compared with chlorambucil alone (hazard ratio 0.41, P=0.002). There was no significant survival benefit of rituximab plus chlorambucil over chlorambucil alone at the time of the report.
  | Table 8 CLL11 trial efficacy |
  | Figure 2 CLL11 trial progression-free survival. |
Patients receiving obinutuzumab were also more likely to achieve MRD negative status after treatment. In the peripheral blood, 37.7% of obinutuzumab plus chlorambucil patients were MRD negative compared with 3.3% of rituximab plus chlorambucil patients (P<0.001). In the bone marrow, 19.5% of obinutuzumab plus chlorambucil patients were MRD negative compared with 2.6% of rituximab plus chlorambucil patients (P<0.001). These results indicate that obinutuzumab plus chlorambucil is more efficacious than either chlorambucil alone or rituximab plus chlorambucil and that the combination has a manageable toxicity profile in this unfit patient population.
Conclusion and future directions
Older patients with CLL, especially those with significant comorbidities, do not derive the same benefit of intensive chemoimmunotherapy regimens than younger patients do. Obinutuzumab plus chlorambucil is an effective treatment option for these unfit patients. Recently, two kinase inhibitors have been approved for the treatment of CLL. Ibrutinib is a first in class, oral covalent inhibitor of Bruton’s tyrosine kinase, an enzyme essential in B-cell signaling.46 Ibrutinib was approved by the FDA in February 2014 for the treatment of patients with CLL who had received at least one prior therapy and for patients (regardless of prior treatment) with deletion of chromosome17p. This approval was based on the results of a Phase III multicenter trial of ibrutinib versus ofatumumab in 391 patients with relapsed or refractory CLL, the RESONATE trial.47 Patients were eligible if they had received at least one prior therapy and were considered inappropriate for purine analogue treatment due to a short progression-free interval after chemoimmunotherapy or because of comorbidities, age greater than 70 years, or deletion of chromosome 17p. Ibrutinib significantly prolonged PFS that, after a median follow-up of 9.4 months, was not reached compared with a median PFS of 8.1 months with ofatumumab (hazard ratio 0.22, P<0.001). Ibrutinib also significantly prolonged overall survival (hazard ratio, 0.43, P=0.005). The most common serious adverse events were infections, which occurred in 24% of ibrutinib and 20% of ofatumumab patients.
The second new agent is idelalisib. Idelalisib is a potent, oral, small molecule inhibitor of the delta isoform of phosphatidylinositol 3-kinase that mediates B-cell receptor signaling and is important in the pathogenesis of CLL.48 In the registration trial, eligible patients had CLL that had progressed within 24 months of their last treatment and were not able to receive cytotoxic agents due to severe neutropenia or thrombocytopenia caused by cumulative myelotoxicity from previous therapies, an estimated creatinine clearance of less than 60 mL/min or a CIRS score of more than 6. Patients received rituximab plus either idelalisib or placebo.49 At 24 weeks, the rate of PFS was 93% in the idelalisib group as compared to 46% in the placebo group (hazard ratio 0.15, P<0.001). The median duration of PFS was not reached in the idelalisib patients compared with 5.5 months in the placebo group. The overall survival was improved in the idelalisib group compared with placebo, 92% versus 80% at 12 months. The most common adverse events in the idelalisib group were fever, fatigue, nausea, chills, and diarrhea.
Although both of these new agents have significant activity, especially in patients with deletion of chromosome17p, they are both reserved for the treatment of patients with relapsed disease at this time. Further studies are needed to determine if they should be given in the front line setting.
Disclosure
The authors report no conflicts of interest in this work.
References
Loken MR, Shah VO, Dattilio KL, Civin CI. Flow cytometric analysis of bone marrow. II. Normal B lymphocyte development. Blood. 1987;70(5):1316–1324. | ||
Campo E, Swerdlow SH, Harris NL, Pileri S, Stein H, Jaffe ES. The 2008 WHO classification of lymphoid neoplasms and beyond: evolving concepts and practical applications. Blood. 2011;117(19): 5019–5032. | ||
Cragg MS, Walshe CA, Ivanov AO, Glennie MJ. The biology of CD20 and its potential as a target for mAb therapy. Curr Dir Autoimmun. 2005;8:140–174. | ||
Cragg MS, Morgan SM, Chan HT, et al. Complement-mediated lysis by anti-CD20 mAb correlates with segregation into lipid rafts. Blood. 2003;101(3):1045–1052. | ||
Tada M, Ishii-Watabe A, Suzuki T, Kawasaki N. Development of a cell-based assay measuring the activation of FcγRIIa for the characterization of therapeutic monoclonal antibodies. PLoS One. 2014;9(4): e95787. | ||
Alduaij W, Ivanov A, Honeychurch J, et al. Novel type II anti-CD20 monoclonal antibody (GA101) evokes homotypic adhesion and actin-dependent, lysosome-mediated cell death in B-cell malignancies. Blood. 2011;117(17):4519–4529. | ||
Abès R, Gélizé E, Fridman WH, Teillaud JL. Long-lasting antitumor protection by anti-CD20 antibody through cellular immune response. Blood. 2010;116(6):926–934. | ||
Niederfellner G, Lammens A, Mundigl O, et al. Epitope characterization and crystal structure of GA101 provide insights into the molecular basis for type I/II distinction of CD20 antibodies. Blood. 2011;118: 358–367. | ||
Robak T. Current and emerging monoclonal antibody treatments for chronic lymphocytic leukemia: state of the art. Expert Rev Hematol. 2014;7(6):841–857. | ||
Herter S, Herting F, Mundigl O, et al. Preclinical activity of the type II CD20 antibody GA101 (obinutuzumab) compared with rituximab and ofatumumab in vitro and in xenograft models. Mol Cancer Ther. 2013;12(10):2013–2042. | ||
Abulayha A, Bredan A, El Enshasy H, Daniels I. Rituximab: modes of action, remaining dispute and future perspective. Future Oncol. 2014;10(15):2481–2492. | ||
Pawluczkowycz AW, Beurskens FJ, Beum PV, et al. Binding of submaximal C1q promotes complement-dependent cytotoxicity (CDC) of B cells opsonized with anti-CD20 mAbs ofatumumab or rituximab: considerably higher levels of CDC are induced by ofatumumab than rituximab. J Immunol. 2009;183(1):749–758. | ||
Mössner E, Brünker P, Moser S, et al. Increasing the efficacy of CD20 antibody therapy through the engineering of a new type II anti-CD20 antibody with enhanced direct and immune effector cell-mediated B-cell cytotoxicity. Blood. 2010;115(22):4393–4402. | ||
Patz M, Isaeva P, Forcob N, et al. Comparison of the in vitro effects of the anti-CD20 antibodies rituximab and GA101 on chronic lymphocytic leukaemia cells. Br J Haematol. 2011;152(3):295–306. | ||
Dalle S, Reslan L, Besseyre de Horts T, et al. Preclinical studies on the mechanism of action and the anti-lymphoma activity of the novel anti-CD20 antibody GA101. Mol Cancer Ther. 2011;10(1):178–185. | ||
Klein C, Lammens A, Schäfer W, et al. Epitope interactions of monoclonal antibodies targeting CD20 and their relationship to functional properties. MAbs. 2013;5(1):22–33. | ||
Bologna L, Gotti E, Manganini M, et al. Mechanism of action of type II, glycoengineered, anti-CD20 monoclonal antibody GA101 in B-chronic lymphocytic leukemia whole blood assays in comparison with rituximab and alemtuzumab. J Immunol. 2011;186(6):3762–3769. | ||
Jak M, van Bochove GG, Reits EA, et al. CD40 stimulation sensitizes CLL cells to lysosomal cell death induction by type II anti-CD20 mAb GA101. Blood. 2011;118(19):5178–5188. | ||
Honeychurch J, Alduaij W, Azizyan M, et al. Antibody-induced nonapoptotic cell death in human lymphoma and leukemia cells is mediated through a novel reactive oxygen species-dependent pathway. Blood. 2012;119(15):3523–3533. | ||
Golay J, Da Roit F, Bologna L, et al. Glycoengineered CD20 antibody obinutuzumab activates neutrophils and mediates phagocytosis through CD16B more efficiently than rituximab. Blood. 2013;122(20):3482–3491. | ||
Kern DJ, James BR, Blackwell S, Gassner C, Klein C, Weiner GJ. GA101 induces NK-cell activation and antibody-dependent cellular cytotoxicity more effectively than rituximab when complement is present. Leuk Lymphoma. 2013;54(11):2500–2505. | ||
Reslan L, Dalle S, Herveau S, Perrial E, Dumontet C. Apoptotic induction by anti-CD20 antibodies in chronic lymphocytic leukemia: comparison of rituximab and obinutuzumab. Leuk Lymphoma. 2014;55(1):188–190. | ||
Herting F, Friess T, Bader S, et al. Enhanced anti-tumor activity of the glycoengineered type II CD20 antibody obinutuzumab (GA101) in combination with chemotherapy in xenograft models of human lymphoma. Leuk Lymphoma. 2014;55(9):2151–2160. | ||
American Cancer Society. Cancer Facts and Figures 2015. Atlanta GA: American Cancer Society; 2015. | ||
Zelenetz AD, Gordon LI, Wierda WG, et al. Chronic lymphocytic leukemia/small lymphocytic lymphoma, version 1. 2015. J Natl Compr Canc Netw. 2015;13(3):326–362. | ||
Wierda WG, O’Brien S, Wang X, et al. Characteristics associated with important clinical end points in patients with chronic lymphocytic leukemia at initial treatment. J Clin Oncol. 2009;27(10):1637–1643. | ||
Ultmann JE, Hyman GA, Gellhorn A. Chlorambucil in treatment of chronic lymphocytic leukemia and certain lymphomas. JAMA. 1956;162(3):178–183. | ||
Montserrat E, Alcalá A, Alonso C, et al. A randomized trial comparing chlorambucil plus prednisone vs cyclophosphamide, melphalan, and prednisone in the treatment of chronic lymphocytic leukemia stages B and C. Nouv Rev Fr Hematol. 1988;30(5–6):429–432. | ||
Hansen MM, Andersen E, Christensen BE, et al. CHOP versus prednisolone + chlorambucil in chronic lymphocytic leukemia (CLL): preliminary results of a randomized multicenter trial. Nouv Rev Fr Hematol. 1988;30(5–6):433–436. | ||
Raphael B, Andersen JW, Silber R, et al. Comparison of chlorambucil and prednisone versus cyclophosphamide, vincristine, and prednisone as initial treatment for chronic lymphocytic leukemia: long-term follow-up of an Eastern Cooperative Oncology Group randomized clinical trial. J Clin Oncol. 1991;9(5):770–776. | ||
Keating MJ, Kantarjian H, O’Brien S, et al. Fludarabine: a new agent with marked cytoreductive activity in untreated chronic lymphocytic leukemia. J Clin Oncol. 1991;9(1):44–49. | ||
Byrd JC, Peterson BL, Morrison VA, et al. Randomized Phase 2 study of fludarabine with concurrent versus sequential treatment with rituximab in symptomatic, untreated patients with B-cell chronic lymphocytic leukemia: results from Cancer and Leukemia Group B 9712 (CALGB 9712). Blood. 2003;101(1):6–14. | ||
Keating MJ, O’Brien S, Albitar M, et al. Early results of a chemoimmunotherapy regimen of fludarabine, cyclophosphamide, and rituximab as initial therapy for chronic lymphocytic leukemia. J Clin Oncol. 2005;23(18):4079–4088. | ||
Tam CS, O’Brien S, Plunkett W, et al. Long-term results of first salvage treatment in CLL patients treated initially with FCR (fludarabine, cyclophosphamide, rituximab). Blood. 2014;124(20):3059–3064. | ||
Eichhorst BF, Busch R, Stilgenbauer S, et al; German CLL Study Group (GCLLSG). First-line therapy with fludarabine compared with chlorambucil does not result in a major benefit for elderly patients with advanced chronic lymphocytic leukemia. Blood. 2009;114(16):3382–3391. | ||
Knauf WU, Lissitchkov T, Aldaoud A, et al. Bendamustine compared with chlorambucil in previously untreated patients with chronic lymphocytic leukaemia: updated results of a randomized phase III trial. Br J Haematol. 2012;159(1):67–77. | ||
Linn BS, Linn MW, Gurel L. Cumulative illness rating scale. J Am Geriatr Soc. 1968;16(5):622–626. | ||
Goede V, Cramer P, Busch R, et al; German CLL Study Group. Interactions between comorbidity and treatment of chronic lymphocytic leukemia: results of German Chronic Lymphocytic Leukemia Study Group trials. Haematologica. 2014;99(6):1095–1100. | ||
Eichhorst B, Fink AM, Busch R, et al. Frontline chemoimmunotherapy with fludarabine, cyclophosphamide and rituximab shows superior efficacy in comparison to bendamustine and rituximab in previously untreated and physically fit patients with advanced CLL. In: Amer Soc Hematol Annual Meeting Abstract, Volume 19, 2014; San Francisco, CA. | ||
Sehn LH, Assouline SE, Stewart DA, et al. A phase 1 study of obinutuzumab induction followed by 2 years of maintenance in patients with relapsed CD20-positive B-cell malignancies. Blood. 2012;119(22):5118–5125. | ||
Salles G, Morschhauser F, Lamy T, et al. Phase 1 study results of the type II glycoengineered humanized anti-CD20 monoclonal antibody obinutuzumab (GA101) in B-cell lymphoma patients. Blood. 2012;119(22):5126–5132. | ||
Salles GA, Morschhauser F, Solal-Céligny P, et al. Obinutuzumab (GA101) in patients with relapsed/refractory indolent non-Hodgkin lymphoma: results from the phase II GAUGUIN study. J Clin Oncol. 2013;31(23):2920–2926. | ||
Morschhauser FA, Cartron G, Thieblemont C, et al. Obinutuzumab (GA101) monotherapy in relapsed/refractory diffuse large b-cell lymphoma or mantle-cell lymphoma: results from the phase II GAUGUIN study. J Clin Oncol. 2013;31(23):2912–2919. | ||
Cartron G, de Guibert S, Dilhuydy MS, et al. Obinutuzumab (GA101) in relapsed/refractory chronic lymphocytic leukemia: final data from the phase 1/2 GAUGUIN study. Blood. 2014;124(14):2196–2202. | ||
Goede V, Fischer K, Busch R, et al. Obinutuzumab plus chlorambucil in patients with CLL and coexisting conditions. N Engl J Med. 2014;370(12):1101–1110. | ||
Ponader S, Chen SS, Buggy JJ, et al. The Bruton tyrosine kinase inhibitor PCI-32765 thwarts chronic lymphocytic leukemia cell survival and tissue homing in vitro and in vivo. Blood. 2012;119:1182–1189. | ||
Byrd JC, Brown JR, O’Brien S, et al; RESONATE Investigators. Ibrutinib versus ofatumumab in previously treated chronic lymphoid leukemia. N Engl J Med. 2014;371(3):213–223. | ||
Hoellenriegel J, Meadows SA, Sivina M, et al. The phosphoinositide 3′-kinase delta inhibitor, CAL-101, inhibits B-cell signaling and chemokine networks in chronic lymphocytic leukemia. Blood. 2011;118:3603–3612. | ||
Furman RR, Sharman JP, Coutre SE, et al. Idelalisib and rituximab in relapsed chronic lymphocytic leukemia. N Engl J Med. 2014;370(11):997–1007. | ||
Rai KR, Peterson BL, Appelbaum FR, et al. Fludarabine compared with chlorambucil as primary therapy for chronic lymphocytic leukemia. N Engl J Med. 2000;343(24):1750–1757. | ||
Catovsky D, Richards S, Matutes E, et al; UK National Cancer Research Institute (NCRI) Haematological Oncology Clinical Studies Group, NCRI Chronic Lymphocytic Leukaemia Working Group. Assessment of fludarabine plus chlorambucil for patients with chronic lymphocytic leukemia (the LRF CLL4 trial): a randomized controlled trial. Lancet. 2007;370(9583):230–239. | ||
Hillmen P, Skotnicki AB, Robak T, et al. Alemtuzumab compared with chlorambucil as first-line therapy for chronic lymphocytic leukemia. J Clin Oncol. 2007;25(35):5616–5623. | ||
Cragg MS. CD20 antibodies: doing the time warp. Blood. 2011;118(2): 219–220. |
 © 2015 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2015 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.


