Back to Journals » Drug Design, Development and Therapy » Volume 14
NVP-BSK805, an Inhibitor of JAK2 Kinase, Significantly Enhances the Radiosensitivity of Esophageal Squamous Cell Carcinoma in vitro and in vivo
Authors Hua Y, Wang W, Zheng X, Yang L, Wu H, Hu Z, Li Y, Yue J, Jiang Z, Zhang X, Hou Q, Wu S
Received 27 January 2019
Accepted for publication 10 January 2020
Published 24 February 2020 Volume 2020:14 Pages 745—755
DOI https://doi.org/10.2147/DDDT.S203048
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 2
Editor who approved publication: Dr Sukesh Voruganti
Yuhui Hua,1 Weijia Wang,1 Xiaoli Zheng,2 Ling Yang,2 Hongjin Wu,2 Zhaoyang Hu,2 Ying Li,2 Jing Yue,2 Zhenzhen Jiang,2 Xiaoyan Zhang,2 Qiang Hou,2 Shixiu Wu3
1Department of Pharmacy, Hangzhou Cancer Hospital, Hangzhou 310002, People’s Republic of China; 2Hangzhou Cancer Institution, Hangzhou Cancer Hospital, Hangzhou 310002, People’s Republic of China; 3National Cancer Center/National Clinical Research Center for Cancer/Cancer Hospital & Shenzhen Hospital, Chinese Academy of Medical Sciences and Peking Union Medical College, Shenzhen 518116, People’s Republic of China
Correspondence: Shixiu Wu
National Cancer Center/National Clinical Research Center for Cancer/Cancer Hospital & Shenzhen Hospital, Chinese Academy of Medical Sciences and Peking Union Medical College, No. 113 Baohe Street Longgang District, Shenzhen, People’s Republic of China
Email [email protected]
Purpose: Radiotherapy is one major curative treatment modality for esophageal squamous cell carcinoma (ESCC) patients. This study aimed to find out small-molecular kinase inhibitors, which can significantly enhance the radiosensitivity of ESCC in vitro and in vivo.
Materials and Methods: Ninety-three kinase inhibitors were tested for their radiosensitizing effect in ESCC cells through high-content screening. The radiosensitizing effect of kinase inhibitors was investigated in vitro by detection of DNA double-strand breaks (DSBs) and clonogenic survival assay. By the establishment of xenograft tumor models in BALB/c nude mice, the radiosensitizing effect of kinase inhibitors was investigated in vivo.
Results: Among the 93 kinase inhibitors tested, we found NVP-BSK805, an inhibitor of JAK2 kinase, significantly radiosensitized ESCC cells through enhancing DSBs, inhibiting DNA damage repair and arresting cell cycle in G2/M or G0/G1 phase. After treatment with NVP-BSK805, ESCC cells showed decreased clonogenic survival and delayed tumor growth in vivo. JAK2 kinase was highly expressed in tumor tissues of ESCC patients, while rarely expressed in matched normal esophageal epithelial tissues. Survival analysis revealed JAK2 kinase as a prognostic factor of ESCC patients treated with chemoradiotherapy.
Conclusion: Our study discovered JAK2 kinase as an attractive target to enhance the radiosensitivity of ESCC cells in vitro and in vivo.
Keywords: ESCC, radioresistance, JAK2, NVP-BSK805, DNA damage repair
Introduction
The 5-year survival rate of esophageal squamous cell carcinoma (ESCC) patients treated with radiotherapy is less than 20% due to tumor radioresistance.1 Small-molecular kinase inhibitors had the ability to restrain tumor growth and enhance tumor response to chemoradiotherapy. Several kinase inhibitors such as tyrosine/phosphoinositide kinase inhibitor PP121 significantly inhibited esophageal cancer cell growth and invasion.2 Janus kinase (JAK), as a member of non-receptor tyrosine kinases, regulated multiple biological processes including cell proliferation, differentiation and survival.3 There are four members in the JAK family containing JAK1, JAK2, JAK3 and tyrosine kinase 2 (TYK2). Upon cytokine receptor ligation by a cognate ligand, receptor-associated JAKs were transphosphorylated and activated, generating docking sites for downstream adaptor and effector proteins such as the signal transducers and activators of transcription (STAT) proteins.4 TG10129, a small molecular inhibitor of JAK2, was demonstrated to increase the radiosensitivity of lung cancer by inhibiting JAK2 downstream signaling.5 Furthermore, TG10129 initiated apoptosis and autophagy in T cell acute lymphoblastic leukemia cells.6 Other JAK2 inhibitors such as AG490 and NS-018 had potent anticancer activities in several human cancer, suggesting JAK2 kinase was an attractive target for cancer therapy.7 In ESCC, Fang et al reported blockage of JAK2/STAT3 pathway with JAK2 kinase inhibitor inhibited cell growth and cancer-related inflammation.8 In our study, 93 kinase inhibitors were screened to explore their radiosensitizing effect in esophageal cancer cells. We found NVP-BSK805, an inhibitor of JAK2 kinase, significantly enhanced the radiosensitivity of ESCC cells both in vitro and in vivo.
Materials and Methods
Cell Culture and Agents
The human esophageal squamous cell carcinoma (ESCC) cells KYSE-150, KYSE-30 and KYSE-180 were obtained from American Type Culture Collection (ATCC) and cultured in RPMI-1640 medium (Gibco, Life Technologies Inc., Grand Island, NY, USA) supplemented with 10% fetal bovine serum (Gibco, Life Technologies Inc., Grand Island, NY, USA) at 37°C in 5% CO2/95% air. The radioresistant esophageal cancer cell line KYSE-150R had been established from KYSE-150 by multiple fractionated radiation.9 The BCA protein assay kit was obtained from Beyotime Institute of Biotechnology (Shanghai, China). The primary antibodies against JAK2, pJAK2 (Tyr 1007/Tyr 1008), GAPDH and goat anti-mouse secondary antibody were purchased from Santa Cruz Company (Dallas, TX, USA). The primary antibody against γH2AX (Ser 139) was purchased from Cell Signaling Technology (Beverly, MA, USA).
Animals and Clinical Specimens of ESCC Patients
Six-week-old female BALB/c nude mice were purchased and maintained under standard conditions in Experimental Animal Center in Zhejiang Chinese Medicine University. All of the animal protocols in our study were performed following institutional guidelines, with the approval by Zhejiang Chinese Medicine University Animal Care and Ethical Committee (Permit Number: SYXK 2018–0012).
The surgically resected tumor tissues of 87 primary ESCC patients and matched normal esophageal epithelial tissues were collected from Hangzhou Cancer Hospital with the written informed consent provided by the patients, and were approval by the Institutional Review Board of Hangzhou Cancer Hospital (Permit Number: HZCH-2016-02). The tissue chips consisting of 50 primary ESCC specimens and matched non-neoplastic tissues were purchased from US Biomax, Inc (Rockville, MD, USA). The clinicopathological parameters of each cohort of ESCC patients used in our study were provided in Supporting Information. All of the human studies in our study were in accordance with the guidelines of the Committees for Ethical Review of Research at Hangzhou Cancer Hospital.
Ionizing Radiation
Irradiation was performed using 6 MV X-rays generated by a Elekta Precise linear accelerator fitted with a 10-mm conical collimator (Elekta, Stockholm, Sweden). The dose delivered (600 cGy/min) to each experimental setup used in our study was verified by radiochromic film dosimetry.10
Kinase Inhibitors Screening
Kinases inhibitors library consisting of 93 chemical compounds was purchased from Selleck (Houston, TX, USA). The compounds were dissolved in DMSO as 30 mM stock solution and kept at −20°C until use. KYSE-150 cells were seeded into 384-wells plate (5×103 cells per well) and cultured until adherent growth. After 4-hr incubation with 30 μM kinase inhibitors, the cells were treated with 6-Gy radiation, and the expression of γ-H2AX, a marker of DNA double-strand breaks (DSBs) was detected 24 h later. We chose the kinase inhibitors which can induce high γ-H2AX expression in KYSE-150 cells following irradiation for further studies.
Immunofluorescence Detection of γ-H2AX and p-ATM Expression
Cells were seeded into six-wells plate and exposed to 6-Gy radiation after adherent growth. After the indicated time intervals, the cells were fixed with acetone/methanol (1:1), and permeabilized with 0.1% Triton-X100 in PBS. The cells were incubated with antibodies against γ-H2AX and p-ATM for 2 hrs. Indirect immunofluorescence was performed by incubation with Alexa Fluor 488-conjugated secondary antibody (Zymed; Invitrogen). The cell nucleus was stained with 1 μg/mL DAPI. Immunofluorescence images were taken using a confocal laser scanning microscope. Data was shown as the average fluorescence intensity of γ-H2AX per cell.
Clonogenic Survival Assay
Exponentially growing tumor cells were seeded into a six-wells plate. After 24-hr incubation, adhesive cells were treated with 5 or 10 μM NVP-BSK805. Four hours later, the cells were exposed to radiation at 0 Gy, 2 Gy, 4 Gy, 6 Gy and 8 Gy with an average dose rate of 600 cGy/min. NVP-BSK805 was removed immediately after radiation by changing the media. The cells were cultured for another 10 days at 37°C in a 5% CO2 environment to allow colony formation. Only colonies containing ≥50 cells were counted as clonogenic survivors. Unirradiated cells were used as a control. The sensitization dose enhancement ratios (DER10) were calculated as the ratio of doses required to achieve 10% surviving fraction for cells without and with NVP-BSK805 treatment.
FCM (Flow Cytometry) Analysis of γ-H2AX Expression
KYSE-150 cells and KYSE-150R cells were seeded into a six-wells plate and pretreated with or without 10 μM NVP-BSK805 4 h before 6-Gy radiation. Then, the cells were collected at different time points after radiation, washed twice with ice-cold PBS and fixed with 70% ethanol diluted in PBS. After kept at −20°C overnight, the cell samples were washed with 0.5% BSA in PBS and then incubated with Alexa Fluor 488-conjugated γ-H2AX antibody (CST, #9719) for 1 h before analysis with FCM (BD, USA).
FCM (Flow Cytometry) Analysis of Cell Cycle Distribution
KYSE-150 cells and KYSE-150R cells were seeded in 6-cm plates. Attached cells pretreated with or without 10-μM NVP-BSK805 were exposed to 6-Gy radiation 4 h later. Then, cells were harvested with 0.05% trypsin-EDTA solution, suspended in the complete culture medium and stained with propidium iodide (PI, BD Biosciences Pharmingen). Cell cycle phase distribution was measured and analyzed with CytoFLEX flow cytometer (Beckman Coulter, 405-nm laser).
Western Blotting Analysis
The protein expressions were analyzed by Western blotting analysis according to the method described by Sui et al.11 Briefly, the cells after indicated treatments were harvested by trypsin-EDTA exposure and washed twice with ice-cold PBS before adding into the protein extraction buffer. Equal amount of proteins was fractionated on 12% SDS-PAGE gel and transferred to polyvinylidence difluoride membranes. The membranes were incubated with the indicated primary and secondary antibodies. The proteins were ultimately visualized by enhanced chemiluminescence and autoradiography (ECL; Thermon Scientific, Waltham, MA, UK).
Immunohistochemical Staining
Immunohistochemical staining was performed on paraffin-embedded sections of tumor tissues according to standard procedures.12 Briefly, sections of 4 um thick were deparaffinized and rehydrated through a series of graded alcohols. Endogenous peroxidase activity was quenched with 3% (v/v) H2O2 for 20 mins. Antigen retrieval was performed with 6.5 mM sodium citrate, pH 6.0, in a pressure cooker. The sections were incubated with the indicated primary antibodies and HRP-conjugated secondary antibodies, and the color reaction was carried out by exposure to DAB. The intensity of p-JAK2 expression was graded as 0, negative; 1+, weak cytoplasmic staining; 2+, strong staining in less than 30% of tumor cells; 3+, strong staining in more than 30% of tumor cells. 0 and 1+ were defined as p-JAK2-negative; 2+ and 3+ as p-JAK2-positive. The slides were scored by a pathologist and two experienced researchers independently.
Xenograft Transplantation and Therapy
To develop xenograft tumors, in vitro growing KYSE-150 cells were harvested by exposure to trypsin-EDTA, washed with ice-cold PBS and implanted into the right flanks of female BALB/c nude mice (1.0×105 cells in 100 μL PBS). When xenograft tumors had reached a mean volume of around 75 mm3, mice were randomly assigned into different groups (seven in each group). Tumors were treated with 12-Gy radiation in six fractions (2 Gy per fraction, once every 2 days), 30 mg/kg NVP-BSK805 by gavage for 11 consecutive days alone or combined with 12-Gy radiation in six fractions (fractionated radiation was performed on day 1,3,5,7,9,11, the day when NVP-BSK805 was administered for the first time was defined as day 1). The mice that were treated with only 0.1% DMSO were used as a control. Each animal was earmarked and followed individually throughout the experiment. Tumor volume (mm3) was calculated using the following formula: V(mm3)= A(mm)×B(mm)2/2, where A and B were the longest and widest diameter of the tumor, respectively, and measured every 2 days by a caliper. The body weights of tumor-bearing mice were also recorded every 2 days during the experiment. All of the tumors were observed until tumor volume ≥1500 mm3 and then the mice were sacrificed according to the institutional guidelines. Tumor growth delay time in each group was determined.
Gene Set Enrichment Analysis (GSEA)
GSEA was analyzed on the datasets (GSE10358) which were downloaded from GEO (gene expression omnibus, http://www.ncbi.nlm.nih.gov/geo/). After Robust Multi-array Average (RMA) normalization, the datasets were assessed by R packages. The GSEA was considered as statistically significant enrichment score at a level of p<0.05.
Statistical Analysis
All of the experiments in our study were independently performed in triplicate and the data were presented as means ± SD. Statistical analyses were performed with SPSS software 16.0. Univariate survival analyses were performed with Kaplan–Meier method and log-rank tests. The other statistical analyses were performed with Student’s t-test unless specified otherwise. Differences were considered statistically significant at a level of p<0.05.
Results
JAK2 Kinase Was a Potent Target to Radiosensitize Esophageal Cancer Cells
DNA double-strand breaks (DSBs) are the major form of cellular damage induced by radiation.13 Our study investigated the expression of γ-H2AX, a marker of DSBs in esophageal cancer cells following radiation with or without pretreatment with an indicated kinase inhibitor. Among the 93 inhibitors tested, we found the expression of γ-H2AX was obviously increased in KYSE-150 cells when pretreated with NVP-BSK805, an inhibitor of JAK2 kinase before radiation (Figure 1). Then, 10 μM NVP-BSK805, which induced over 75% cell viability after 24-hr incubation, was used to study its radiosensitizing effect on the parental KYSE-150 cells and the radioresistant KYSE-150R cells (Supplementary Figure 1). As shown in Figure 1B and in Supplementary Figure 2, both KYSE-150 cells and KYSE-150R cells had significantly increased γ-H2AX expression while decreased p-ATM expression when pretreated with 10 μM NVP-BSK805 before irradiation. These results suggested that inhibition of JAK2 kinase increased irradiation-induced DSBs while suppressed DNA damage repair. When KYSE-150 cells and KYSE-150R cells were pretreated with 10 μM NVP-BSK805 4 h before irradiation, their cell cycle was arrested at the most radiosensitive G2/M phase or G0/G1, respectively (Figure 2).
Following 6-Gy irradiation, the expression and activation of JAK2 were significantly increased in KYSE-150 cells and in KYSE-150R cells (Figure 1C and Supplementary Figure 3A and B). When pretreated with 5 or 10 μM NVP-BSK805 4 h before irradiation, the expression of JAK2 was inhibited while the expression of γ-H2AX was increased in KYSE-150 cells and in KYSE-150R cells, suggesting inhibition of JAK2 kinase by NVP-BSK805 enhanced irradiation-induced DNA damage (Figure 1D and Supplementary Figure 3C–F). By clonogenic survival assay, we found pretreatment with NVP-BSK805 significantly reversed the radioresistance of the radioresistant KYSE-150R cells and improved the radiosensitivity of other esophageal cancer cell lines including KYSE-150 cells, KYSE-30 cells and KYSE-180 cells (Figure 3). And the dose enhancement ratios (DER10) defined as the ratio of doses required to achieve 10% surviving fraction for cells without and with NVP-BSK805 treatment were determined in each group. The DER10 of 10 μM NVP-BSK805 was 1.728 and 14.251 for KYSE-150 cells and KYSE-150R cells, respectively (Figure 3A). For KYSE-30 cells, the DER10 of 5 μM NVP-BSK805 and 10 μM NVP-BSK805 was 2.4542 and 5.3514, respectively (Figure 3B). For KYSE-180 cells, the DER10 of 5 μM NVP-BSK805 and 10 μM NVP-BSK805 was 3.2509 and 26.0088, respectively (Figure 3C). These results suggested JAK2 kinase may be a potent target to radiosensitize esophageal cancer cells.
Inhibition of JAK2 Kinase Delayed the Growth of ESCC Xenograft Tumors
To confirm the radiosensitizing effect of JAK2 kinase inhibitor NVP-BSK805 in vivo, xenograft tumor models implanted with KYSE-150 cells had been established in BALB/c nude mice. When treated with the combination therapy of NVP-BSK805 and 12 Gy of fractionated radiation (IR) (12 Gy of fractionated radiation was performed according to 2 Gy per fraction on day 1,3,5,7,9,11, respectively, and the day when NVP-BSK805 was administered for the first time was defined as day 1), the time of xenograft tumors grown to 1500 m3 was significantly delayed compared with IR alone (59 d vs 37 d, p=0.0328), suggesting NVP-BSK805 had potent ability to improve the radiosensitivity of KYSE-150 xenograft tumors (Figure 4). Treatment with NVP-BSK805 alone had no significant delay effect on tumor growth compared with control group (15 d vs 19 d, p=0.3175) (Figure 4). These results suggested NVP-BSK805, the inhibitor of JAK2 kinase, also had a potent radiosensitizing effect.in vivo.
JAK2 Kinase Was a Prognostic Factor of ESCC Patients Treated with Chemoradiotherapy
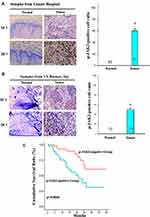
The clinical significance of JAK2 kinase was assessed by analysis of tumor tissues of ESCC patients. IHC (immunohistochemical) analysis revealed the JAK2-positive ratio in tumor tissues was significantly higher than in the matched normal esophageal epithelial tissues of ESCC patients (Figure 5A and B). Survival analysis showed those p-JAK2-positive ESCC patients had a significantly poorer prognosis than those p-JAK2-negative patients after chemoradiotherapy (Figure 5C). These results suggested JAK2 kinase may be used as a prognostic factor of ESCC patients treated with chemoradiotherapy.
Discussion
It has remained a tough problem to overcome the radioresistance of esophageal carcinoma during the past years.14–16 Several kinase inhibitors such as wee1 kinase inhibitor AZD1775, inhibitor of DNA-PKcs kinase and ATR inhibitor were proven to enhance the radiosensitivity of human cancers.17–19 In our study, by screening of 93 kinase inhibitors, we found NVP-BSK805, a specific inhibitor of JAK2 kinase,20 significantly improved the radiosensitivity of esophageal cancer cells in vitro and in vivo. As found in other models,21 JAK2 inhibition by NVP-BSK805 efficiently blocked the phosphorylation of STAT5. In this study, we illustrate the mechanisms underlying the radiosensitivity of esophageal cancer cells. Upon radiation, γ-H2AX, a marker of DSBs (DNA double-strand breaks), was highly expressed and initiated the activation of DNA damage response in tumor cells. γ-H2AX interacted with the MRE11-RAD50–NBS1 (MRN) complex, leading to the activation of ataxia telangiectasia mutated (ATM) kinase and/or ataxia telangiectasia and Rad3-related (ATR) kinase.22 ATM and ATR kinases phosphorylated their downstream target genes such as BRCA1, p53, Chk1 and Chk2 to induce cell cycle arrest and/or cell apoptosis. Accumulating evidence have suggested the initiation of damage repair response was associated with tumor radioresistance.23–26 Inhibition of DNA damage repair radiosensitized several types of human cancers. Our study found inhibition of JAK2 kinase increased irradiation-induced DSBs while suppressed DNA damage repair in KYSE-150 cells and in KYSE-150R cells. Furthermore, inhibition of JAK2 kinase arrested the cell cycle of KYSE-150 cells at the most radiosensitive G2/M phase. Whereas in the KYSE-150R cells this occurred at the G1/G0 phase. This result suggests that KYSE-150 cells and KYSE-150R cells might use different mechanisms to regulate the cell cycle. Future studies will help further decipher the role of JAK2 kinase repression in cell cycle arrest. Following NVP-BSK805 treatment, the lower levels of JAK2 were found in KYSE-150R cells. Clonogenic survival assay showed a large discrepancy between KYSE-150 cells and KYSE-150R cells. These results imply that the JAK2 inhibitor preferentially inhibits colony formation ability of ESCC cells after irradiation. Therefore, we propose JAK2 kinase-mediated the radioresistance of esophageal cancer cells possibly through regulating DNA damage and repair.
In summary, our study discovered JAK2 kinase as an attractive target to improve the radiosensitivity of esophageal carcinoma and these findings may provide novel strategies against tumor radioresistance in esophageal carcinoma.
Acknowledgments
This work was financially supported by National Natural Science Foundation of China (No. 81672994), Zhejiang Provincial Natural Sciences Foundation of China (No. LZ15H220001 and No. LY18H310012), Zhejiang Provincial Medical Scientific Research Foundation of China (No. 2015PYA009), Hangzhou City Medical Scientific Research Foundation of Zhejiang Province, China (No. 20180533B67) and Hangzhou City Scientific Technology Research Foundation of Zhejiang Province, China (No. 20150733Q63).
The abstract of this paper was presented at ASTRO’s 2017 Annual Meeting as a poster presentation/conference talk with interim findings. The poster’s abstract was published in “Poster Abstracts” in International Journal of Radiation Oncology • Biology • Physics, Vol. 99, Issue 2, E630–E631.
Disclosure
The authors declare that they have no potential or actual conflicts of interest.
References
1. Minsky BD, Pajak TF, Ginsberg RJ, et al. INT 0123 (radiation therapy oncology group 94-05) Phase III trial of combined-modality therapy for esophageal cancer: high-dose versus standard-dose radiation therapy. J Clin Oncol. 2002;20:1167–1174.
2. Peng Y, Zhou Y, Cheng L, et al. The anti-esophageal cancer cell activity by a novel tyrosine/phosphoinositide kinase inhibitor PP121. Biochem Biophys Res Commun. 2015;465:137–144. doi:10.1016/j.bbrc.2015.07.147
3. Buchert M, Burns CJ, Ernst M. Targeting JAK kinase in solid tumors: emerging opportunities and challenges. Oncogene. 2016;35:939–951. doi:10.1038/onc.2015.150
4. Babon JJ, Lucet IS, Murphy JM, Nicola NA, Varghese LN. The molecular regulation of Janus kinase (JAK) activation. Biochem J. 2014;462:1–13. doi:10.1042/BJ20140712
5. Sun Y, Moretti L, Giacalone NJ, et al. Inhibition of JAK2 signaling by TG101209 enhances radiotherapy in lung cancer models. J Thorac Oncol. 2011;4:699–706. doi:10.1097/JTO.0b013e31820d9d11
6. Cheng Z, Yi Y, Xie S, Yu H, Peng H, Zhang G. The effect of the JAK2 inhibitor TG101209 against T cell acute lymphoblastic leukemia (T-ALL) is mediated by inhibition of JAK-STAT signaling and activation of the crosstalk between apoptosis and autophagy signaling. Oncotarget. 2017;63:106753–106763.
7. Furqan M, Mukhi N, Lee B, Liu D. Dysregulation of JAK-STAT pathway in hematological malignancies and JAK inhibitors for clinical application. Biomarker Res. 2013;1:5. doi:10.1186/2050-7771-1-5
8. Fang JM, Chu L, Li CY, et al. JAK2 inhibitor blocks the inflammation and growth of esophageal squamous cell carcinoma in vitro through the JAK/STAT3 pathway. Oncol Rep. 2015;33:494–502. doi:10.3892/or.2014.3609
9. Jing Z, Gong L, Xie C-Y, et al. Reverse resistance to radiation in KYSE-150R esophageal carcinoma cell after epidermal growth factor receptor signal pathway inhibition by cetuximab. Radiother Oncol. 2009;93:468–473. doi:10.1016/j.radonc.2009.08.008
10. Tomic N, Gosselin M, Wan JF, et al. Verification of cell irradiation dose deposition using a radiochromic film. Phys Med Biol. 2007;52:3121–3131. doi:10.1088/0031-9155/52/11/013
11. Sui M, Huang Y, Park BH, Davidson NE, Fan W. Estrogen receptor alpha mediates breast cancer cell resistance to paclitaxel through inhibition of apoptotic cell death. Cancer Res. 2007;67:5337–5344. doi:10.1158/0008-5472.CAN-06-4582
12. Konigshoff M, Kramer M, Balsara N, et al. WNT1-inducible signaling protein-1 mediates pulmonary fibrosis in mice and is upregulated in humans with idiopathic pulmonary fibrosis. J Clin Investig. 2009;119:772–787. doi:10.1172/JCI33950
13. Santivasi WL, Xia F. Ionizing radiation-induced DNA damage, response, and repair. Antioxid Redox Signal. 2014;21:251–259. doi:10.1089/ars.2013.5668
14. Zhou S, Ye W, Ren J, et al. MicroRNA-381 increases radiosensitivity in esophageal squamous cell carcinoma. Am J Cancer Res. 2015;5:267–277.
15. Qian D, Zhang B, Zeng XL, et al. Inhibition of human positive cofactor 4 radiosensitizes human esophageal squmaous cell carcinoma cells by suppressing XLF-mediated nonhomologous end joining. Cell Death Dis. 2014;5:e1461.
16. Dong Q, Sharma S, Liu H, et al. HDAC inhibitors reverse acquired radio resistance of KYSE-150R esophageal carcinoma cells by modulating Bmi-1 expression. Toxicol Lett. 2014;224:121–129. doi:10.1016/j.toxlet.2013.10.014
17. Yang L, Liu Y, Sun C, et al. Inhibition of DNA-PKcs enhances radiosensitivity and increases the levels of ATM and ATR in NSCLC cells exposed to carbon ion irradiation. Oncol Lett. 2015;10:2856–2864. doi:10.3892/ol.2015.3730
18. Karnak D, Engelke CG, Parsels LA, et al. Combined inhibition of Wee1 and PARP1/2 for radiosensitization in pancreatic cancer. Clin Cancer Res. 2014;20:5085–5096. doi:10.1158/1078-0432.CCR-14-1038
19. Salovska B, Fabrik I, Durisova K, et al. Radiosensitization of human leukemic HL-60 Cells by ATR kinase inhibitor (VE-821): phosphoproteomic analysis. Int J Mol Sci. 2014;15:12007–12026. doi:10.3390/ijms150712007
20. Baffert F, Régnier CH, De Pover A, et al. Potent and selective inhibition of polycythemia by the quinoxaline JAK2 inhibitor NVP-BSK805. Mol Cancer Ther. 2010;7:1945–1955. doi:10.1158/1535-7163.MCT-10-0053
21. Andraos R, Qian Z, Bonenfant D, et al. Modulation of activation-loop phosphorylation by JAK inhibitors is binding mode dependent. Cancer Discov. 2012;6:512–523. doi:10.1158/2159-8290.CD-11-0324
22. Hosoya N, Miyagawa K. Targeting DNA damage response in cancer therapy. Cancer Sci. 2014;105:370–388. doi:10.1111/cas.2014.105.issue-4
23. Nagelkerke A, Bussink J, van der Kogel AJ, Sweep FCGJ, Span PN. The PERK/ATF4/LAMP3-arm of the unfolded protein response affects radioresistance by interfering with the DNA damage response. Radiother Oncol. 2013;108:415–421. doi:10.1016/j.radonc.2013.06.037
24. Lundholm L, Haag P, Zong D, et al. Resistance to DNA-damaging treatment in non-small cell lung cancer tumor-initiating cells involves reduced DNA-PK/ATM activation and diminished cell cycle arrest. Cell Death Dis. 2013;4:e478.
25. Sugrue T, Brown JAL, Lowndes NF, Ceredig R. Multiple facets of the DNA damage response contribute to the radioresistance of mouse mesenchymal stromal cell lines. Stem Cells. 2013;31:137–145. doi:10.1002/stem.1222
26. Zhang P, Wei Y, Wang L, et al. ATM-mediated stabilization of ZEB1 promotes DNA damage response and radioresistance through CH K1. Nat Cell Biol. 2014;16:864–875. doi:10.1038/ncb3013
 © 2020 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2020 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.