Back to Journals » Clinical Interventions in Aging » Volume 11
Nonmotor gastrointestinal disorders in older patients with Parkinson’s disease: is there hope?
Authors Georgescu D, Ancusa OE , Georgescu LA, Ionita I, Reisz D
Received 12 February 2016
Accepted for publication 23 March 2016
Published 11 November 2016 Volume 2016:11 Pages 1601—1608
DOI https://doi.org/10.2147/CIA.S106284
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 2
Editor who approved publication: Dr Richard Walker
Doina Georgescu,1 Oana Elena Ancusa,1 Liviu Andrei Georgescu,2 Ioana Ionita,3 Daniela Reisz4
1Department of Internal Medicine, 2Department of Urology, 3Department of Hematology, 4Department of Neurology, “Victor Babes” University of Medicine and Pharmacy, Timisoara, Romania
Abstract: Despite the fact that nonmotor symptoms (NMS) like gastrointestinal (GI) complaints are frequently reported in Parkinson’s disease (PD), no therapeutic guidelines are available. This study aimed to manage some lower GI-NMS in a group of patients with PD. A total of 40 patients (17 males, 23 females; mean age 76.05±2.09 years) were randomly selected for this study. Patients were confirmed to have PD (modified Hoehn–Yars scale: 2.075±0.4) who had undergone levodopa or dopamine agonist treatment. In the non-motor symptoms questionnaire (NMS-Quest), regarding GI complaints, the following were recorded: abdominal pain, bloating, and constipation of mild-to-moderate severity. Laboratory studies, abdominal ultrasound, and upper and lower digestive endoscopies were performed to rule out organic issues. All patients increased their water intake to 2 L/d and alimentary fiber to 20–25 g/d. Twenty patients received trimebutine 200 mg three times daily half an hour before meals. The other 20 patients received probiotics (60 mg per-tablet of two lactic bacteria: Lactobacillus acidophilus and Bifidobacterium infantis), 2×/d, 1 hour after meals for 3 months along with the reassessment of GI complaints. Our results demonstrated that there were significant statistical differences in all assessed symptoms in the first group: 1.55±0.51 vs 0.6±0.5 (P<0.0001) for abdominal pain; 1.6±0.5 vs 0.45±0.51 (P<0.0001) for bloating; and 1.5±0.51 vs 0.85±0.67 (P=0.0014) for constipation with incomplete defecation. The second group displayed statistical differences only for abdominal pain 1.45±0.51 vs 1.05±0.69 (P=0.00432) and bloating 1.4±0.5 vs 0.3±0.47 (P<0.0001). For constipation with incomplete defecation, there was a slight improvement. Thus, there was no significant statistical difference: 1.35±0.49 vs 1.15±0.49 (P=0.2040). In conclusion, lower GI-NMS are frequently present, isolated or associated with other autonomic issues, even before the diagnosis of PD. Treatment with probiotics could improve abdominal pain and bloating as much as with trimebutine, but less for constipation with incomplete evacuation, where trimebutine showed better results.
Keywords: PD, lower GI-NMS, probiotics, prokinetics, trimebutine
Background
Parkinson’s disease (PD), as a common neurodegenerative disease, is characterized by motor issues like tremor at rest, rigidity, bradykinesia, and also by various types of nonmotor symptoms (NMS).1
NMS may show at any stage of the disease. Sometimes, they are reported before the onset of specific motor disturbances of PD. NMS are a supplementary burden and are often very difficult to manage satisfactorily. They have even greater significance when assessed by quality-of-life measures and institutionalization rates.2
Some of NMS are classified as neuropsychiatric problems (depression, anxiety, sleep disorders, and cognitive impairment), while others are being included in the autonomic dysfunction category of the non-motor symptoms questionnaire (NMS-Quest).3
The autonomic problems of PD manifest as nonmotor features including gastrointestinal (GI) and urinary dysfunction. Autonomic problems also include a variety of other aspects, such as sweating, sexual dysfunction, and drooling, which represent additional features lowering the quality of life of these patients.4
GI dysfunction can be observed in patients who complain about disturbances of intestinal habit with constipation, bloating, flatulence, and abdominal pain. Consequently, some studies reported a high prevalence of constipation (58%) among patients with PD.5,6
Despite the fact that NMS-like GI dysfunction is frequently reported in PD, no therapeutic guidelines are yet available.
Objective
The aim of this study was to attempt the management of NMS of lower GI complaints in a group of patients with PD.
Patients and methods
Research participants
A total of 40 patients (17 males, 23 females; mean age 76.05±2.09 years) were randomly selected for this study between 2013 and 2014.7 They were confirmed to have PD (modified Hoehn–Yars scale: 2.075±0.4) and had been treated with levodopa or dopamine agonists. At the NMS-Quest the following GI complaints were recorded: abdominal pain, bloating, constipation with sensation of incomplete defecation.
In the first group, the study population included seven males (35%) and 13 females (65%). They had a mean age of 75.65±9.66 years, with 14 living in urban areas (70%) and six living in rural areas (30%).
The second group consisted of ten males (50%) and ten females (50%). Eleven were from urban location (55%), while nine from rural location (45%), with a mean age of 69.80±5.64 years.
All participants had good access to medical support for the duration of this study.
The study was approved by the ethical Board of the University of Medicine and Pharmacy “Victor Babes” Timisoara. Written informed consent was obtained from all participants before the commencement of the study.
Data recording and study design
A thorough clinical examination was performed. All patients were assessed by the same two doctors (neurologist and gastroenterologist) at the time of the data collection. The record obtained included information on disease duration and clinical onset, neurological therapy, disease severity assessment, NMS (duration and severity), and other comorbidities.
Subsequently, a scoring of the symptoms was set using a scale from 0 to 3, with 0 indicating no symptoms, 1 indicating mild symptoms, 2 indicating moderate symptoms, and 3 indicating severe symptoms.
Inclusion criteria
The inclusion criteria included the following: patients with PD irrespective of the severity or duration of the disease, already treated with the specific drugs such as levodopa or dopaminergic agonists, having mild-to-moderate GI symptoms, and not having any signs of organic digestive disease. Other factors were: negative Helicobacter pylori antigen, negative giardia antigen, negative coproparasitologic examinations, negative hemoccult test, and calprotectin levels in normal ranges.
Exclusion criteria
Patients with severe heart, liver, or kidney conditions; cancer; diabetes mellitus; and thyroid issues were excluded from the study.
Patients underwent the following laboratory examinations: blood smear, serum ferritin, C-reactive protein, transaminases (alanine aminotransferase), international normalized ratio, fasting glucose, total cholesterol, triglycerides, thyroid-stimulating hormone, creatinine, urine and stool examinations (coproparasitological, H. pylori antigen, Giardia lamblia antigen, hemoccult test, and calprotectin), abdominal ultrasonography with a high-resolution General Electric ultrasound machine, upper digestive endoscopy, and colonoscopy (Karl Storz; Olympus, Tokyo, Japan). However, the colonoscopy was aimed to rule out organic digestive issues.
All patients increased their fluid intake to 2 L/d and augment dietary fiber in the range of 20–25 g/d. Patients were randomly assigned into two groups. Twenty patients received trimebutine 200 mg three times daily half an hour before meals, while the other 20 received probiotics (60 mg as a mixture of two lactic bacteria: Lactobacillus acidophilus and Bifidobacterium infantis), 2×/day, 1 hour after meals. The treatment began during hospitalization and continued in outpatient care for 3 months. No side effects or dropout cases were recorded.
Scoring of the severity of the symptoms was done before and after the completion of the therapy.
Statistical analysis
GraphPad InStat 3 and Graph Pad Prism 6 software (GraphPad Software, Inc., La Jolla, CA, USA) were used with the panel for continuous data. This was accompanied with descriptive statistics and calculation of mean values and standard deviation. The unpaired t-test was run for statistical distribution and interpretation of P-values with confidence interval =95% to set the statistical significance. Nonparametric Spearman correlation test was also used, with the calculation of r coefficient to quantify the magnitude and direction of correlation, if any.
Results
The baseline clinical data are listed in Table 1. These were presented as the mean values and the standard deviation. However, no statistically significant differences between the two groups was observed (P>0.001).
The presence of the GI symptoms either prior to, or after the diagnosis of PD is illustrated in Figures 1 and 2.
Consequently, the relationship between the onset of GI complaints of constipation with incomplete evacuation, occurred long before the diagnosis of PD. This was such that in both groups, patients recorded symptoms that existed before the neurological diagnosis (75% in the first group versus 85% in the second group). The other symptoms were noticed either prior to or after the PD was diagnosed.
As can be seen from Table 2, at the start of this study, there was no statistical difference concerning the severity of symptoms between the two groups. After completing the therapy, the same situation was recorded: ie, no statistically significant difference between the two groups was observed.
The presence of other autonomic NMS like dysphagia, nausea, urinary urgency, nocturia, orthostatic hypotension, and excessive sweating at the time of PD diagnosis is shown in Figure 3.
  | Figure 3 Other NMS associated with GI symptoms. |
It is clearly seen that nausea and dysphagia were very frequently associated with NMS. About 75% of patients from the first group and 70% of patients from the second group had nausea. Dysphagia was recorded in 50% of the patients from the first group and in 40% of the patients from the second group. The other symptoms such as urinary urgency, nocturia, orthostatic hypotension, and excessive sweating were present in less than 35% of all the patients.
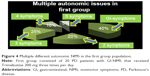
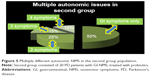
At the beginning of the study, some patients exhibited many different autonomic NMS associated with GI symptoms, which are illustrated in Figures 4 and 5.
The other NMS associated with GI complaints were present at different ranges: 20%–25% of the patients had more than one autonomic complaint, 15%–25% had more than two complaints, and 10% had more than three complaints. In the first group, there was one patient with four more autonomic issues.
From the point of view of therapeutic results, as shown in Figure 6, we can easily observe that the first group showed statistically significant differences in all assessed symptoms. Thus, this shows good improvement after therapy.
Abdominal pain was significantly alleviated. In addition, scoring severity was improved, with the complaint intensity decreasing from 1.55±0.51 to 0.6±0.5 points (P<0.0001).
The next symptom assessed was bloating. This also shows an improvement from 1.6±0.5 points at initial scoring to 0.45±0.51 points after completion of therapy (P<0.0001).
Incomplete defecation evaluated prior to therapy showed a scoring of 1.5±0.51 points. After administering prokinetics, we recorded an important decrease to 0.85±0.67 points (P=0.0014).
Results observed in the second group (treated with probiotics), as illustrated in Figure 7, showed statistical differences only for abdominal pain: 1.45±0.51 points vs 1.05±0.69 points (P=0.00432). Regarding bloating, the differences were statistically very significant: 1.4±0.5 points vs 0.3±0.47 points (P<0.0001). Thus, the outcome for this symptom is very promising.
However, for incomplete defecation, there was no statistically significant difference: 1.35±0.49 points vs 1.15±0.49 points (P=0.2040).
Concerning the first group, there was no correlation between patient age and PD scoring r=0.02785 (P=0.7835 not statistically significant), between PD scoring and duration of disease r=0.9842 (P=0.33 not statistically significant), and between the onset of the disease and the intensity of the constipation with incomplete evacuation r=0.0078 (P=0.878).
Similar results were seen in the second group: age and PD scoring: r=0.01848 (P=0.9099), PD scoring and duration of disease: r=0.1609 (P=0.3213), and the onset of the disease and the intensity of the constipation with incomplete evacuation: r=0.0056 (P=0.73).
Discussion
The importance of NMS in patients with PD has gained a greater awareness in recent years.8 GI-NMS when assessed could be very frequent and has been estimated in some studies at the range of 75% in advanced PD patients.9 Lower GI symptoms such as constipation are very frequent in patients with PD. However, in some authors’ opinions, they are likely to worsen with advancing age. Some studies reported a possible increase of constipation severity related to PD severity and duration.10
Our results did not confirm a clear correlation between the duration and the severity of PD with severity of constipation. This is probably because we had no cases of advanced or severe PD.
In addition, a number of papers reported an increased incidence of other NMS, either an autonomic or a neuropsychiatric domain issue.11,12
In this study, we did observe that many patients exhibited other kinds of NMS at the time of PD diagnosis, with the most frequent being nausea and dysphagia. Other symptoms like urinary urgency, nocturia, orthostatic hypotension, and excessive sweating were only recorded sporadically. Almost half the patients also experienced other associated autonomic disorders.
Even if constipation is probably the most common nonmotor GI symptom with about half of PD patients experiencing it, there is no consensus of opinion regarding this matter. However, there are authors advocating that some of the symptoms such as sialorrhea and constipation may occur at later stages of the disease as opposed to major symptoms like orthostatic hypotension, which is one of the earlier signs or symptoms.13
In contrast to the general view, we consider the fact that constipation can predate the motor onset of PD. In addition, some evidence has shown that men who have bowel movements less than once daily compared with two or more times daily have a four times higher risk of PD after a mean time of 10 years.14,15
In our study, the patient’s history clearly showed that constipation was present in both groups. This was seen in the vast majority long before the diagnosis of PD. It is possible that the severity of constipation can increase with the advancement of severity and duration of PD. Eventually, it increases, leading to complications such as pseudo-obstruction and megacolon. Drugs used for PD, including the anticholinergics, can also exacerbate constipation. Autonomic symptoms may be due to the abnormal deposition of α-synuclein aggregates in a number of key autonomic regulatory areas (namely, the hypothalamus, parabrachial nucleus, intermediate reticular zone of the medulla, locus coeruleus, and raphe), preganglionic parasympathetic, and also in the paravertebral autonomic ganglia autonomic ganglia, which could directly cause symptoms. Nevertheless, it should be noted that antiparkinsonian medications might also play a role. Dopaminergic receptors are widely distributed throughout both the central and peripheral autonomic nervous systems. In addition, dopamine lowers systemic blood pressure by decreasing catecholamine release and reducing gastric migrating motor complex and gastric motility; however, it may increase colonic motor activity.16
At this point, while the evolution of motor symptoms has been well characterized, an understanding of GI-NMS is still not so clear. From a clinical point of view, there is also an important heterogeneity among patients; little is yet known about the NMS progression.17
This matter is also complicated by the fact that the dopaminergic treatment used for the motor symptoms of PD can cause or worsen a number of NMS, including orthostatic hypotension, nausea, sleep disturbances, hallucinations, or impulsive–compulsive NMS behaviors.18
Therefore, the changes that underlie the NMS still remain unclear and are not treated effectively with dopamine-based drug therapies.19 Concerning the management of GI-NMS, there is not much information that has been made available. Apart from a well-balanced diet with plenty of fiber (in the range of 20–35 g daily) and fluid intake, macrogol can be used to treat constipation.20,21
Therefore, one of the questions that was raised regarding macrogol is: Could this compound that acts as an osmotic substance be taken as a long-standing therapy? What about GI side effects like augmentation of bloating and borborygmus that already exist, and sometimes more abdominal pain, nausea, and vomiting?
There are some studies advocating the good results after prokinetics, like domperidone in mitigating dyspepsia, which is secondary to gastric motility disorders; however, no sustainable data have been recorded regarding the effects of prokinetics on intestinal motility disorders as NMS in PD patients.22
Although it is possible that there are nonmotor GI complaints in PD patients and functional bowel disorders like irritable bowel syndrome (IBS), we did not apply Rome III criteria for the assessment of functional constipation because this classification has its own limitations.23
We considered that NMS-Quest with its section dedicated to constipation description would be more appropriate in the evaluation of cases with neurological conditions. Considering the fact that this study was designed for PD patients who are having minor GI symptoms, we made the decision to use some drugs and nutritional supplements that are already used for symptom management in patients with IBS.
In a meta-analysis published in 2007, the significant treatment benefit in favor of prokinetic agents, including trimebutine, in patients with functional dyspepsia was clearly highlighted.24
Trimebutine is a therapeutic agent prescribed mostly in patients with functional dyspepsia, IBS, chronic constipation, and other functional GI disorders. This is due to its effect on the regulation of the motility of the digestive system acting on the GI tract via 1) an agonist effect on peripheral mu, kappa, and delta opiate receptors and 2) releasing of GI peptides such as motilin and the modulation of the release of other peptides, including vasoactive intestinal peptide, gastrin, and glucagon. Trimebutine accelerates gastric emptying, induces premature phase III of the migrating motor complex in the intestine, and modulates the contractile activity of the colon.25
We were also encouraged by our previous data, where we studied some upper GI-NMS in patients with PD and dyspeptic complaints, with promising outcome seen in patients treated with trimebutine.26
This study showed promising results of prokinetics like trimebutine in the management of some mild-to-moderate lower GI-NMS like constipation, bloating, and abdominal pain.
Recent data presented an interesting hypothesis regarding the brain–gut axis that could be modulated by the gut microbiota via immunological, neuroendocrine, and direct neural mechanisms.27 In this review, there are authors suggesting that gut microbiota could be a potential biomarker for the predictability of motor PD phenotype.28 So far, gut microbiota has been studied more as a pathogenic pathway related to PD or as a possible biomarker for particular phenotypes in PD, and less as a potential target for therapeutic purpose.
At this point, we are aware of the existence of one pilot study using probiotics as a single strain. L. casei Shirota, from fermented milk, addressed the management of constipation in PD patients and significantly improved stool consistency and bowel habits.29 In this regard, using probiotics to rebalance a dysregulated gut microbiome in patients with PD seemed to be reasonable. Our experience with probiotics was very encouraging, as it showed good results in mitigating some GI symptoms like bloating and abdominal pain.
It would be very interesting also, to assess the characteristics of each individual’s microbiome before and after long-term usage of probiotics, to observe if those changes could interfere with other symptoms and signs that characterize PD.
It could be possible that association of prokinetics with probiotics is much more beneficial to a certain category of patients. It is hard to anticipate and evaluate if this schedule of treatment would be satisfactory enough in improving more severe lower GI-NMS.
Limitations
This study has limitations related to the relatively small cohort of patients with PD and lower GI-NMS. Another aspect that could also be considered as a limitation is the fact that we enrolled only patients with mild-to-moderate severity of the GI-NMS. This experience should be extended to attempt managing more severe lower GI complaints in PD patients, and should ideally be addressed in further discussions and debates.
Conclusion
On the basis of our results, lower GI-NMS are frequently present, alone or associated with other autonomic issues. Thus, many of them were recorded long before the diagnosis of PD. The treatment with trimebutine showed promising results in alleviating all assessed symptoms. However, probiotics also showed good potential in mitigating many abdominal complaints.
Author contributions
DG contributed to creating the study design, drafting the article, and carrying out the interpretation of the data. OEA, LAG, and DR contributed to the interpretation and the statistical analysis of the data. II assisted in the proofreading of the manuscript and the reviewing of all the tables and figures. Therefore, all authors contributed in reviewing this manuscript and in carrying out the interpretation of the statistical analysis of the data. In addition, all authors hold rights to the intellectual content of this article. All authors contributed toward data analysis, drafting and critically revising the paper and agree to be accountable for all aspects of the work.
Disclosure
The authors report no conflicts of interests in this work.
References
Borek LL, Amick MM, Friedman JH. Non-motor aspects of Parkinson’s disease. CNS Spectr. 2006;11(7):541–554. | ||
Pressley JC, Louis ED, Tang MX, et al. The impact of comorbid disease and injuries on resource use and expenditures in parkinsonism. Neurology. 2003;60(1):87–93. | ||
Martinez-Martin P, Schapira AH, Stocchi F, et al. Prevalence of nonmotor symptoms in Parkinson’s disease in an international setting; study using nonmotor symptoms questionnaire in 545 patients. Mov Disord. 2007;22(11):1623–1629. | ||
Schrag A, Jahanshahi M, Quinn N. What contributes to quality of life in patients with Parkinson’s disease? J Neurol Neurosurg Psychiatry. 2000;69(3):308–312. | ||
Magerkurth C, Schnitzer R, Braune S. Symptoms of autonomic failure in Parkinson’s disease: prevalence and impact on daily life. Clin Auton Res. 2005;15:76–82. | ||
Cersosimo MG, Benarroch EE. Neural control of the gastrointestinal tract: implications for Parkinson disease. Mov Disord. 2008;23:1065–1075. | ||
Goetz CG, Poewe W, Rascol O, et al. Movement Disorder Society task force report on the Hoehn and Yahr staging scale: status and recommendations. The Movement Disorder Society Task Force on rating scales for Parkinson’s disease. Mov Disord. 2004;19(9):1020–1028. | ||
Lyons KE, Pahwa R. The impact and management of nonmotor symptoms of Parkinson’s disease. Am J Manag Care. 2011;12:S308–S314. | ||
Barone P, Antonini A, Colosimo C, et al; PRIAMO study group. The PRIAMO study: a multicenter assessment of nonmotor symptoms and their impact on quality of life in Parkinson’s disease. Mov Disord. 2009;24(11):1641–1649. | ||
Salat-Foix D, Andrews CN, Meddings J, Suchowersky O. Gastrointestinal symptoms in Parkinson disease: clinical aspects and management. Can J Neurol Sci. 2011;38(4):557–564. | ||
Simuni T, Sethi K. Nonmotor manifestations of Parkinson’s disease. Ann Neurol. 2008;64(Suppl 2):S65–S80. | ||
Lim SY, Lang AE. The nonmotor symptoms of Parkinson’s disease – an overview. Mov Disord. 2010;25(Suppl 1):S123–S130. | ||
Gołab-Janowska M, Budzianowska A, Honczarenko K. Autonomic disorders in Parkinson’s disease. Ann Acad Medicare Steninensis. 2011;1:11–15. | ||
Cersosimo MG, Raina GB, Pecci C, et al. Gastrointestinal manifestations in Parkinson’s disease: prevalence and occurrence before motor symptoms. J Neurol. 2013;260(5):1332–1338. | ||
Abbott RD, Petrovitch H, White LR, et al. Frequency of bowel movements and the future risk of Parkinson’s disease. Neurology. 2001;57(3):456–462. | ||
Asahina M, Vichayanrat E, Low DA, et al. Autonomic dysfunction in parkinsonian disorders: assessment and pathophysiology. J Neurol Neurosurg Psychiatry. 2013;84(6):674–680. | ||
Antonini A, Barone P, Marconi R, et al. The progression of non-motor symptoms in Parkinson’s disease and their contribution to motor disability and quality of life. J Neurol. 2012;259(12):2621–2631. | ||
Chaudhuri KR, Healy DG, Schapira AH. Non-motor symptoms of Parkinson’s disease: diagnosis and management. Lancet Neurol. 2006;5(3):235–245. | ||
Breen KC, Drutyte G. Non-motor symptoms of Parkinson’s disease: the patient’s perspective. J Neural Transm. 2013;120(4):531–535. | ||
Zangaglia R, Martignoni E, Glorioso M, et al. Macrogol for the treatment of constipation in Parkinson’s disease. A randomized placebo-controlled study. Mov Disord. 2007;22:1239–1244. | ||
Chaudhuri KR, Schapira AH. Non-motor symptoms of Parkinson’s disease: dopaminergic pathophysiology and treatment. Lancet Neurol. 2009;8(5):464–474. | ||
Barone JA. Domperidone: a peripherally acting dopamine2-receptor antagonist. Ann Pharmacother. 1999;33:429–440. | ||
Wang AJ, Liao XH, Xiong LS, et al. The clinical overlap between functional dyspepsia and irritable bowel syndrome based on Rome III criteria. BMC Gastroenterol. 2008;8:43. | ||
Hiyama T, Yoshihara M, Matsuo K, et al. Meta-analysis of the effects of prokinetic agents in patients with functional dyspepsia. J Gastroenterol Hepatol. 2007;22(3):304–310. | ||
Schmulson M, Chang L. The treatment of functional abdominal bloating and distension. Aliment Pharmacol Ther. 2011;33(10):1071–1086. | ||
Georgescu D, Simu M, Lighezan D. Understanding dyspepsia in PD. Eur J Neurol Suppl. 2011;92–99. | ||
Mulak A, Bonaz B. Brain-gut-microbiota axis in Parkinson’s disease. World J Gastroenterol. 2015;7;21(37):10609–10620. | ||
Scheperjans F, Aho V, Pereira PA, et al. Gut microbiota are related to Parkinson’s disease and clinical phenotype. Mov Disord. 2015;30(3):350–358. | ||
Cassani E, Privitera G, Pezzoli G, et al. Use of probiotics for the treatment of constipation in Parkinson’s disease patients. Minerva Gastroenterol Dietol. 2011;57(2):117–121. |
 © 2016 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2016 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.