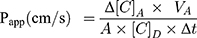Back to Journals » Drug Design, Development and Therapy » Volume 15
New Approach in Ocular Drug Delivery: In vitro and ex vivo Investigation of Cyclodextrin-Containing, Mucoadhesive Eye Drop Formulations
Authors Bíró T, Bocsik A, Jurišić Dukovski B, Gróf I, Lovrić J, Csóka I , Deli MA , Aigner Z
Received 5 June 2020
Accepted for publication 18 November 2020
Published 3 February 2021 Volume 2021:15 Pages 351—360
DOI https://doi.org/10.2147/DDDT.S264745
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 3
Editor who approved publication: Professor Manfred Ogris
Tivadar Bíró,1 Alexandra Bocsik,2 Bisera Jurišić Dukovski,3 Ilona Gróf,2,4 Jasmina Lovrić,3 Ildikó Csóka,1 Mária A Deli,2 Zoltán Aigner1
1Institute of Pharmaceutical Technology and Regulatory Affairs, Faculty of Pharmacy, University of Szeged, Szeged, Hungary; 2Institute of Biophysics, Biological Research Centre,, Szeged, Hungary; 3Department of Pharmaceutical Technology, Faculty of Pharmacy and Biochemistry, University of Zagreb, Zagreb, Croatia; 4Doctoral School of Biology, University of Szeged, Szeged, Hungary
Correspondence: Zoltán Aigner
Institute of Pharmaceutical Technology and Regulatory Affairs, University of Szeged, 6 Eötvös Street, Szeged, H-6720, Hungary
Tel +3662545577
Email [email protected]
Background: Optimal transcorneal penetration is necessary for ocular therapy; meanwhile, it is limited by the complex structure and defensive mechanisms of the eye. Antimicrobial stability of topical ophthalmic formulations is especially important. According to previous studies, the mostly used preservative, benzalkonium-chloride is irritative and toxic on corneal epithelial cells; therefore, novel non-toxic, antimicrobial agents are required. In this study, prednisolone-containing ophthalmic formulations were developed with expected optimal permeation without toxic or irritative effects.
Methods: The toxicity and permeability of prednisolone-containing eye drops were studied on a human corneal epithelial cell line (HCE-T) and ex vivo cornea model. The lipophilic drug is dissolved by the formation of cyclodextrin inclusion complex. Zinc-containing mucoadhesive biopolymer was applied as an alternative preservative agent, whose toxicity was compared with benzalkonium-chloride.
Results: As the results show, benzalkonium-chloride-containing samples were toxic on HCE-T cells. The biopolymer caused no cell damage after the treatment. This was confirmed by immunohistochemistry assay. The in vitro permeability was significantly higher in formulations with prednisolone-cyclodextrin complex compared with suspension formulation. According to the ex vivo permeability study, the biopolymer-containing samples had significantly lower permeability.
Conclusion: Considering the mucoadhesive attribute of target formulations, prolonged absorption is expected after application with less frequent administration. It can be stated that the compositions are innovative approaches as novel non-toxic ophthalmic formulations with optimal drug permeability.
Keywords: prednisolone, cyclodextrin, ocular drug delivery, mucoadhesion, human corneal epithelial cell line, ex vivo cornea model
Introduction
Ocular drug delivery is a difficult challenging task in the field of pharmaceutical research and development. The main goal is to meet the requirements of patient-based therapy and technological formulation aspects. The special environment of the eye makes the optimization difficult. Several methods have been developed for enhanced ocular drug delivery. Mainly topical solutions are in the focus of research laboratories.1–4 Besides the advantages of eye drop formulations (self-applicable, non-invasive, convenient, economical), many difficulties are known, which need to be overcome (short retention time, low drug absorption, and bioavailability and problematic microbiological stability).
Drug delivery from eye drops is limited because of the special anatomical structure and reflex mechanisms, such as eye-blinking and lachrymal secretion.5,6 Sufficient amounts of the active pharmaceutical ingredient (API) need to be ensured on the eye surface, therefore the API is able to pass the hydrophilic tear film. The corneal and non-corneal pathways for topically applied drug absorption are complex, and consist of lipophilic and hydrophilic layers. For optimal drug penetration the required physicochemical characteristics of the drug delivery system must be strictly designed. Optimally a lipophilic-hydrophilic balance is needed in the system to induce the required therapeutic effect without minimal precorneal drug elimination.7–10
In ocular postoperative therapy, glucocorticoids are applied for treatment and prevention of inflammation. These steroids are poorly soluble in water, commonly steroid-containing formulations are suspensions on the market. Eye drops with prednisolone (PR), prednisolone-acetate, dexamethasone, fluorometholone, and fluocinolone are licensed worldwide.11–13 PR is mostly used in acetate-form as a suspension; however, it is more water-soluble than the base form. Problems of suspension formulations are well known. After application, increased tear secretion and reflex blinking are induced, causing rapid drug elimination from the surface of the eye. Increasing the efficiency could be possible with a selection of appropriate additives.12
The efficiency can be increased by optimization of the amount of dissolved API molecules at the site of drug absorption. By using cyclodextrin (CD) derivatives the solubility of drug can be increased, therefore a solution as a dosage form can be prepared with minimal irritative effect and optimal permeation of API.14 CDs are cyclic oligosaccharides, which consist of α-(1,4) linked α-D-glucopyranose units, which form a water soluble inclusion complex with lipophilic molecules. Due to the hydrophilic external surface, the molecular complex is soluble in aqueous medium. Thermodynamic interactions are formed between the CD and API molecule, and equilibrium is created between the free and complexed molecules.15–17 Due to complex formation, a more dissolved, lipophilic API molecule can permeate through the epithelium, meanwhile the hydrophilic cyclodextrin is eliminated.4,16 Considering the toxicological toleration, hydroxypropyl-, sulfobutyl-ether-β-CD, and hydroxypropyl-γ-CD derivatives are mostly applied in ocular drug delivery. The amount of CD concentration must be set strictly to ensure the optimal drug permeation. Unnecessarily high amount restricts the drug permeation, meanwhile low concentration cannot keep the API in solution, which causes limited efficiency.18,19
Besides enhancement of permeation, increasing the residence time on the eye surface induces a higher therapeutic effect. Residence time is increasable by mucoadhesive polymers, which can ensure prolonged drug absorption; therefore, less frequent administration is needed during the therapy. By addition of these compounds, the viscosity is increased which is also favorable considering the increment of contact time on the eye surface.20–22 However, if the viscosity of the applied formulation significantly differs from the tear, the defensive mechanisms of the eye are induced, thus the elimination rate of the API will be increased. Besides the increased viscosity, mucoadhesion also provides enhanced retention on the eye surface due to the interpenetration of proteoglycan and polymer chains.23,24 Nowadays, mucoadhesive biopolymers, such as hyaluronic acid (HA), are widely used in ophthalmic formulations. As a biocompatible polymer, HA is tolerable, non-toxic, and protects the cornea with a proliferative effect.25,26
The microbiological stability is especially important in the case of ophthalmic formulations, because of the special sensitivity of the eye. Mostly, benzalkonium-chloride (BK) is applied as a preservative compound, meanwhile its destructive effect on corneal epithelial cells is proven. BK damages the eye barrier through breaking the DNA single- and double-strands in the epithelial cells. Application of BK-containing eye drops may induce irritation and high lachrymal secretion.27,28 As an alternative preservative, Zn2+ ion-containing systems can be added. In aqueous environment, the dissolved Zn2+ ion destabilizes the cell wall of microbes. Therefore zinc-containing compounds like zinc-hyaluronate (ZnHA) and zinc-gluconate (ZnGlu) may ensure the antimicrobial stability in the formulation with less irritative and toxic effect in the corneal epithelial cells in comparison with BK.20,29
Previously, formulation and investigation of innovative eye drop were published by our research group, where innovative PR-containing ophthalmic solution was developed in aqueous medium by the addition of CD derivatives and ZnHA-ZnGlu system. The physiological parameters (surface tension, pH, osmolality) were set with optimized mucoadhesive, rheological, and preservative attributes.12 The aim of this study was to continue and complete the research by investigating the cytotoxicity and API permeability of the formulation on an in vitro human corneal cell line and ex vivo, porcine cornea model.
Materials and Methods
Materials
PR was purchased from Henan Lihua Pharmaceutical Company (Henan, China). hydroxypropyl-γ-cyclodextrin (HPGCD) was donated by Cyclolab Ltd. (Budapest, Hungary), ZnHA and ZnGlu from Gedeon Richter Plc (Budapest, Hungary). Hydroxypropyl-β-cyclodextrin (HPBCD) was obtained from Wacker-Chemie GmbH (Munich, Germany). Dimethyl sulfoxide (DMSO), BK, NaCl, boric acid, and borax (for borate buffer) were obtained from Molar Chemical Ltd. (Halásztelek, Hungary), KCl, NaHCO3, D-glucose monohydrate from Kemika (Zagreb, Croatia), CaCl2 × 2H2O and MgCl2 × 6H2O from Sigma-Aldrich, Chemie GmbH (Steinheim, Germany). Ringer-buffer was used as medium during the experiments. The pH of Ringer buffer was set to 7.4.
Sample Preparation
0.1% PR was used as API. Defined amounts of HPGCD or HPBCD were dissolved in borate buffer (prepared by water for injection filtered on 0.22 µm membrane filter). After addition of PR products were sonicated for 10 minutes, until total dissolution of API. Then 0.5% ZnHA and 0.5% ZnGlu was added to the system. Osmolality was set with NaCl to about 300 mOsm/kg, the pH was about 6.20 in every formulation. Every eye drop was prepared in aseptic environment. The containers were stored in the fridge for at least 24 hours for completely wetting of ZnHA. Final composition is shown in Table 1.
 |
Table 1 Composition of Target Eye Drop Formulation |
Preparation of Human Corneal Epithelial Cell Line (HCE-T) Model
Human corneal epithelial cells (HCE-T; RCB 2280; RIKEN BRC, Tsukuba, Japan) were immortalized by transfection with a recombinant SV40-adenovirus vector, established and characterized by Araki-Sasaki.30 The cells were grown in Dulbecco’s Modified Eagle’s Medium/F-12 (Gibco, Life Technologies, Carlsbad, CA, USA) supplemented with 10% fetal bovine serum (Gibco), 0.5% DMSO, 5 µg/mL recombinant human insulin, and 10 ng/mL recombinant human epidermal growth factor (EGF) in a humidified incubator with 5% CO2 at 37°C. All plastic surfaces were coated with 0.05% rat tail collagen in sterile distilled water before cell seeding in culture dishes. The culture medium was changed every second day. The air–liquid interface is crucial for the development of a tight multilayer epithelium in HCE-T cells.31 HCE-T cells were cultured first in liquid–liquid condition for 5–8 days. To create the air–liquid condition the medium from the upper compartment was removed and only 1 mL of medium was added to the lower compartment to keep the liquid level at the appropriate height for the next 5–8 days. To measure TEER cells were fed with 500 µL medium in the upper compartment every second day.
Treatment of Cultured Cells
The final concentration of the PR in the formulations for cell culture experiments was 100 µg/mL. The formulations were diluted in Ringer buffer (pH=7.4) (150 mM NaCl, 2.2 mM CaCl2, 0.2 mM MgCl2, 5.2 mM KCl, 5 mM glucose, 6 mM NaHCO3). We tested the following samples (Table 2).
 |
Table 2 Composition of Investigated Samples by Toxicity and Permeability Studies. Defined Amounts Were Completely Dissolved or Suspended in Ringer Buffer |
Cell Viability Measurement by Impedance
Impedance was measured at 10 kHz by an RTCA SP instrument (ACEA Biosciences, San Diego, CA, USA). This method is label-free, non-invasive, and monitors cell adherence, growth, and viability real time. We have successfully tested the cellular effects of pharmaceutical excipients and peptides by impedance kinetics in our previous studies.32–34 For background measurements 50 μL of cell culture medium was added to the wells, then cells were seeded at a density of 5×103 cells/well to 96-well plate with gold electrodes (E-plate 96, ACEA Biosciences) coated with collagen. Cells were cultured for 4–5 days in a CO2 incubator at 37°C and monitored every 10 minutes until the end of experiments. Cells were treated at the beginning of the plateau phase of growth. The treatment solutions were dissolved in Ringer buffer. Triton X-100 (TX-100) detergent (1 mg/mL) was used as a reference compound to induce cell toxicity. Cell index was defined as Rn-Rb at each time point of measurement, where Rn is the cell-electrode impedance of the well when it contains cells and Rb is the background impedance of the well with the medium alone.
Immunohistochemistry
To evaluate morphological changes in HCE-T cells caused by the different formulations, cell viability assay was followed by immunostaining for junctional proteins zonula occludens protein-1 (ZO-1), occludin, β-catenin, and E-cadherin. Cells were grown on glass coverslips (Menzel-Glaser, Braunschweig, Germany) at a density of 4×104 cells/coverslips and treated with different formulations containing PR for 30 minutes. After the treatment, coverslips were washed with phosphate buffer (PBS) and the cells were fixed with 3% paraformaldehyde solution for 15 minutes at room temperature. The cells were permeabilized by 0.2% TX-100 solution for 10 minutes and the non-specific binding sites were blocked with 3% bovine serum albumin in PBS. Primary antibodies rabbit anti-ZO-1 (AB_138452, 1:400; Life Technologies, Carlsbad, CA, USA), rabbit anti-β-catenin (AB_476831, 1:400), rabbit anti-occludin (AB_2533977, 1:100; Life Technologies), and mouse anti-E-cadherin (AB_397580, 1:400; Life Technologies) were applied as overnight treatment. Incubation with secondary antibodies Alexa Fluor-488-labeled anti-mouse (AB_2534088, 1:400; Life Technologies) and anti-rabbit IgG Cy3 conjugated (AB_258792, 1:400) lasted for 1 hour. Hoechst dye 33,342 was used to stain cell nuclei. After mounting the samples (Fluoromount-G; Southern Biotech, Birmingham, IL, USA) staining was visualized by a Visitron spinning disk confocal system (Visitron Systems GmbH, Germany).
Permeability Study on HCE-T Cell Culture Model
Transepithelial electrical resistance (TEER) reflects the tightness of the intercellular junctions closing the paracellular cleft, therefore the overall tightness of cell layers of biological barriers. TEER was measured to check the barrier integrity by an EVOM volt-ohmmeter (World Precision Instruments, Sarasota, FL, USA) combined with STX-2 electrodes, and was expressed relative to the surface area of the monolayers as Ω × cm2. TEER of cell-free inserts was subtracted from the measured data.
HCE-T cells were seeded at a density of 105 cells onto Transwell inserts (polycarbonate membrane, 0.4 µm pore size, 1.12 cm2 surface area; 3401, Corning Life Sciences, Tewksbury, MA, USA) and cultured for 5–8 days at liquid–liquid and for 5–8 days at air–liquid interface. The culture medium was changed and TEER was checked every second day.
For the permeability experiments the inserts were transferred to 12-well plates containing 1.5 mL Ringer buffer in the acceptor (lower/basal) compartments. In the donor (upper/apical) compartments 0.5 mL buffer was pipetted containing different formulations (F1–F5) of PR for 30 minutes. To avoid unstirred water layer effect, the plates were kept on a horizontal shaker (120 rpm) during the assay. Samples from both compartments were collected and the PR concentration was detected by HPLC.
To determine the tightness of the cornea epithelial culture model two marker molecules were tested.34 In the donor compartments 0.5 mL buffer containing fluorescein (10 μg/mL; Mw: 376 Da) and Evans blue labeled albumin (167.5 μg/mL Evans blue dye and 10 mg/mL bovine serum albumin; Mw: 67.5 kDa) was added. The inserts were kept in the multiwell plates on a horizontal shaker (120 rpm) for 30 minutes, then the concentrations of the marker molecules in the samples from the compartments were determined by a fluorescence multiwell plate reader (Fluostar Optima, BMG Labtechnologies, Germany; fluorescein: excitation wavelength: 485 nm, emission wavelength: 520 nm; Evans-blue labeled albumin: excitation wavelength: 584 nm, emission wavelength: 680 nm).
The apparent permeability coefficients (Papp) were calculated as described previously.34 Briefly, cleared volume was calculated from the concentration difference of the tracer in the acceptor compartment (Δ[C]A) after 30 minutes and donor compartments at 0 hour ([C]D), the volume of the acceptor compartment (VA; 1.5 mL) and the surface area available for permeability (A; 1.1 cm2) using Equation 1:
Ex vivo Permeability Assay
The ex vivo permeability model was published previously by Juretić et al.35,36 Fresh porcine eyes were collected from Large White Pigs (weight 90–115 kg, male and female, 6–7 months) from a local slaughterhouse. Enucleated eyeballs were washed by isotonic saline solution (NaCl, 0.9%; B. Braun, Melsungen, Germany) and stored in Ringer buffer in a container held on ice until application. Transport and excision were performed within 2 hours after the death of animals. Excised corneas were placed into vertical diffusion chambers (Standard Vertical Ussing/Diffusion Chambers, made of acrylic with 0.64 cm2 diffusion surface, Harvard Apparatus, Holliston, MA, USA). The epithelial side of the cornea was faced to the donor phase of the system. Donor and acceptor phase volume were equally 3.5 mL. After 30 minutes incubation of corneas (Ringer buffer, 37°C), the donor compartment was removed, and 3.5 mL sample was injected. Then 500 µL samples were collected from the acceptor compartment at defined time intervals (0 minutes, 30 minutes, 1, 1.5, 2, 2.5, 3, 3.5, 4, 4.5, and 5 hours) and refilled with 500 µL Ringer-buffer at each sample collection. Continuous oxygenation was ensured during the experiment for mixing of the system and mimicking physiological circumstances for the tissue. Six parallels were measured for each type of formulation (F1–F5). PR content of samples was analyzed by HPLC. Apparent permeability was calculated at each sample by using Equation 1.
TEER Measurement in ex vivo Model
During the ex vivo permeability assay, the integrity of porcine corneas was monitored by TEER measurement, to check whether the compounds affect the barrier properties of the model. Ag/AgCl electrodes were used connected with Millicell® ERS-2 Epithelial Volt-Ohm Meter (EMD Millipore Corporation, Billerica, MA, USA). The resistance was measured at 0, 15, 150, and 300 minutes in each cornea containing vertical diffusion chambers. Blank resistance was measured and subtracted to obtain exactly the TEER of ex vivo cornea-based model.
Quantification by High-Performance Liquid Chromatography
The quantitative measurement of PR was performed by high-performance liquid chromatography (HPLC) using Agilent Infinity 1260 (Agilent, Santa Clara, CA, USA). Phenomenex Gemini NX C18 column (150x4.6 mm, 5 µm) was used with the official method of European Pharmacopoeia.37 The following conditions were applied during the analysis: highly purified and filtered water in channel A, HPLC grade acetonitrile/HPLC grade methanol 50%/50% in channel B, 1 mL/min flow rate, 25°C temperature. Gradient elution was used for the separation. Samples were collected from in vitro HCE-T and ex vivo cornea models; 20 µL volume of samples was injected and analyzed on 254.4 nm wavelength.
Statistical Analysis
All data presented are means±SD. The values were compared using the one-way ANOVA followed by Dunnett test by GraphPad Prism 5.0 software (GraphPad Software Inc., San Diego, USA). Changes were considered statistically significant at P<0.05.
Results
Barrier Properties of the Cornea Epithelial Cell Culture Model
HCE-T cell layers showed good barrier properties as reflected by the TEER values (246±7 Ω×cm2, n=3) after 6 days of air-liquid interface condition (Figure 1A). The permeability of HCE-T cell layers was low (Figure 1B) for the hydrophilic marker molecules fluorescein (Papp: 1.05±0.11×10−6 cm/s) and the large biomolecule albumin (Papp: 0.10±0.04×10−6 cm/s).
Cell Viability Assay
Impedance measurement, as a sensitive method to detect cellular effects, showed significant cell damage after treatment with all three formulations containing BK (III, IV, VI). PR containing formulations with HPBCD, HPGCD, ZnHA, and ZnGlu did not show any cytotoxic effect. The normalized cell index was significantly higher in ZnHA/ZnGlu containing sample (V) than in formulation with BK (VI). As a comparison, maximal toxicity was detected in cells treated with the reference damaging agent Triton X-100 detergent (Figure 2).
Immunohistochemistry
The cornea epithelial cells formed a tight paracellular barrier visualized by the localization of the junctional proteins ZO-1, β-catenin, E-cadherin, and occludin. The cells were tightly apposed, and all junctional proteins were localized at the intercellular connections forming pericellular belts in the control groups (Figure 3). Morphological change can be observed in the case of BK-containing sample (VI) by the localization of E-cadherin protein. No major morphological change was seen for the treatment of other groups.
Permeability Study on HCE-T Cell Culture Model
The permeability of PR given in different formulations was tested on the HCE-T cell model (Figure 4). After 30-minute treatment only dissolved PR-CD complex containing formulation 2 (F2) and formulation 3 (F3) showed significantly higher Papp values (F2: 5.97×10−6 cm/s, F3: 5.97×10−6 cm/s) compared with PR suspension (F1: 5.02×10−6 cm/s). Papp values were minimally lower in ZnHA/ZnGlu-containing formulations (F4: 5.59×10−6 cm/s, F5: 5.31×10−6 cm/s). The model showed low Papp values for the two hydrophilic paracellular marker molecules indicating a good barrier.
Ex vivo Permeability Assay
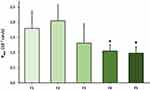
Permeability of PR was tested on an ex vivo porcine cornea model in the case of previously mentioned formulations (F1–F5) (Figure 5). In the case of PR-HPBCD (F2), permeability was higher (2.05×10−7 cm/s) compared with PR-containing suspension (F1, 1.79×10−7 cm/s). Permeability in PR-HPGCD (F3) complex-containing samples was lower (1.31×10−7 cm/s). Considering the relatively high standard deviations in F1–F3, no significant difference can be stated between their Papp values. In the presence of ZnHA/ZnGlu (F4–F5), significantly lower permeability values were measured (F4: 1.05×10−7 cm/s, F5: 0.98×10−7 cm/s). The monitored TEER values were in the range of 1052–2818 Ω cm2.
Discussion
Glucocorticoids are widely used as anti-inflammatory drugs in ophthalmology, mostly applied in the therapy of diseases affecting the anterior segment of the eye. Due to the poor water solubility of steroids, they can be found in suspension formulations on the market. Disadvantageous attributes of suspensions like irritation on the eye may induce fast elimination of drug, therefore frequent application is needed to ensure the proper therapy.4,11 Our aim was a pharmaceutic formulation development, where PR is in water dissolved form with expected increased therapeutic effect. In the early phase of the study, PR-CD inclusion complex was formed and dissolved in a borate-buffer-based environment. The formulations contain ZnHA and ZnGlu to increase the residence time by mucoadhesion and the antimicrobial stability by the preservative effect Zn2+ ion. In this ophthalmic solution, the physiological parameters were set. Previous investigations confirmed that the formulations have adequate mucoadhesiveness with suitable antimicrobial stability (Ph Eur. requirement B).12 Continuing the project, our aim was to test the formulations for toxicity and permeability on an in vitro cell culture model and ex vivo cornea to predict the safety and expected absorption after topical application on the eye.
An immortalized human corneal epithelial cell line was used by in vitro toxicity and permeability tests. The cell culture was established by Araki-Sasaki, whose optimal grown attributes are favorable in the studies of ophthalmic formulations. The toxicity was investigated by impedance measurement after the treatment of several types of formulations. According to the calculated normalized cell index, ZnHA-ZnGlu-containing samples are not toxic, meanwhile BK-containing samples show significantly lower values and the toxic effect can be observed on the HCE-T cells. The toxicity of BK-containing formulations was also demonstrated by immunohistochemistry. Major morphological changes were seen on E-cadherin junctional protein by the cells treated by BK-containing formulations. In the case of target eye drops formulated with ZnHA-ZnGlu, no morphological change and no toxic effect were detected on the cell culture. It can be stated that the ZnHA-ZnGlu combination is a non-toxic alternative preservative system, which is tolerable on HCE-T cells in the applied concentration. The results of BK-containing samples confirmed the previously published toxic and expected irritative effect on the eye surface.
Furthermore, the permeability of PR was tested on HCE-T model in different formulations. The applied in vitro model is suitable for the prediction of drug absorption through the lipophilic epithelial cell layer of the cornea. It needs to be mentioned that this in vitro model only shows the permeation through the epithelial layer, for prediction of transcorneal permeation, ex vivo cornea model is preferred. Meanwhile, testing the samples on cell culture, the absorption through the first obstacle of corneal barrier can be simulated. PR-CD complex-containing solutions and PR-CD-ZnHA-ZnGlu-containing target formulations were studied compared with sample which contains the same amount of PR in suspension form. The electric resistance was monitored, and it confirmed the barrier integrity of cell layers. The permeability was significantly higher in samples containing the dissolved PR-CD complex compared with the suspension. Because the concentration of dissolved API is higher in PR-CD solutions, and higher towards the corneal epithelial cell layer, faster drug permeation is expected through the epithelial layer. In the target formulations with biopolymer, the permeability is minimally lower, due to the diffusion restrictive effect of the polymer structure.
Transcorneal permeation of PR was investigated by ex vivo porcine cornea model. Freshly resected corneas were put into vertical diffusion chambers. O2 was continuously circulated in the chambers to ensure the respiration of cornea, and the donor and receptor phases are mixed during the experiment. The same compositions were tested as in the HCE-T model. The monitored TEER was increased during the experiment, the integrity of the cornea was acceptable, samples did not cause damage and toxic effects on the barrier, which can affect the permeability of PR. Compared with published results, these values meet the requirement of barrier properties.35,37 According to the results, no significant difference was found between the eye drop suspension and solutions. Permeability values were significantly lower in ZnHA-ZnGlu-containing formulations in comparison with the suspension form. Considering the continuous mixing, vertical position, and complexity of porcine cornea, the optimal attributes are not observed in this model in solution-based samples. The permeability is lower in target formulations because polymer structure affects the drug diffusion negatively; however, the retention time is increased on the eye surface due to the increased viscosity and mucoadhesion. In the case of ex vivo study the whole porcine cornea is used, which is complex, formed by lipophilic and hydrophilic layers. Therefore, the difference shall be observed in the permeability on in vitro HCE-T and ex vivo porcine cornea models. The formulations can be optimal in vivo, because less irritation and lachrymal secretion are expected due to the not irritative solution form of eye drops.
As the eye drop contacts for longer time, reflex lachrymal secretion and eye blinking are limited, prolonged drug absorption is expected after administration, which results in less frequent application. We assume that the permeability of PR is optimal in the target formulations, which may result in an enhanced therapeutic effect.
Conclusions
In summary, novel ophthalmic formulations were developed, where PR is dissolved in a water-based environment as CD inclusion complex. Eye drops contain a mucoadhesive and alternative preservative ZnHA-ZnGlu system. We found that the formulations are non-toxic with optimal permeability according to the results of in vitro and ex vivo models. These compositions are promising for overcoming the challenges of ocular drug delivery by increased bioavailability, ensured microbiological stability, minimal irritation, and acceptable patient compliance.
Author Contributions
All authors contributed to the data analysis, drafting, or revising of the article, have agreed on the journal to which the article will be submitted, gave final approval of the version to be published, and agree to be accountable for all aspects of the work.
Funding
This work was supported by the UNKP-19-3-SZTE-26 New National Excellence Program of the Ministry for Innovation and Technology, Campus Mundi Program of Tempus Foundation (EFOP-3.4.2-VEKOP-15-2015-00001) and by the project entitled Topical nanodelivery systems funded by the University of Zagreb (Z169).
Disclosure
The authors declare no conflicts of interest.
References
1. Maharjan P, Cho KH, Maharjan A, Shin MC, Moon C, Min KA. Pharmaceutical challenges and perspectives in developing ophthalmic drug formulations. Int J Pharm Investig. 2018. doi:10.1007/s40005-018-0404-6
2. Ali J, Fazil M, Qumbar M, Khan N, Ali A. Colloidal drug delivery system: amplify the ocular delivery. Drug Deliv. 2016;23(3):700–716. doi:10.3109/10717544.2014.923065
3. Nayak K, Misra M. A review on recent drug delivery systems for posterior segment of eye. Biomed Pharmacother. 2018;107:1564–1582. doi:10.1016/j.biopha.2018.08.138
4. Bíró T, Aigner Z. Current approaches to use cyclodextrins and mucoadhesive polymers in ocular drug delivery—a mini-review. Sci Pharm. 2019;87(3):15. doi:10.3390/scipharm87030015
5. Patel A. Ocular drug delivery systems: an overview. World J Pharmacol. 2013;2(2):47. doi:10.5497/wjp.v2.i2.47
6. Yellepeddi VK, Palakurthi S. Recent advances in topical ocular drug delivery. J Ocul Pharmacol Ther. 2016;32(2):67–82. doi:10.1089/jop.2015.0047
7. Gaudana R, Ananthula HK, Parenky A, Mitra AK. Ocular drug delivery. AAPS J. 2010;12(3):348–360. doi:10.1208/s12248-010-9183-3
8. Prausnitz MR, Noonan JS. Permeability of cornea, sclera, and conjunctiva: a literature analysis for drug delivery to the eye. J Pharm Sci. 1998;87(12):1479–1488. doi:10.1021/js9802594
9. Torrecilla J, Del Pozo-Rodríguez A, Vicente-Pascual M, Solinís MÁ, Rodríguez-Gascón A. Targeting corneal inflammation by gene therapy: emerging strategies for keratitis. Exp Eye Res. 2018;176:130–140. doi:10.1016/j.exer.2018.07.006
10. Lee VHL, Robinson JR. Topical ocular drug delivery: recent developments and future challenges. J Ocul Pharmacol Ther. 1986;2(1):67–108. doi:10.1089/jop.1986.2.67
11. Johannsdottir S, Jansook P, Stefansson E, et al. Topical drug delivery to the posterior segment of the eye: dexamethasone concentrations in various eye tissues after topical administration for up to 15 days to rabbits. J Drug Deliv Sci Technol. 2018;45:449–454. doi:10.1016/j.jddst.2018.04.007
12. Bíró T, Horvát G, Budai-Szűcs M, et al. Development of prednisolone-containing eye drop formulations by cyclodextrin complexation and antimicrobial, mucoadhesive biopolymer. Drug Des Devel Ther. 2018;12:2529–2537. doi:10.2147/DDDT.S165693
13. Mazet R, Choisnard L, Levilly D, Wouessidjewe D, Gèze A. Investigation of combined cyclodextrin and hydrogel formulation for ocular delivery of dexamethasone acetate by means of experimental designs. Pharmaceutics. 2018;10(4):249. doi:10.3390/pharmaceutics10040249
14. Loftsson T, Stefánsson E. Cyclodextrins and topical drug delivery to the anterior and posterior segments of the eye. Int J Pharm. 2017;531(2):413–423. doi:10.1016/j.ijpharm.2017.04.010
15. Loftsson T, Duchene D. Cyclodextrins and their pharmaceutical applications. Int J Pharm. 2007;329(1–2):1–11. doi:10.1016/j.ijpharm.2006.10.044
16. Loftsson T, Stefánsson E. Effect of cyclodextrins on topical drug delivery to the eye. Drug Dev Ind Pharm. 1997;23(5):473–481. doi:10.3109/03639049709148496
17. Szente L. Highly soluble cyclodextrin derivatives: chemistry, properties, and trends in development. Adv Drug Deliv Rev. 1999;36(1):17–28. doi:10.1016/S0169-409X(98)00092-1
18. Adelli GR, Balguri SP, Majumdar S. Effect of cyclodextrins on morphology and barrier characteristics of isolated rabbit corneas. AAPS PharmSciTech. 2015;16(5):1220–1226. doi:10.1208/s12249-015-0315-z
19. Loftsson T. Drug permeation through biomembranes: cyclodextrins and the unstirred water layer. Pharmazie. 2012;5:363–370. doi:10.1691/ph.2012.1698
20. Horvát G, Budai-Szűcs M, Berkó S, et al. Comparative study of nanosized cross-linked sodium-, linear sodium- and zinc-hyaluronate as potential ocular mucoadhesive drug delivery systems. Int J Pharm. 2015;494(1):321–328. doi:10.1016/j.ijpharm.2015.08.024
21. Horvát G, Gyarmati B, Berkó S, et al. Thiolated poly(aspartic acid) as potential in situ gelling, ocular mucoadhesive drug delivery system. Eur J Pharm Sci. 2015;67:1–11. doi:10.1016/j.ejps.2014.10.013
22. Andrews GP, Laverty TP, Jones DS. Mucoadhesive polymeric platforms for controlled drug delivery. Eur J Pharm Biopharm. 2009;71(3):505–518. doi:10.1016/j.ejpb.2008.09.028
23. Khutoryanskiy VV. Advances in mucoadhesion and mucoadhesive polymers. Macromol Biosci. 2011;11(6):748–764. doi:10.1002/mabi.201000388
24. Cook MT, Khutoryanskiy VV. Mucoadhesion and mucosa-mimetic materials—a mini-review. Int J Pharm. 2015;495(2):991–998. doi:10.1016/j.ijpharm.2015.09.064
25. Elbahwy IA, Lupo N, Ibrahim HM, et al. Mucoadhesive self-emulsifying delivery systems for ocular administration of econazole. Int J Pharm. 2018;541(1–2):72–80. doi:10.1016/j.ijpharm.2018.02.019
26. Mansuri S, Kesharwani P, Jain K, Tekade RK, Jain NK. Mucoadhesion: a promising approach in drug delivery system. React Funct Polym. 2016;100:151–172. doi:10.1016/j.reactfunctpolym.2016.01.011
27. Cha S-H, Lee J-S, Oum B-S, Kim C-D. Corneal epithelial cellular dysfunction from benzalkonium chloride (BAC) in vitro. Clin Experiment Ophthalmol. 2004;32(2):180–184. doi:10.1111/j.1442-9071.2004.00782.x
28. Ye J, Wu H, Zhang H, et al. Role of benzalkonium chloride in DNA strand breaks in human corneal epithelial cells. Graefes Arch Clin Exp Ophthalmol. 2011;249(11):1681–1687. doi:10.1007/s00417-011-1755-0
29. Pasquet J, Chevalier Y, Pelletier J, Couval E, Bouvier D, Bolzinger M-A. The contribution of zinc ions to the antimicrobial activity of zinc oxide. Colloids Surf a Physicochem Eng Asp. 2014;457:263–274. doi:10.1016/j.colsurfa.2014.05.057
30. Araki-Sasaki K, Ohashi Y, Sasabe T, Hayashi K, Watanabe H, Tano Y. An SV40-immortalized human corneal epithelial cell line and its characterization. Invest Ophthalmol Vis Sci. 1995;36(3):614–621.
31. Ban Y, Cooper LJ, Fullwood NJ, et al. Comparison of ultrastructure, tight junction-related protein expression and barrier function of human corneal epithelial cells cultivated on amniotic membrane with and without air-lifting. Exp Eye Res. 2003;76(6):735–743. doi:10.1016/S0014-4835(03)00033-2
32. Kürti L, Veszelka S, Bocsik A, et al. The effect of sucrose esters on a culture model of the nasal barrier. Toxicol in Vitro. 2012;26(3):445–454. doi:10.1016/j.tiv.2012.01.015
33. Kiss L, Walter FR, Bocsik A, et al. Kinetic Analysis of the toxicity of pharmaceutical excipients cremophor EL and RH40 on endothelial and epithelial cells. J Pharm Sci. 2013;102(4):1173–1181. doi:10.1002/jps.23458
34. Bocsik A, Walter FR, Gyebrovszki A, et al. Reversible opening of intercellular junctions of intestinal epithelial and brain endothelial cells with tight junction modulator peptides. J Pharm Sci. 2016;105(2):754–765. doi:10.1016/j.xphs.2015.11.018
35. Juretić M, Cetina-Čižmek B, Filipović-Grčić J, Hafner A, Lovrić J, Pepić I. Biopharmaceutical evaluation of surface active ophthalmic excipients using in vitro and ex vivo corneal models. Eur J Pharm Sci. 2018;120:133–141. doi:10.1016/j.ejps.2018.04.032
36. Juretić M, Jurišić Dukovski B, Krtalić I, et al. HCE-T cell-based permeability model: a well-maintained or a highly variable barrier phenotype? Eur J Pharm Sci. 2017;104:23–30. doi:10.1016/j.ejps.2017.03.018
37. Rodríguez I, Vázquez JA, Pastrana L, Khutoryanskiy VV. Enhancement and inhibition effects on the corneal permeability of timolol maleate: polymers, cyclodextrins and chelating agents. Int J Pharm. 2017;529(1–2):168–177. doi:10.1016/j.ijpharm.2017.06.075
 © 2021 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2021 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.