Back to Journals » International Journal of Nanomedicine » Volume 14
Nanodelivery and anticancer effect of a limonoid, nimbolide, in breast and pancreatic cancer cells
Authors Patra A, Satpathy S, Hussain MD
Received 13 March 2019
Accepted for publication 28 August 2019
Published 7 October 2019 Volume 2019:14 Pages 8095—8104
DOI https://doi.org/10.2147/IJN.S208540
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 5
Editor who approved publication: Prof. Dr. Anderson Oliveira Lobo
Arjun Patra,1,2 Swaha Satpathy,1,2 Muhammad Delwar Hussain1
1Department of Pharmaceutical and Biomedical Sciences, College of Pharmacy, California Health Sciences University, Clovis, CA, USA; 2Institute of Pharmacy, Guru Ghasidas University, Bilaspur, CG, India
Correspondence: Muhammad Delwar Hussain
Department of Pharmaceutical and Biomedical Sciences, California Health Sciences University, College of Pharmacy, Mailing address: 120 North Clovis Avenue, Clovis, CA 93612, USA
Tel +1 559 369 2715
Fax +1 559 473 1487
Email [email protected]
Introduction: Nimbolide (Nim), a limonoid obtained from the neem tree, Azadirachta indica, has several pharmacological properties, including anticancer effects in different type of cancers. No drug-delivery system has been reported for enhancing the therapeutic application of this novel hydrophobic molecule.
Methods: In the present research, poly(lactic-co-glycolic acid) (PLGA) nanoparticles of Nim (Nim-nano) were formulated by nanoprecipitation, characterized for physicochemical properties, and screened for anticancer potential in breast (MCF-7 and MDA-MB-231) and pancreatic (AsPC-1) cancer cell lines.
Results: The Nim-nano had a particle size of 183.73±2.22 nm and 221.20±11.03 nm before and after lyophilization, respectively. Cryoprotectants (mannitol and sucrose) significantly inhibited growth in particle size due to lyophilization. The ζ-potential of the Nim-nano was −22.40±4.40 mV. Drug loading and encapsulation efficiency of Nim-nano were 5.25%±1.12% and 55.67%±12.42%, respectively. The Nim-nano exhibited sustained release of Nim for more than 6 days in PBS (pH 7.4) and showed two- to three-fold enhanced cytotoxicity in breast and pancreatic cancer cell lines compared with free Nim.
Conclusion: The Nim-nano formulation has great potential for treatment of cancers, such as pancreatic and breast cancer. Further, the PLGA-polymer surface can be modified by conjugation with polyethylene glycol, receptor-binding ligands (eg, folic acid), and other that which may lead to targeted delivery of Nim in the treatment of cancer.
Keywords: nimbolide, PLGA, nanoparticles, breast cancer, pancreatic cancer
Introduction
Cancer is the most dreadful disease worldwide and the primary cause of death in developed and developing countries.1,2 Globocan 2012 data estimated 14.1 million cancer-diagnosed people worldwide and 8.2 million deaths due to the disease.3 If the current trend continues, the estimated number of new cases would be 22 million and deaths of 13.2 million every year across the world by 2030.4 The current concept of cancer treatment is to use drug combinations or a molecule that can modulate several targets, because cancer is a hyperproliferative disorder mediated through dysregulation of a number of genes and cell-signaling pathways.5 Secondary plant metabolites have been used for a long time for management of various ailments, including cancer, because of their safety, efficacy, easy availability, and low cost.6 Some phytochemicals used clinically as anticancer drugs are vinca alkaloids (vincristine and vinblastine), taxols (paclitaxel and docetaxel), etoposide, and camptothecin derivatives. Furthermore, phytoconstituents can target multiple signaling pathways without serious side effects or toxicity.7
Nimbolide (Nim; 5,7,4’-trihydroxy-3’,5’-diprenylflavanone, Figure 1), a tetranortriterpenoid, was first obtained from flowers and leaves of the neem tree. This bioactive compound belongs to the limonoid group and has a classic limonoid skeleton with an α,β-unsaturated ketone system and δ-lactone nucleus.8 The α,β-unsaturated ketone system of Nim contributes to its anticancer potential.9 Nim is a very hydrophobic drug (molecular weight 466.52) with solubility of ~50 mM in DMSO and ~8 mM in ethanol (product brochure; BioVision, Milpitas, CA, USA). The oral bioavailability of Nim has not been reported. Gupta et al reported only a single-point plasma concentration (2 hours from the last dose) of 222 and 409 ng/mL in mice after 10 days of repetitive intraperitoneal administration of 5 and 20 mg/kg/day of Nim, respectively.6
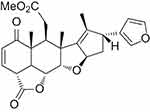 |
Figure 1 Chemical structure of nimbolide. |
Nim has been used as an antimicrobial,10,11 anti-HIV,12 antifeedant,13 antimalarial,14 insecticidal,15 antioxidant and free radical–scavenging,16 anticolitis,17 and anticancer agent. The anticancer nature of Nim has been widely screened against various types of cancer, such as lung carcinoma, melanoma, lymphoma, choriocarcinoma, colon carcinoma, promyleocytic leukemia, cervical carcinoma, hepatoma, hepatic carcinoma, fibrosarcoma, nasopharyngeal carcinoma, breast carcinoma, neuroblastoma, ovarian carcinoma, lymphocytic leukemia, prostrate carcinoma, monocytic leukemia, histiocytic lymphoma, glioblastoma, osteosarcoma, and pancreatic cancer.7,18–22 Besides the anticancer activities of Nim as a single agent, it also shows additive or synergistic properties against cancer in combination with other anticancer agents (5-fluorouracil, thalidomide), various cytotoxic stimuli like TNFα, and TRAIL.6,23 The cellular and molecular mechanism by which Nim produces cytotoxicity includes inhibition of cell-cycle progression, cell survival, migration, invasion, and initiation of apoptosis. The different targets altered by Nim are transcription factors, cytokines, growth factors and their receptors, enzymes, and genes controlling cell propagation and apoptosis.7,18,19
In addition to in vitro investigation in different cell lines, Nim has also been explored in vivo against colorectal cancer, lymphoma, brain cancer, Waldenström's macroglobulinemia tumor–xenografted mouse models, 7,12-dimethylbenz[a]anthracene-induced buccal pouch carcinogenesis, and oral carcinogenesis.6,16,24–26 The LD50 of Nim administered intraperitoneally in adult male mice is 225 mg/kg and intravenously 24 mg/kg. Toxicity is markedly decreased when administered via oral, subcutaneous, and intramuscular routes, with the LD50 >600 mg/kg.18
Novel drug-delivery systems of drug molecules are at the center of pharmaceutical research and development for treatment of diseases like cancer. Recent developments in nanotechnology have radically changed the method of treatment, diagnosis, and prevention of cancers. Developing nanoparticle (NP) formulations have several advantages such as increased solubility and bioavailability, protection from physical and chemical degradation, improved tissue distribution, sustained delivery, enhancement of pharmacological activity, targeted delivery, and decreased toxicity.27,28
Polymeric NPs are useful nanocarriers for targeted delivery of drugs. Commonly used polymers for formulation of NPs include poly(lactic-co-glycolic acid) (PLGA), polylactic acid, dextran, and chitosan. The polymer should be easily metabolized and removed from the body after administration of polymeric formulations. In this regard, biodegradable polymers are most suitable for formulation development.29–31 NP surfaces can also be sterically stabilized to decrease hepatic uptake and improve the enhanced permeability–retention effect.32–34 PLGA is a US Food and Drug Administration (FDA) approved polymer for delivery of drugs, and is one of the most commonly used polymers because of its excellent biodegradability, biocompatibility, and mechanical strength.35–37 PLGA NPs have also been explored for incorporation of single drug38 and dual agents to decrease the toxicity and enhance the efficacy of a drug.39
The effects of Nim and its mechanisms of action have been studied extensively in breast (MCF-7 and MDA-MB-231)40–42 and pancreatic cancer cells (PANC-1).22 No drug-delivery system has been reported for enhancing the therapeutic application of this novel hydrophobic molecule. NP formulation of Nim may help in delivery to cancer cells, sustained release, enhanced pharmacological activity, and decreased toxicity of the moiety. Also, PLGA is used in FDA approved drug products. In the current study, we formulated and characterized PLGA-based NPs of Nim (Nim-nano) to enhance its therapeutic potential. The formulation was evaluated for its cytotoxicity in breast (MCF-7 and MDA-MB-231), and pancreatic (AsPC-1) cancer cells.
Methods
Preparation of PLGA nanoparticles of Nim
NPs were prepared using nanoprecipitation.33,43 Briefly, 1 mg Nim (BioVision Incorporation) and 9 mg PLGA (50:50, inherent viscosity range 0.15–0.25 dL/g; Durect, Pelham, AL, USA) were dissolved in 1 mL acetone. Ten mL of a 1.5% w:v solution of polyvinyl alcohol (Alfa Aesar, Ward Hill, MA, USA) was taken in a beaker and the drug–polymer solution was added in a controlled manner (0.1 mL/min). The mixture was stirred for 5 hours at 500 rpm on a magnetic stirrer to evaporate acetone. The resulting suspension of NPs was centrifuged at 25,000 g (Allegra 25R centrifuge; Beckman Coulter, Brea, CA, USA) for 1 hour at 8°C. The NP pellet was suspended in deionized (DI) water after discarding the supernatant and centrifuged again in the same conditions. The resulting pellet was suspended in 3 mL DI water by vortexing. The suspension was transferred to a clean and dry amber-colored bottle, kept at −80°C for 2 hours, and then lyophilized overnight (FreeZone Plus; Labconco). Blank NPs were also prepared similarly by adding 10 mg PLGA to 1 mL acetone without the drug. NP preparation was performed in triplicate under light-protected conditions.
Determination of particle size and ζ-potential
Mean particle size, size distribution and ζ-potential of NPs were determined using a NanoBrook 90 Plus PALS (Brookhaven Instruments, Holtsville, NY, USA). All measurements were performed in triplicate. Freeze-drying increases particle size, which can be reduced by addition of cryoprotectants before lyophilization. The effect of mannitol and sucrose as cryoprotectants on particle size of NPs was determined by taking a small volume of NP suspension in an amber vial, to which an equal volume of either sucrose or mannitol solution was added to make final concentrations of 5% or 10% w:v, respectively.33 Suspensions were lyophilized as mentioned earlier. Sizes of the lyophilized NPs with or without cryoprotectant were determined by reconstitutition in 3 mL DI water and sonication for a few seconds.
Determination of drug loading and encapsulation efficiency
For drug-loading and encapsulation-efficiency determination, 1 mg lyophilized NPs was dissolved in 1 mL acetone by sonication in an amber glass vial. The content was kept at room temperature for 1 hour and then filtered through a 0.22 µM PVDF membrane filter (Millex GV syringe-driven filter unit; Millipore, Bedford, MA, USA). Absorbance of the filtrate was measured by ultraviolet-visible spectrophotometry (Varioskan Flash; Thermo Fisher Scientific) at 207 nm against a blank (empty-NP solution prepared similarly in the same concentration). Encapsulation efficiency was calculated by measuring the amount of drug present in the NPs compared to the amount of drug used for preparation of the same amount of NPs. Drug loading was calculated by measuring the amount of drug present in the NPs compared to the total amount of polymer and drug taken for the preparation of the same amount of NP formulation.
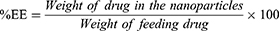
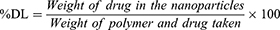
In vitro release
A dialysis-bag method was employed for determining in vitro release of drug from NPs. PBS (pH 7.4) was used as the release media. NPs containing 500 µg Nim in 0.5 mL PBS were put in a dialysis bag (molecular-weight cutoff 6,000–8,000 Da; Spectrum Laboratories) and sealed from both ends. PBS (30 mL) was added to an amber glass container, and then the sealed bag containing NPs was transferred into it. The glass container was allowed to shake horizontally at 37°C and 100 rpm on a horizontally shaking incubator (VWR). Release medium (1 mL) was taken out at predetermined time intervals (1, 2, 4, 8, 24, 48, 72, 96, 120, and 144 hours) and the amount of Nim in the media measured by ultraviolet-visible spectrophotometry. The same amount of fresh medium was added to the containers after each sample withdrawal. The percentage of drug released was calculated from the equation:
% drug released = (amount of Nim in the medium [µg]/amount of Nim loaded in the NPs [µg]) × 100
In vitro cytotoxicity
In vitro anticancer activity of Nim PLGA NPs (Nim-nano) was evaluated in AsPC-1 (pancreatic cancer cell line), and breast cancer cell lines (MCF-7 and MDA-MB-231) by MTT assay. All cell lines were obtained from the American Type Culture Collection (Manassas, VA, USA). Pancreatic cells were grown in RPMI 1640 medium and breast cancer cells in DMEM (Mediatech, Manassas, VA). Media were supplemented with 10% FBS and 1% penicillin–streptomycin. Breast (3,000 cells/well) and pancreatic (4,000 cells/well) cancer cells were transferred to 96-well culture plates and incubated at 37°C in a humidified atmosphere of 5% CO2 for 24 hours. The culture medium was then taken out carefully and the cells treated with fresh medium (control) or different concentrations of pure Nim in medium or various concentrations of Nim-nano in medium. Plates were again incubated for 72 hours in similar conditions, then the medium was removed and the cells washed with PBS (pH 7.4). Fifty microliter of a 0.5 mg/mL solution of 3-(4,5-dimethylthiazol-2-yl)-3,5-diphenyl tetrazolium bromide (MTT, Sigma-Aldrich) prepared in respective media was added to each well and further incubated for 4 hours. Purple formazan was formed by reaction of MTT with mitochondrial succinate dehydrogenase enzymes of the live cells. This formazan complex was dissolved by adding 100 µL DMSO to each well after removing the medium carefully. Percentage cell viability with different treatments was calculated by measuring absorbance at 570 nm on a micro-plate reader (Varioskan Flash; Thermo Fisher Scientific, USA), considering absorbance of the blank (treatment with fresh medium only, control) as 100% growth. IC50 was calculated by GraphPad Prism 7.0 (GraphPad Software, La Jolla, CA, USA).
Statistical analysis
Data are presented as means ± SD/SEM. Student's t-test was used for statistical analysis. P<0.05 was considered statistically significant.
Results
Characterization of Nim-nano
The average particle size of Nim-nano was 183.73±2.22 nm and 221.20±11.03 nm before and after lyophilization, respectively (Figure 2). Lyophilization increased particle size by 20%. Polydispersity-index (PDI) values before and after lyophilization were 0.058±0.022 and 0.168±0.033, respectively. Lyophilized Nim-nano had negative ζ-potential (−22.40±4.40 mV) (Figure 3). This may improve the stability of the Nim-nano, as negative ζ-potential of particles cause electricals repulsion among them and prevents their aggregation.44 Average drug loading and encapsulation efficiency of the lyophilized Nim-nano were 5.25%±1.12% w:w (0.0113 M Nim/100 g Nim-nano) and 55.67%±12.42%, respectively. Varied percentages of drug loading (5.43%–16.98%) and encapsulation efficiency (37.8%–88.4%) have been reported in the literature for different drugs.34,45–47
The effect of mannitol (10% w:v) and sucrose (5% w:v) as cryoprotectants on particle-size growth of lyophilized Nim-nano was assessed, and particle-size increase was significantly decreased by both cryoprotectants. Mannitol 10% was more effective in reducing the size of the NPs after lyophilization (Figure 4). Both cryoprotectants also reduced the PDI of the Nim-nano (0.083±0.050 and 0.143±0.045, respectively, for mannitol and sucrose) after lyophilization.
 |
Figure 2 Particle size of Nim-nano before lyophilization (A) and after lyophilization (B). |
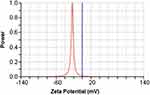 |
Figure 3 ζ-potential of Nim-nano after lyophilization. |
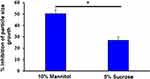 |
Figure 4 Effect of cryoprotectants on size of Nim-nano after lyophilization (means ± SD, n=3 each batch). *P<0.05 vs sucrose. |
In vitro drug release
In vitro release of Nim from the Nim-nano exhibited an initial burst release of drug from the NPs: approximately 20% of drug was released after 1 hour, followed by sustained release (Figure 5). After 6 days, the percentage cumulative release of Nim was >80%. The initial fast release of Nim may have been because of the surface-bound Nim on the NPs, which released quickly by diffusion. The remaining drugs are embedded in the NP structure, and for release of these drugs, the polymer needs to be degraded by hydrolysis. This caused the drug to be released over a few days.
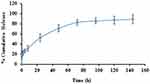 |
Figure 5 Release study of Nim-nano in PBS, pH 7.4 (means ± SEM, n=3 each batch). |
In vitro cytotoxicity
In vitro cytotoxic effects of free Nim, Nim-nano, and empty PLGA NPs (without drug) were evaluated in breast and pancreatic cancer cell lines by MTT assays and are illustrated in Figure 6. Nim-nano exhibited significantly higher cytotoxicity than pure Nim against all three cell lines tested. IC50 of Nim in MCF-7, MDA-MB-231, and AsPC-1 cell lines was 4.02, 2.24, and 2.30 µM, respectively. IC50 of Nim-nano was approximately three-fold, two-fold, and three-fold, respectively, less than the pure drug (P<0.05, Figure 7). Further, the empty PLGA NPs showed negligible cytotoxicity in all three cell lines (Figure 6).
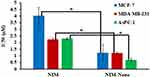 |
Figure 7 IC50 values of Nim and Nim-nano in MCF-7, MDA-MB-231, and AsPC-1 cells after 72 hours of incubation (means ± SD, n=3 different each batch). *P<0.05. |
Discussion
Physicochemical characteristics, such as size, size distribution, and surface charge, are functional performance features for NP-based delivery of drugs.48,49 The observed particle size of the Nim-nano was in the acceptable size range for uptake in tumor cells by enhanced permeation–retention effects.50 The non-solvent used in the preparation of NPs contributes to mean particle size.38 In the present study, acetone–water (a commonly used pairing)48 was employed for formulation of Nim-nano. We did not determine the morphology or shape of the NPs. PLGA NPs are usually spherical, as confirmed by SEM and TEM analyses in earlier studies.34,46 ζ-Potential is an indicator of NP, stability and high ζ-potential causes electrostatic repulsion among NPs, preventing aggregation and increase in particle size.44 Further, negative ζ-potential of formulations is beneficial for increasing circulation time and drug delivery.51 During freeze-drying, the size of NPs increases by aggregation, due to considerable changes in physical stress and introduction of freezing and drying.33,52,53 Particle size plays an important role in the tissue distribution and cellular internalization of NPs.54 The smaller the NPs, the better they will be on therapeutic efficacy, because of the enhanced permeability–retention effect. Cryoprotectant, normally a sugar, may prevent NPs from aggregation and extreme conditions of freezing by forming hydrogen bonds with the NPs and hence decreasing growth.53,55 In the present study, the effect of mannitol (10% w:v) and sucrose (5% w:v) as cryoprotectants on particle-size growth was studied, and particle-size increase due to lyophilization was significantly inhibited by both cryoprotectants. Similarly to our earlier studies,33 10% mannitol was more effective in reducing the size of NPs after lyophilization. We did not study the role of cryoprotectants in cumulative release and cell viability. The NPs characterized and used for cell-viability assays were the lyophilized NPs without cryoprotectants. In the current study, we reported the role of cryoprotectants in controlling the increase in particle size due to lyophilization. This will be valuable for further development of the PLGA NPs for pharmaceutical development.
The release of Nim from Nim-nano was sustained, due to encapsulation of Nim inside the PLGA NPs (Figure 5). More than 90% of naïve Nim (dissolved in DMSO) was released within 2 hours from the dialysis bag to the release media (data not shown, as this period was very short compared to the days of sustained release of Nim from the Nim-nano). Drug release from the polymeric NPs can take place either by diffusion from the polymer surface and matrix or by diffusion and swelling of the polymer,56 which are controlled by the nature of the polymer and physicochemical characteristics and surface-erosion properties of the NPs.57 A biphasic release pattern of Nim from Nim-nano was observed in the present study, which has also been reported for other hydrophobic drugs.33,58 The initial burst release may have been because of the release of weakly bound drugs on/near the surface of the NPs by diffusion from the surface layer. The remaining drug embedded in the core of Nim-nano showed controlled/slow release from NPs, most likely due to degradation of the polymer by hydrolysis and diffusion.34
The anticancer activity in different cell lines of Nim-nano was significantly higher than pristine Nim, as indicated in cell-viability and IC50 values. The enhanced cytotoxicity of Nim-nano may have been due to sustained release of the drug,51 increased cellular uptake, and less efflux of the drug by Pgp pumps.59 The effect of both Nim and Nim-nano was investigated only after 72 hours of treatment to compare the effect of Nim and Nim-nano. This time for in vitro anticancer activity was selected, as release of the drug from Nim-nano had been sustained at least for 72 hours in the drug-release study (around 80% Nim released, Figure 5). In future, we would like to investigate and compare the effects of Nim and Nim-nano earlier, such as at 24 and 48 hours.
In the presence of highest concentrations (6.25 and 12.5 µM), the cytotoxic effect was similar for Nim and Nim-nano in MDA-MB-231, whereas in MCF-7 and AsPC-1 cells Nim-nano was more active at these concentrations (Figure 6). Also, the reduction in IC50 of Nim-nano compared to Nim was more pronounced in MCF-7 and AsPC-1 cells than MDA-MB-231 cells (Figure 7). This may have been due to different potency and mechanism of action of Nim against different cell lines, which remains to be studied in future.
Earlier studies have demonstrated that Nim reduces the movement and invasive potential of MCF-7 and MDA-MB-231 cell lines by downregulation of uPA, uPAR, chemokines, pEGFR, VEGFR, NFкB, IKKα, IKKβ, MMP2, and MMP9 and upregulation of TIMP2.40 Nim also inhibits cell propagation and cell survival by downregulation of IGF1R–Akt–ERK signaling, which accumulates G0/G1 cells and downregules cyclin protein expression,41 causes apoptosis by both intrinsic and extrinsic pathways, increases levels of proapoptotic proteins Bax, Bad, FasL, TRAIL, FADDR, and cytochrome C, and downregulates, the anti-apoptotic proteins Bcl2, BclxL, Mcl1 and XIAP1.42 In the present study, the enhanced effect of Nim-nano might have been due to increased cellular uptake and less efflux by Pgp pumps with the same mode of action.
The present research involved the formulation, characterization, and in vitro cytotoxicity of Nim-nano. No NP-based drug-delivery system has been reported for this novel molecule. Further in vitro studies such as cell-uptake studies, molecular mechanisms of anticancer effect, elucidation of the type of cell death (necrosis or apoptosis), and flow cytometry are warranted, in addition to in vivo investigations of Nim and Nim-nano in breast and pancreatic cancer.
Conclusion
We formulated and characterized a nanodelivery system of the potent natural anticancer molecule Nim. Nim-nano was more effective than pure Nim in breast and pancreatic cancer cell lines. Nim-nano exhibited sustained release of Nim in PBS. Although in vitro studies are reliable, correlating these studies with in vivo experiments would be of great value. The future scope of this project would be optimization of the PLGA-based nanoformulation of the drug and preclinical studies in animal models of cancer. The current NP formulation of Nim has potential for development into a pharmaceutical product. Also, this research will initiate further research into other drug-delivery systems for Nim and improvement of the current Nim-nano. In future, the PLGA-polymer surface can be modified by conjugation with polyethylene glycol, receptor-binding ligands (eg, folic acid), and other moieties. This will lead to targeted delivery of Nim and effective treatment of breast and pancreatic cancer.
Acknowledgment
Financial support from the University Grants Commission, New Delhi, India to Arjun Patra for postdoctoral research under Raman Fellowship (F.NO.5-63/2016[IC]) is greatly acknowledged.
Disclosure
The authors report no conflicts of interest in this work.
References
1. Jemal A, Bray F, Center MM, Ferlay J, Ward E, Forman D. Global cancer statistics. CA Cancer J Clin. 2011;61(2):69–90. doi:10.3322/caac.20107
2. Hanahan D, Weinberg RA. Hallmarks of cancer: the next generation. Cell. 2011;144(5):646–674. doi:10.1016/j.cell.2011.02.013
3. GLOBOCAN. Estimated Cancer Incidence, Mortality and Prevalence Worldwide in 2012; 2012 Available from: http://globocan.iarc.fr.
4. Ferlay J, Shin HR, Bray F, Forman D, Mathers C, Parkin DM. Estimates of worldwide burden of cancer in 2008: GLOBOCAN 2008. Int J Cancer. 2010;127(12):2893–2917. doi:10.1002/ijc.25516
5. Hasima N, Aggarwal BB. Cancer-linked targets modulated by curcumin. Int J Biochem Molec Biol. 2012;3(4):328–351.
6. Gupta SC, Prasad S, Sethumadhavan DR, Nair MS, Mo YY, Aggarwal BB. Nimbolide, a limonoidtriterpene, inhibits growth of human colorectal cancer xenografts by suppressing the proinflammatory microenvironment. Clin Cancer Res. 2013;19(16):4465–4476. doi:10.1158/1078-0432.CCR-13-0080
7. Bodduluru LN, Kasala ER, Thota N, Barua CC, Sistla R. Chemopreventive and therapeutic effects of nimbolide in cancer: the underlying mechanisms. Toxicol In Vitro. 2014;28(5):1026–1035. doi:10.1016/j.tiv.2014.04.011
8. Anitha G, Josepha LRJ, Narasimhan S, Anand SK, Rajan SS. Nimbolide and isonimbolide. J Asian Nat Prod Res. 2006;8(5):445–449. doi:10.1080/10286020500173267
9. Sastry BS, Babu KS, Babu TH, et al. Synthesis and biological activity of amide derivatives of nimbolide. Bioorg Med Chem Lett. 2006;16(16):4391–4394. doi:10.1016/j.bmcl.2006.05.105
10. Biswas K, Chattopadhyay I, Banerjee RK, Bandyopadhyay U. Biological activities and medicinal properties of neem (Azadirachta indica). Curr Sci. 2002;82(11):1336–1345.
11. Sarkar P, Acharyya S, Banerjee A, et al. Intracellular, biofilm-inhibitory and membrane-damaging activities of nimbolide isolated from Azadirachta indica A. Juss (Meliaceae) against meticillin-resistant Staphylococcus aureus. J Med Microbiol. 2016;65(10):1205–1214. doi:10.1099/jmm.0.000343
12. Udeinya IJ, Mbah AU, Chijioke CP, Shu EN. An antimalerial extract from neem leaves is antiretroviral. Trans R Soc Trop Med Hyg. 2004;98(7):435–437. doi:10.1016/j.trstmh.2003.10.016
13. Suresh G, Gopalakrishnan G, Wesley SD, Singh PN, Malathi R, Rajan S. Insect antifeedant activity of tetranortriterpenoids from the rutales. A perusal of structural relations. J Agric Food Chem. 2002;50(16):4484–4490. doi:10.1021/jf025534t
14. Rochanakij S, Thebtaranonth Y, Yenjai C, Yuthavong Y. Nimbolide, a constituent of Azadirachta indica, inhibits Plasmodium falciparum in culture. Southeast Asian J Trop Med Public Health. 1985;16(1):66–72.
15. Cohen E, Quistad GB, Jefferies PR, Casida JE. Nimbolide is the principal cytotoxic component of neem seed insecticide preparations. Pest Manag Sci. 1996;48:135–140. doi:10.1002/(SICI)1096-9063(199610)48:2<135::AID-PS451>3.0.CO;2-J
16. Priyadarsini RV, Manikandan P, Kumar GH, Nagini S. The neem limonoids azadirachtin and nimbolide inhibit hamster cheek pouch carcinogenesis by modulating xenobiotic-metabolizing enzymes, DNA damage, antioxidants, invasion and angiogenesis. Free Radic Res. 2009;43(5):492–504. doi:10.1080/10715760902870637
17. Seo JY, Lee C, Hwang SW, Chun J, Im JP, Kim JS. Nimbolide inhibits nuclear factor-КB pathway in intestinal epithelial cells and macrophages and alleviates experimental colitis in mice. Phytother Res. 2016;30(10):1605–1614. doi:10.1002/ptr.v30.10
18. Wang L, Phan DDK, Zhang J, et al. Anticancer properties of nimbolide and pharmacokinetic considerations to accelerate its development. Oncotarget. 2016;7(28):44790–44802.
19. Elumalai P, Arunakaran J. Review on molecular and chemopreventive potential of nimbolidein cancer. Genomics Inform. 2014;12(4):156–164. doi:10.5808/GI.2014.12.4.156
20. Singh PR, Priya ES, Balakrishnan S, et al. Nimbolide inhibits androgen independent prostate cancer cells survival and proliferation by modulating multiple pro-survival signaling pathways. Biomed Pharmacother. 2016;84:1623–1634. doi:10.1016/j.biopha.2016.10.076
21. Singh PR, Priya ES, Balakrishnan S, et al. Inhibition of cell survival and proliferation by nimbolide in human androgen-independent prostate cancer (PC-3) cells: involvement of the PI3K/Akt pathway. Mol Cell Biochem. 2017;427(1–2):69–79. doi:10.1007/s11010-016-2898-4
22. Subramani R, Gonzalez E, Arumugam A, et al. Nimbolide inhibits pancreatic cancer growth and metastasis through ROS-mediated apoptosis and inhibition of epithelialtomesenchymal transition. Sci Rep. 2016;6:19819. doi:10.1038/srep19819
23. Gupta SC, Prasad S, Reuter S, et al. Modification of cysteine 179 of IkBa kinase by nimbolide leads to down-regulation of NF-kB-regulated cell survival and proliferative proteins and sensitization of tumor cells to chemotherapeutic agents. J Biol Chem. 2010;285(46):35406–35417. doi:10.1074/jbc.M110.161984
24. Chitta K, Paulus A, Caulfield TR, et al. Nimbolide targets BCL2 and induces apoptosis in preclinical models of waldenstroms macroglobulinemia. Blood Cancer J. 2014;4:e260. doi:10.1038/bcj.2014.74
25. Karkare S, Chhipa RR, Anderson J, et al. Direct inhibition of retinoblastoma phosphorylation by nimbolide causes cell-cycle arrest and suppresses glioblastoma growth. Clin Cancer Res. 2014;20(1):199–212. doi:10.1158/1078-0432.CCR-13-0762
26. Kumar GH, Priyadarsini RV, Vinothini G, Letchoumy PV, Nagini S. The neem limonoids azadirachtin and nimbolide inhibit cell proliferation and induce apoptosis in an animal model of oral oncogenesis. Invest New Drugs. 2010;28(4):392–401. doi:10.1007/s10637-009-9263-3
27. Kushwaha SKS, Rastogi A, Rai AK, Singh S. Novel drug delivery system for anticancer drug: a review. Int J PharmTech Res. 2012;4(2):542–553.
28. Estanqueiro M, Amaral MH, Conceicao J, Lobo JMS. Nanotechnological carriers for cancer chemotherapy: the state of the art. Colloids Surf B Biointerfaces. 2015;126:631–648. doi:10.1016/j.colsurfb.2014.12.041
29. Duncan R. Polymer conjugates as anticancer nanomedicines. Nat Rev Cancer. 2006;6(9):688–701. doi:10.1038/nrc1958
30. Lammers T, Subr V, Ulbrich K, Hennink WE, Storm G, Kiessling F. Polymeric nanomedicines for image-guided drug delivery and tumor targeted combination therapy. Nano Today. 2010;5(3):197–212. doi:10.1016/j.nantod.2010.05.001
31. Panyam J, Zhou WZ, Prabha S, Sahoo SK, Labhasetwar V. Rapid endolysosomal escape of poly(DL-lactide-co-glycolide) nanoparticles: implications for drug and gene delivery. Faseb J. 2002;16(10):1217–1226. doi:10.1096/fj.02-0088com
32. Gref R, Luck M, Quellec P, et al. Stealth’corona-core nanoparticles surface modified by polyethylene glycol (PEG): influences of the corona (PEG chain length and surface density) and of the core composition on phagocytic uptake and plasma protein adsorption. Colloids Surf B Biointerfaces. 2000;18:301–313. doi:10.1016/S0927-7765(99)00156-3
33. Saxena V, Naguib Y, Hussain MD. Folate receptor targeted 17-allylamino-17-demethoxygeldanamycin (17-AAG) loaded polymeric nanoparticles for breast cancer. Colloids Surf B Biointerfaces. 2012;94:274–280. doi:10.1016/j.colsurfb.2012.02.001
34. El-Hammadi MM, Delgado AV, Melguizo C, Prados JC, Arias JL. Folic acid-decorated and PEGylated PLGA nanoparticles for improving the antitumour activity of 5-fluorouracil. Int J Pharm. 2017;516(1–2):61–70. doi:10.1016/j.ijpharm.2016.11.012
35. Athanasiou KA, Niederauer GG, Agrawal C. Sterilization, toxicity, biocompatibility and clinical applications of polylactic acid/polyglycolic acid copolymers. Biomaterials. 1996;17(2):93–102. doi:10.1016/0142-9612(96)85754-1
36. Jain RA. The manufacturing techniques of various drug loaded biodegradable poly (lactide-co-glycolide)(PLGA) devices. Biomaterials. 2000;21(23):2475–2490. doi:10.1016/S0142-9612(00)00115-0
37. Bala I, Hariharan S, Ravi K. PLGA nanoparticles in drug delivery: the state of the art. Crit Rev Ther Drug Carrier Syst. 2004;21(5):387–422. doi:10.1615/CritRevTherDrugCarrierSyst.v21.i5.20
38. Bilati U, Allemann E, Doelker E. Development of a nanoprecipitation method intended for the entrapment of hydrophilic drugs into nanoparticles. Eur J Pharm Sci. 2005;24(1):67–75. doi:10.1016/j.ejps.2004.09.011
39. Song X, Zhao Y, Wu W, et al. PLGA nanoparticles simultaneously loaded with vincristine sulfate and verapamil hydrochloride: systematic study of particle size and drug entrapment efficiency. Int J Pharm. 2007;350(1–2):320–329. doi:10.1016/j.ijpharm.2007.08.034
40. Elumalai P, Mercy AB, Arunkamar R, et al. Nimbolide inhibits invasion and migration, and down-regulates uPAR chemokine gene expression, in two breast cancer cell lines. Cell Prolif. 2014;47(6):540–552. doi:10.1111/cpr.2014.47.issue-6
41. Elumalai P, Arunkumar R, Benson CS, Sharmila G, Arunakaran J. Nimbolide inhibits IGF-I-mediated PI3K/Akt and MAPK signalling in human breast cancer cell lines (MCF-7 and MDA-MB-231). Cell Biochem Funct. 2014;32(5):476–484.
42. Elumalai P, Gunadharini DN, Senthilkumar K, et al. Induction of apoptosis in human breast cancer cells by nimbolide through extrinsic and intrinsic pathway. Toxicol Lett. 2012;215(2):131–142. doi:10.1016/j.toxlet.2012.10.008
43. Song X, Zhao Y, Hou S, et al. Dual agents loaded PLGA nanoparticles: systematic study of particle size and drug entrapment efficiency. Eur J Pharm Biopharm. 2008;69(2):445–453. doi:10.1016/j.ejpb.2008.01.013
44. Honary S, Zahir F. Effect of zeta potential on the properties of nano-drug delivery systems - a review (Part 2). Trop J Pharm Res. 2013;12(2):265–273.
45. Patel J, Amrutiya P, Bhatt P, Javia A, Jain M, Misra A. Targeted delivery of monoclonal antibody conjugated docetaxel loaded PLGA nanoparticles into EGFR overexpressed lung tumour cells. J Microencapsul. 2018;35(2):204–217. doi:10.1080/02652048.2018.1453560
46. Kang BS, Choi JS, Lee SE, et al. Enhancing the in vitro anticancer activity of albendazole incorporated into chitosan-coated PLGA nanoparticles. Carbohyd Polym. 2017;159:39–47. doi:10.1016/j.carbpol.2016.12.009
47. Sun SB, Liu P, Shao FM, Miao QL. Formulation and evaluation of PLGA nanoparticles loaded capecitabine for prostate cancer. Int J Clin Exp Med. 2015;8(10):19670–19681.
48. Perrault SD, Walkey C, Jennings T, Fischer HC, Chan WC. Mediating tumor targeting efficiency of nanoparticles through design. Nano Lett. 2009;9(5):1909–1915. doi:10.1021/nl900031y
49. Galindo-Rodriguez S, Allemann E, Fessi H, Doelker E. Physicochemical parameters associated with nanoparticle formation in the salting-out, emulsification-diffusion, and nanoprecipitation methods. Pharm Res. 2004;21(8):1428–1439. doi:10.1023/B:PHAM.0000036917.75634.be
50. Yan F, Zhang C, Zheng Y, et al. The effect of poloxamer 188 on nanoparticle morphology, size, cancer cell uptake, and cytotoxicity. Nanomedicine. 2010;6(1):170–178. doi:10.1016/j.nano.2009.05.004
51. Pimple S, Manjappa AS, Ukawala M, Murthy RSR. PLGA nanoparticles loaded with etoposide and quercetin dihydrate individually: in vitro cell line study to ensure advantage of combination therapy. Cancer Nano. 2012;3(1–6):25–36. doi:10.1007/s12645-012-0027-y
52. Beirowski J, Inghelbrecht S, Arien A, Gieseler H. Freeze-drying of nanosuspensions, 1: freezing rate versus formulation design as critical factors to preserve the original particle size distribution. J Pharm Sci. 2011;100(5):1958–1968. doi:10.1002/jps.22425
53. Hirsjarvi S, Peltonen L, Hirvonen J. Effect of sugars, surfactant, and tangential flow filtration on the freeze-drying of poly(lactic acid) nanoparticles. AAPS PharmSciTech. 2009;10(2):488–494. doi:10.1208/s12249-009-9236-z
54. Tang L, Yang X, Yin Q, et al. Investigating the optimal size of anticancer nanomedicine. PNAS. 2014;111(43):15344–15349. doi:10.1073/pnas.1411499111
55. Kamiya S, Kurita T, Miyagishima A, Itai S, Arakawa M. Physical properties of griseofulvin-lipid nanoparticles in suspension and their novel interaction mechanism with saccharide during freeze-drying. Eur J Pharm Biopharm. 2010;74(3):461–466. doi:10.1016/j.ejpb.2009.12.004
56. Mu L, Feng SS. PLGA/TPGS nanoparticles for controlled release of paclitaxel: effect of emulsifier and drug loading ratio. Pharm Res. 2003;20(11):1864–1872. doi:10.1023/B:PHAM.0000003387.15428.42
57. Muthu MS, Kulkarni SA, Raju A, Feng SS. Theranostic liposomes of TPGS coating for targeted co-delivery of docetaxel and quantum dots. Biomaterials. 2013;33(12):3494–3501. doi:10.1016/j.biomaterials.2012.01.036
58. Gõmez-Gaete C, Tsapis N, Besnard M, Bochot A, Fattal E. Encapsulation of dexamethasone into biodegradable polymeric nanoparticles. Int J Pharm. 2007;331(2):153–159. doi:10.1016/j.ijpharm.2006.11.028
59. Fojo T, Coley HM. The role of efflux pumps in drug-resistant metastatic breast cancer: new insights and treatment strategies. Clin Breast Cancer. 2007;7(10):749–756. doi:10.3816/CBC.2007.n.035
 © 2019 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2019 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.

