Back to Journals » Drug Design, Development and Therapy » Volume 9
Myofibrotic malformation vessels: unique angiodysplasia toward the progression of hemorrhoidal disease
Authors Li S, Jing F, Ma L, Guo L, Na F, An S, Ye Y, Yang J, Bao M, Kang D, Sun X, Deng Y
Received 9 June 2015
Accepted for publication 2 July 2015
Published 13 August 2015 Volume 2015:9 Pages 4649—4656
DOI https://doi.org/10.2147/DDDT.S90209
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 2
Editor who approved publication: Prof. Dr. Wei Duan
Sheng-Long Li,1,* Fang-Yan Jing,1–3,* Li-Li Ma,2,3 Li-Li Guo,2,3 Feng Na,2,3 Sheng-Li An,4 Yan Ye,5 Jun-Ming Yang,1 Ming Bao,1 Dong Kang,1 Xiao-Lan Sun,1 Yong-Jian Deng2,3
1Department of Anorectal Surgery, 2Department of Pathology, Nanfang Hospital, 3Department of Pathology, School of Basic Medical Sciences, 4Department of Biostatistics, Southern Medical University, 5Department of General Surgery, Xintang Hospital, Zengcheng, Guangzhou, People’s Republic of China
*These authors contributed equally to this work
Background: The etiology and pathogenesis of hemorrhoids is unclear, although hemorrhoids are a worldwide disease in men and women, with peak prevalence at 45–65 years of age. Hemorrhoidal cushions as the anal venous plexi are normal anatomical structures from infancy. This study attempts to reveal the angiodysplasia and other pathological changes in association with different degrees of symptomatic hemorrhoids.
Materials and methods: A total of 281 patients with internal hemorrhoids from degree I to IV underwent hemorrhoidectomy. The vascular changes were analyzed by microscopic assessment and software analysis, with Masson’s trichrome, CD34, and smooth muscle actin.
Results: The hemorrhoidal tissues exhibited abnormal vessels in the mucosae and submucosae that we termed them as myofibrotic malformation vessels (MMVs). MMVs are not ascribed to arteries or veins because they exhibit enlarged and tortuous lumens with smooth muscle dysplasia and fibrotic deposition in the walls without overlying mucosal ulceration. The muscularis mucosae also showed smooth muscle dysplasia and fibrosis, even if it were interrupted by the intruding MMVs. The statistical data indicated that the severity of all the changes correlate positively with the progression of hemorrhoids (P<0.001). Hemorrhoidal patients are prone for reoccurrence even with prolapsing hemorrhoid when compared with the conventional hemorrhoidectomy. Multiple logistic regression analysis showed that MMVs in mucosal propria, mean thickness of mucosal muscularis layer, and fibrotic changes in MMV were independent risk factors for MMVs in hemorrhoidal disease.
Conclusion: MMVs and muscularis mucosae dysplasia reciprocally contribute to hemorrhoidal exacerbation. The novel findings of this study propose that the characteristic features of MMVs and muscularis mucosae dysplasia of the anorectal tube ultimately cause symptomatic hemorrhoids, which could affect the clinical management of hemorrhoidal disease through the use of surgery to target the malformed vessels.
Keywords: internal hemorrhoids, hemorrhoidal progression, myofibrotic malformation vessels, muscularis mucosae dysplasia, anorectal disease
Introduction
The etiology and pathogenesis of hemorrhoids is unclear, although hemorrhoids are a worldwide disease in men and women, with peak prevalence at 45–65 years of age.1,2 Hemorrhoidal cushions as the anal venous plexi are normal anatomical structures from infancy,3 and the term “hemorrhoidal disease” indicates a pathological process. Prolapse of the anal cushions and vascular hyperplasia, first proposed by Thomson3 and modified by Haas et al4 appears to be the pathogenesis of hemorrhoidal disease. Neovasculature in the expression of CD105 might contribute to the development of hemorrhoids.5 Underlying morphopathophysiological abnormalities require more understanding to clarify anorectal symptoms, such as bleeding, prolapse, and pain, as being secondary to hemorrhoidal disease.
The procedure for prolapsing hemorrhoids (PPHs) is progressively used,6–9 and it is merely a compromised treatment for hemorrhoids, especially in degree II and III hemorrhoids. PPH is accompanied by a high ratio of postoperative recurrence, although it has advantages in maintaining functional hemorrhoidal anatomy over the traditional hemorrhoidectomy.6,7,10,11–14 The sliding anal lining theory is hypothesized to explain the prolapse of hemorrhoids; however, the theory is unlikely to explicate repeated bleeding, in particular, in patients with nonprolapsed hemorrhoids. Pathologically, venous distension is the predominant change observed in hemorrhoids under colonoscopic and microscopic observation.3,4,15 However, the fundamental insight into the interpretation of hemorrhoidal vasculature is the lack of convincing evidence to explain the abnormal vessels, so that even the researches of hemorrhoidal diseases have not been paid as much attention to as cardiac or cerebral vascular diseases.
Angiodysplasia/varices are another cause of bleeding in hemorrhoidal patients.15 Anorectal varices were previously hypothesized to be associated with portal hypertension in cirrhotic patients; however, prospective studies showed that the hepatic venous pressure gradient of cirrhotic patients with anorectal varices was similar to that of cirrhotic patients without anorectal varices16 and that hemorrhoids and anorectal varices are separate and distinct entities.17 Pathological studies of hemorrhoids have emphasized the anchoring connective tissue system, whereas vascular changes per se have not been seriously addressed concerning the pathogenetic mechanisms. Novel findings of hemorrhoidal angiodysplasia might have some effect on the clinical management of hemorrhoidal disease.
In this study, we focused on the unique vessels with smooth muscle dysplasia and sclerosing of internal hemorrhoids, and these vessels have not been well described in any published literatures, so we defined them to be myofibrotic malformation vessels (MMVs). The abnormal vessels in hemorrhoids are not accompanied with ulceration, and we have also found that the MMVs are indicators of the clinical stages of internal hemorrhoids in association with dysplasia of the muscularis mucosa by microscopic analysis combined with the histochemical/immunohistochemical features of the tissues removed by hemorrhoidectomy. MMVs might cause recurrent bleeding and the prolapse of hemorrhoids.
Materials and methods
Internal hemorrhoid samples were obtained from 281 patients with hemorrhoidectomy performed from January 2009 to March 2013 at Nanfang Hospital, Southern Medical University, Guangzhou, People’s Republic of China. Approval was obtained from the Ethics Committee of Southern Medical University to use clinical materials for research purposes. Informed consent and written approval for the clinical and research use of the human specimens were obtained from each patient before this study began. Prolapsing internal hemorrhoids are classified into four degrees according to a grading system of current clinical application based on the degree of prolapse during defecation as follows:18–20
Degree I: hemorrhoids are considered to be complicated with intermittent bloody stool, which do not prolapse.
Degree II: hemorrhoids which prolapse during defecation, but spontaneously return to their original position after defecation.
Degree III: hemorrhoids which do not return without manual help after prolapse.
Degree IV: hemorrhoids which remain prolapsed permanently.
There were only four patients with degree IV hemorrhoids in this study because other studies also recruited a few patients with degree IV hemorrhoids,1 and these four patients were integrated into the degree III group. The patients with a history of other diseases in the anal canal, pelvic radiotherapy, Crohn’s disease, ulcerative colitis, abdominal surgeries, sclerotherapy history, or liver cirrhosis were excluded. The patients of degree I were defined as Group 1, degree II as Group 2, and degree III/IV as Group 3.
However, it is very hard to recruit appropriate controls to match the tissues removed by hemorrhoidectomy. The controls in the published studies seemed to be inappropriate, such as conducting with anal tissues for controls, from Miles’ surgical samples of rectal cancer.5 Recruitment of adult volunteers without anorectal diseases for anal cushion resection is unreachable for our research, as in Shafik’s study.21 We have chosen relatively matchable samples for the controls in this study, the normal anorectal tissues from surgical resection in the control group were obtained from 12 adult patients (age ranging from 22 to 42 years) with anal fistula without a history or anoscopic findings of hemorrhoids.
The internal hemorrhoid lesions were fixed in 10% buffered formalin and subsequently subjected to alcohol-dehydration and paraffin-embedding procedures. The serial sections were sliced into 4 μm thickness for hematoxylin and eosin (H&E) staining, Masson’s trichrome staining, and immunohistochemistry. Mouse monoclonal antibodies against human actin (smooth muscle, 1:100, clone 1A4, Invitrogen, Camarillo, CA, USA) and CD34 (1:200, clone BI-3C5, Invitrogen) were immunostained in the serial sections. Immunohistochemical staining was performed with the EnVision Detection System (peroxidase/3,3′-diaminobenzidine reaction, anti-rabbit/mouse, ready-to-use, K500711, DAKO Corporation, Copenhagen, Denmark).
The slides were reviewed independently by three pathologists who were not informed of the clinical degrees of the hemorrhoids, and each pathologist took ten photos for every case, the results were obtained from the measurement and calculation by using Image-Pro Plus (IPP) version 6.0 (Media Cybernetics, Inc., Rockville, MD, USA). In each section, ten hot-spot fields of 10× objective lens were examined for the assessment of vessels with a cross section of more than 0.5 mm under a microscopic micrometer (Olympus, Tokyo, Japan) and the photos were verified with IPP software analysis, and the mean thickness of muscularis mucosa layer was reviewed (the thickness of the control group tissues was 67.9±12.4 μm). The items were stratified into grades, as depicted in Table 1. The vessels with a partially or completely thickened wall and an irregular cross section were classified as MMVs by excluding the normal veins and arteries. After summarizing the MMVs into ten fields, each specimen was classified into a corresponding grade. In the assessment of the submucosae, the MMVs were easily observed, and the mean of the MMVs per field was calculated in each case. The submucosal MMV changes with the actin staining or Masson’s trichrome testing in each case were combined to obtain the cumulative value (CV). Considering the high grade of the MMVs related to the severity of the hemorrhoids, we proposed a formula to calculate the CV, referring to the calculation methods of the extent and intensity of cancer cell immunostaining.22 The formula is as follows: CV =1 (Grade 1) × vessel number +2 (Grade 2) × vessel number +3 (Grade 3) × vessel number, and Grade 0 is defined as zero.
The results are shown as the mean ± standard deviation (SD) of the quantitative data. The chi-square test was performed in the analysis by sex. A univariate analysis of variance in the general linear model and a multinomial logistic regression were performed to analyze the relationship of the histological changes to the clinical stages. After screening for the multivariable linear regression analysis, a one-way analysis of variance was performed to analyze the quantitative variables. The ranked data of the MMVs and the muscularis mucosa changes were measured using a nonparametric test (the Kruskal–Wallis test); P-values <0.01 were considered statistically significant. All the analyses were performed using the SPSS software program (version 13.0; SPSS Inc., Chicago, IL, USA).
Results
Based on the patient’s self-report and anoscopic examination, 281 patients with hemorrhoidal disease were subdivided into three groups. Group 1 and 2 consisted of 91 and 95 cases of patients with degree I and II prolapsing internal hemorrhoids, respectively. Group 3 consisted of 91 cases of degree III and four cases of degree IV hemorrhoids. There were 147 male and 134 female patients in this study, with a mean age of 37.2±10.5 years and a mean disease history of 4.7±4.9 years. The statistical examination demonstrates that the degree of hemorrhoids is not associated with age, sex, or disease history among the groups. The clinical data and histological characteristics are shown in Table 2.
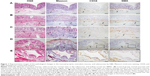
The histological examination revealed that the remarkable malformed vessels that we defined as MMVs appeared in the internal hemorrhoidal tissues; the MMVs were not observed in the control cases. In the control group, capillaries were found in the mucosal propria, and discernable arteries were not found; the muscularis mucosa showed a uniform smooth muscle layer without interruption or thinning/thickening, with a mean thickness of 67.9 μm and SD value of 12.4 μm (mean +1.96 SD as referred to be abnormal thickness, more than 100 μm); the walls of the vessels are uniform, without smooth muscle dysplasia and fibrosis, and the vessels are easily classified into veins, arteries, or capillaries (Figure 1A). The MMVs were increased in the mucosae propria, muscularis mucosa, and submucosae of the tissues removed by hemorrhoidectomy. The muscularis mucosa became thin and loose with fibrotic deposition, which was divided by the intruding MMVs. The walls of the MMVs became partially or completely thickened with smooth muscle dysplasia or sclerotic deposition. The MMVs had tortuous lumens, and it was difficult to classify them into veins or arteries. The MMVs in internal hemorrhoidal tissue were embedded in the connective tissues without ulceration. These changes were increasingly remarkable under microscopic observation in the Masson’s trichrome testing, CD34, and smooth muscle actin staining from degree I to III hemorrhoids (Figure 1B–D). Figure 1E shows enlarged and tortuous lumen with fibrotic wall and dysplasia of smooth muscle cells in degree III hemorrhoids. Pathological changes were not found in the walls of the arteries and veins in the control group and the hemorrhoidal tissues, and the amount of normal veins or arteries in the submucosae were not associated with the degrees of hemorrhoids (P=0.050, 0.453, Table 2).
MMVs are very commonly observed in this study. Mucosal propria had an increase of MMVs with luminal dilation with disease progression (P<0.001), in which we did not find arterioles. MMVs are generally distributed as single vessels without accompanying arteries and veins. Some changes have been discovered in the submucosal vessels, including that the vascular walls are thickened with smooth muscle dysplasia and fibrosis. Masson’s trichrome staining exhibits blue-stained material deposited on muscularis, which suggests fibrosis. Grade 0–3 with actin staining and Masson’s trichrome staining were attributed to the different vessels in a given case under microscopic observation. The statistical analysis shows that the MMV grades and the amount of increased MMVs in the submucosae are associated with the progression of hemorrhoids (P<0.001, Table 2). Smooth muscle dysplasia and fibrosis in MMVs correlate to the degree of disease (P<0.001, Table 2).
The muscularis mucosae showed observable changes in the internal hemorrhoidal tissues on histological examination. The muscularis mucosa becomes fibrotic, thickened, or disperses distribution of smooth muscle cells, and it is frequently divided by the MMVs intruding into the mucosal propria, especially with the smooth muscle layer being interrupted radially in some areas with the deposition of fibrotic materials. The grades of the muscularis mucosa changes are statistically significant with the degree of the disease (P<0.001, Table 2). Hemorrhoidal degrees progressively correspond with high grades of MMVs in the mucosa lamina propria, muscularis mucosa changes, and MMVs in the submucosae by multinomial logistic regression analysis (P<0.001). This correspondence indicates that MMVs and muscularis mucosa dysplasia mutually degrade anorectal tissues and cause subsequent complications.
We then performed a multiple logistic regression analysis using the following parameters: age, history, sex, MMVs in mucosal propria, mean thickness of mucosal muscularis layer, and fibrotic changes in MMV. Table 3 indicated the results of the multiple logistic regression analysis. Multiple logistic regression analysis showed that MMVs in mucosal propria (odds ratio: 3.453, 95% confidence interval [CI]: 1.232–7.153, P<0.0001), mean thickness of mucosal muscularis layer (odds ratio: 2.984, 95% CI: 1.631–8.032, P<0.0001), and fibrotic changes in MMV (odds ratio: 2.543, 95% CI: 1.231–5.578, P=0.0123) were independent risk factors for malformation vessels in hemorrhoidal disease. However, age (odds ratio: 1.157, 95% CI: 0.612–2.415, P=0.0732), history (odds ratio: 1.329, 95% CI: 0.714–2.543, P=0.0579), and sex (odds ratio: 0.984, 95% CI: 0.578–1.875, P=0.0854) were not the independent risk factors for malformation vessels.
  | Table 3 The results of logistic regression analysis for variables of the malformation vessels in hemorrhoidal disease |
Discussion
This study presents novel findings on internal hemorrhoidal vessels. MMVs comprise a remarkable finding that has not been well described based on histological examination by previous studies, and the MMVs are not a result of secondary diseases such as angiodysplasia/varices derived from liver cirrhosis. Aigner et al23 found similar dilated vessels with focal thickening of the muscular wall in the submucosae of hemorrhoidal samples in only five hemorrhoidectomy specimens, but they did not notice the contact between the pathological mechanism of hemorrhoids. We found that MMVs were demonstrated in the mucosae and the submucosae of hemorrhoids in a consecutive cohort of 281 patients. The MMVs, which were coupled with muscularis mucosa dysplasia, were closely related to the progression of hemorrhoids. The discernable changes in hemorrhoidal pathology found in this study could help improve surgical management.
The MMVs in hemorrhoidal tissues, which suggest an association with Dieulafoy lesions of the unusual causes of gastrointestinal bleeding,24–27 are not identical pathologically to the arteries of Dieulafoy lesions. A Dieulafoy lesion is a very rare entity, with only 25 described cases of rectal Dieulafoy lesions reported in a review of Ruiz-Tovar et al.28 The Dieulafoy lesion is referred to as a large submucosal artery in the gastrointestinal tract that is otherwise histologically normal, but lies in close contact with the mucous membrane typically coupled with an overlying mucosal ulceration.29,30 We found that the MMVs in internal hemorrhoids did not show overlying ulcers, and the MMVs were frequently observed in the mucosa lamina propria, where normal anatomical structures in human do not show muscular vasculature or thickened wall vessels, including arterioles and veins, except for capillaries and lymphatics.31 The MMVs should not be defined as arteries of Dieulafoy lesions or veins because they exhibited irregular enlarged lumens, focal dysplasia of smooth muscle cells, or even a deficiency accompanying fibrotic deposition. The MMVs showed characteristic morphology in hemorrhoidal tissues, which might be unique pathological changes contributing to repeat bleeding and prolapse of hemorrhoids.
MMVs are coupled with muscularis mucosae changes, including the muscular layer becoming slim, loose, fibrotic, or partially interrupted. The defect of muscularis mucosae might result from MMVs intruding into the mucosal propria. Engelhardt et al19 hypothesized in a study of vascular corrosion casts in hemorrhoids that tortuous veins with a diameter of up to 1 mm would lead to massive bleeding. However, we found that MMVs predominantly involved vessels with a diameter of more than 0.5 mm. The difference in vascular diameter between this study and Engelhardt’s study19 might result from sample management. The vessels in the vascular corrosion casts of Engelhardt’s study19 were enlarged from their actual appearance, and the vessels in the formalin fixed, paraffin-embedded tissues in our study were shrunken compared to the normal status. Our results indicate that the MMVs in the mucosal propria and submucosae are associated with the exacerbation of hemorrhoids.
Bleeding is one of the most common symptoms of internal hemorrhoids, with the exact pathogenesis being poorly understood. Clinically, hemorrhoid-associated bleeding frequently resembles arterial breakdown, and the blood is hypothesized to be arterial blood delivered via arteriovenous anastomoses.32 Arteriovenous anastomoses were not confirmed in anorectal vascular corrosion casts of inner hemorrhoids.19 Venous dilation and the capillary bed within the submucosae have been suggested to be the source of bleeding.3 According to our observations, the MMVs in the mucosae and submucosae are tortuous vessels that have irregularly thickened walls with deposition of fibrotic material. MMVs would be susceptible to injuries during defecation and hemostatic failure because of the decrease in elasticity and constriction compliance. We did not find that MMVs had accompanying arteries or veins, and MMVs should not be arteriovenous anastomoses.
Prolapse, sometimes with thrombotic pain, is another symptom of hemorrhoids. The sliding anal lining appears to reveal partially the reason for prolapse.3,4 However, hemorrhoidal patients are prone to a reoccurrence even with PPH compared with the traditional hemorrhoidectomy.6,7 MMVs would result in irreversible swelling in the anal cushions. Muscularis mucosae dysplasia destroys the support system of the anus. MMVs and muscularis mucosae dysplasia reciprocally contribute to recurrent bleeding and prolapse. Malformed vessels provoke blood turbulence and would be susceptible to thromboses. The adult anus is inevitably exposed to intermittent damage, including injury from constipation and diarrhea, and mucosal injury with smooth muscle dysplasia and fibrosis might promote MMVs development and subsequent prolapse of hemorrhoids. If the irritating factors were not corrected, hemorrhoids would develop again after medical intervention.
Hemorrhoidectomy is widely applied for the treatment of prolapsing internal hemorrhoids. Traditional ligation or sclerotherapy for internal hemorrhoids to restrain bleeding is suggested by pathological evidence of malformed vessels. Being unaware of targeted vessels would result in unavoidable latent complications such as ulceration and secondary bleeding. Traditionally, in European countries, the preferred conventional hemorrhoidectomy technique was the Milligan–Morgan procedure.33 Though effective in treating hemorrhoidal prolapse, Milligan–Morgan hemorrhoidectomy is associated with significant postoperative pain, often requiring a prolonged hospital admission and protracted recovery.34 However, in the other parts of the world, another technique, Ferguson hemorrhoidectomy, was designed to leave a less painful perianal wound.35 The PPH procedure removes a ring or sleeve of rectal tissue just proximal to the anal canal; however, it does not target a lesion such as those of MMVs, which might be another reason that hemorrhoids tend to be refractory, or even relapse, after PPH.36 Ligation or sclerotherapy of MMVs by visual guidance or surgical removal of MMVs tissue might substantially inhibit the bleeding and prolapse of internal hemorrhoids. Therefore, in this study, we reported the consequent vascular abnormalities of hemorrhoids and illustrated a novel target for hemorrhoidal therapy.
Though we have investigated some interesting data, there are also some limitations for our study. For example, when we choose a treatment, the main problem is to understand if MMVs should be considered a malformation or the consequence of constipation, diarrhea, or other causes. We have not investigated the above points. Therefore, we would perform some related analysis for investigating the pathological regression that could remove the causes.
In adults, MMVs and muscularis mucosae dysplasia may ultimately lead to symptomatic internal hemorrhoids with prolapse, bleeding, and pain. These pathological changes might be a result of intermittent damages of the anorectal tube, though the etiological factors of hemorrhoids are not determined. The consequent vascular abnormalities of hemorrhoids reported in this study present a novel target for hemorrhoidal therapy, rather than conventional PPH. Due to the lack of effective animal models of hemorrhoids at present, mechanistic findings in the future would be needed to explore the pathogenesis of MMVs in hemorrhoids.
Acknowledgment
We thank Dr Bharath R (faculty of the Department of Pathology, Southern Medical University, Guangzhou, People’s Republic of China) for correcting the grammatical errors in this paper.
Disclosure
The authors report no conflicts of interest in this work.
References
Riss S, Weiser FA, Schwameis K, et al. The prevalence of hemorrhoids in adults. Int J Colorectal Dis. 2012;27(2):215–220. | ||
Johanson JF, Sonnenberg A. The prevalence of hemorrhoids and chronic constipation. An epidemiologic study. Gastroenterology. 1990;98(2):380–386. | ||
Thomson WH. The nature of haemorrhoids. Br J Surg. 1975;62(7):542–552. | ||
Haas PA, Fox TA, Haas GP. The pathogenesis of hemorrhoids. Dis Colon Rectum. 1984;27(7):442–450. | ||
Chung YC, Hou YC, Pan AC. Endoglin (CD105) expression in the development of haemorrhoids. Eur J Clin Invest. 2004;34(2):107–112. | ||
Rowsell M, Bello M, Hemingway DM. Circumferential mucosectomy (stapled haemorrhoidectomy) versus conventional haemorrhoidectomy: randomised controlled trial. Lancet. 2000;355(9206):779–781. | ||
Van de Stadt J, D’Hoore A, Duinslaeger M, Chasse E, Penninckx F; Belgian Section of Colorectal Surgery Royal Belgian Society for Surgery. Long-term results after excision haemorrhoidectomy versus stapled haemorrhoidopexy for prolapsing haemorrhoids; a Belgian prospective randomized trial. Acta Chir Belg. 2005;105(1):44–52. | ||
Ross NP, Hildebrand DR, Tiernan JP, Brown SR, Watson AJ. Haemorrhoids: 21st-century management. Colorectal Dis. 2012;14(8):917–919. | ||
Madoff RD, Fleshman JW; Clinical Practice Committee AGA. American Gastroenterological Association technical review on the diagnosis and treatment of hemorrhoids. Gastroenterology. 2004;126(5):1463–1473. | ||
Festen S, van Geloven AA, Gerhards MF. Redo procedure for prolapse and haemorrhoids (PPH) for persistent and recurrent prolapse after PPH. Digest Surg. 2009;26(5):418–421. | ||
Wolthuis AM, Penninckx F, Cornille JB, Fieuws S, D’Hoore A. Recurrent symptoms after stapled haemorrhoidopexy and the impact on patient satisfaction after a minimum of 2 years follow-up. Acta Chir Belg. 2012;112(6):419–422. | ||
Ganio E, Altomare DF, Gabrielli F, Milito G, Canuti S. Prospective randomized multicentre trial comparing stapled with open haemorrhoidectomy. Br J Surg. 2001;88(5):669–674. | ||
Chen HH, Wang JY, Changchien CR, Chen JS, Hsu KC, Chiang JM. Risk factors associated with posthemorrhoidectomy secondary hemorrhage: a single-institution prospective study of 4,880 consecutive closed hemorrhoidectomies. Dis Colon Rectum. 2002;45(8):1096–1099. | ||
Raahave D, Jepsen LV, Pedersen IK. Primary and repeated stapled hemorrhoidopexy for prolapsing hemorrhoids: follow-up to five years. Dis Colon Rectum. 2008;51(3):334–341. | ||
Koning MV, Loffeld RJ. A survey of abnormalities in the colon and rectum in patients with haemorrhoids. BMC Gastroenterol. 2010;10(1):74. | ||
Wang TF, Lee FY, Tsai YT, Lee SD, Wang SS, Hsia HC. Relationship of portal pressure, anorectal varices and hemorrhoids in cirrhotic patients. J Hepatol. 1992;15(1–2):170–173. | ||
Hosking SW, Smart HL, Johnson AG, Triger DR. Anorectal varices, haemorrhoids, and portal hypertension. Lancet. 1989;1(8634):349–352. | ||
Banov L Jr, Knoepp LF Jr, Erdman LH, Alia RT. Management of hemorrhoidal disease. J S C Med Assoc. 1985;81(7):398–401. | ||
Engelhardt V, Lametschwandtner A, Böhler U, Wienert V. Microangioarchitecture of haemorrhoids: a scanning electron microscopy study of vascular corrosion casts. Phlebologie. 2010;39(1):12–17. | ||
Hulme-Moir M, Bartolo DC. Hemorrhoids. Gastroenterol Clin North Am. 2001;30(1):183–197. | ||
Shafik A. The pathogenesis of hemorrhoids and their treatment by anorectal bandotomy. J Clini Gastroenterol. 1984;6(2):129–137. | ||
Soumaoro LT, Uetake H, Higuchi T, Takagi Y, Enomoto M, Sugihara K. Cyclooxygenase-2 expression: a significant prognostic indicator for patients with colorectal cancer. Clin Cancer Res. 2004;10(24):8465–8471. | ||
Aigner F, Gruber H, Conrad F, Eder J, Wedel T, Zelger B. Revised morphology and hemodynamics of the anorectal vascular plexus: impact on the course of hemorrhoidal disease. Int J Colorectal Dis. 2009;24(1):105–113. | ||
Garg R. Bleeding from a gastric Dieulafoy lesion. Emerg Med J. 2007;24(7):520. | ||
Linhares MM, Filho BH, Schraibman V, et al. Dieulafoy lesion: endoscopic and surgical management. Surg Laparosc Endosc Percutan Tech. 2006;16(1):1–3. | ||
Sone Y, Kumada T, Toyoda H, Hisanaga Y, Kiriyama S, Tanikawa M. Endoscopic management and follow up of Dieulafoy lesion in the upper gastrointestinal tract. Endoscopy. 2005;37(5):449–453. | ||
Chiu HH, Jao YT, Mo LR. Endoscopic management of bleeding from colonic Dieulafoy-like lesion. J Clin Gastroenterol. 2003;37(1):91. | ||
Ruiz-Tovar J, Die-Trill J, Lopez-Quindos P, Rey A, Lopez-Hervas P, Devesa JM. Massive low gastrointestinal bleeding due to a Dieulafoy rectal lesion. Colorectal Dis. 2008;10(6):624–625. | ||
Lara LF, Sreenarasimhaiah J, Tang SJ, Afonso BB, Rockey DC. Dieulafoy lesions of the GI tract: localization and therapeutic outcomes. Dig Dis Sci. 2010;55(12):3436–3441. | ||
Stark ME, Gostout CJ, Balm RK. Clinical features and endoscopic management of Dieulafoy’s disease. Gastrointest Endosc. 1992;38(5):545–550. | ||
Fenoglio-Preiser CM, Noffsinger AE, Stemmermann GN, Lantz PE, Isaacson PG. The nonneoplastic colon. In: Fenoglio-Preiser CM, Noffsinger AE, Stemmermann GN, Lantz PE, Isaacson PG, editors. Gastrointestinal Pathology: An Atlas and Text. 3rd ed. Philadelphia, PA: Lippincott Williams & Wilkins; 2008:744. | ||
Thulesius O, Gjores JE. Arterio-venous anastomoses in the anal region with reference to the pathogenesis and treatment of haemorrhoids. Acta Chir Scand. 1973;139(5):476–478. | ||
Milligan ETC, Morgan CN, Jones LE, Officer R. Surgical anatomy of the anal canal and operativetreatment of hemorrhoids. Lancet. 1937;2:1119–1124. | ||
Ribaric G, Kofler J, Jayne DG. Stapled hemorrhoidopexy, an innovative surgical procedure for hemorrhoidal prolapse: cost-utility analysis. Croat Med J. 2011;52:497–504. | ||
Gencosmanoglu R, Sad O, Koc D, Inceoglu R. Haemorrhoidectomy: open or closed technique? A prospective, randomized clinical trial. Dis Colon Rectum. 2002;45:7075. | ||
Lim YK, Eu KW, Ho KS, Ooi BS, Tang CL. PPH03 stapled hemorrhoidopexy: our experience. Tech Coloproctol. 2006;10:43–46. |
 © 2015 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2015 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.