Back to Journals » Drug Design, Development and Therapy » Volume 13
Muscone Promotes The Adipogenic Differentiation Of Human Gingival Mesenchymal Stem Cells By Inhibiting The Wnt/β-Catenin Signaling Pathway
Authors Yuan WX, Wang XX, Zheng DH, Ma D, Cui Q, Yang F, Zhang J
Received 28 June 2019
Accepted for publication 6 September 2019
Published 18 September 2019 Volume 2019:13 Pages 3291—3306
DOI https://doi.org/10.2147/DDDT.S220970
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 3
Editor who approved publication: Prof. Dr. Cristiana Tanase
Wen-Xiu Yuan,1 Xu-Xia Wang,2 De-Hua Zheng,1 Dan Ma,1 Qun Cui,1 Fan Yang,1 Jun Zhang1
1Department of Orthodontics, School and Hospital of Stomatology, Shandong University and Shandong Provincial Key Laboratory of Oral Tissue Regeneration and Shandong Engineering Laboratory for Dental Materials and Oral Tissue Regeneration, Jinan, Shandong Province, People’s Republic of China; 2Department of Oral and Maxillofacial Surgery, School and Hospital of Stomatology, Shandong University and Shandong Provincial Key Laboratory of Oral Tissue Regeneration and Shandong Engineering Laboratory for Dental Materials and Oral Tissue Regeneration, Jinan, Shandong Province, People’s Republic of China
Correspondence: Jun Zhang
Department of Orthodontics, School and Hospital of Stomatology, Shandong University and Shandong Provincial Key Laboratory of Oral Tissue Regeneration, No. 44-1 Wenhua Road West, Jinan, Shandong Province, People’s Republic of China
Tel +86 139 5310 9816
Email [email protected]
Objectives: This study was performed to evaluate the effects of muscone on the proliferation, migration and differentiation of human gingival mesenchymal stem cells (GMSCs) and to explore the relevant mechanisms.
Materials and methods: We performed studies to determine the effects and mechanisms of muscone on GMSC proliferation, migration and differentiation. We conducted CCK-8, colony formation, transwell chamber, scratch wound, alkaline phosphatase (ALP) staining and activity, and alizarin red and oil red O staining assays, as well as real-time quantitative polymerase chain reaction (qRT-PCR), to ascertain the effects of muscone on GMSC proliferation, migration and differentiation in vitro. The mechanism by which muscone influences the osteogenic and adipogenic differentiation of GMSCs was elucidated by qRT-PCR and Western blotting.
Results: We found that muscone significantly promoted GMSC proliferation, chemotaxis, wound healing and fat droplet formation and inhibited ALP activity and mineral deposition. Notably, we observed that the Wnt/β-catenin pathway was closely related to the ability of muscone to inhibit the osteogenic differentiation and promote the adipogenic differentiation of GMSCs. The effect of muscone on the multidirectional differentiation capacity of GMSCs was significantly reversed by the agonist lithium chloride through the Wnt/β-catenin signaling pathway.
Conclusion: Muscone effectively increased the proliferation and migration, promoted the adipogenic differentiation and inhibited the osteogenic differentiation of GMSCs by inhibiting the Wnt/β-catenin signaling pathway. These results may provide a theoretical basis for the application of GMSCs and muscone in tissue engineering and regenerative medicine.
Keywords: muscone, gingival mesenchymal stem cells, GMSCs, differentiation, Wnt/β-catenin signaling pathway, proliferation, chemotaxis
Introduction
Tissue engineering is one of the most popular research fields to emerge in recent years, and this field is mainly concerned with generating new structures for damaged or lost tissues that include three important features: seed cells, scaffold materials and cytokines.1,2 One goal of regenerative medicine is to regenerate and repair tissues damaged or lost due to various diseases.3 The key to tissue engineering and regenerative medicine is finding stem cells with the potential for self-renewal and multidirectional differentiation. Among numerous seed cells, the most widely used are adult mesenchymal stem cells (MSCs), including bone marrow MSCs (BMSCs),4 adipose-derived MSCs,5 human amniotic membrane-derived MSCs and umbilical cord-derived MSCs.6 Although these MSCs come from a wide range of sources, a limited number of cells are available from the tissue source. Among different populations of stem cells, gingival mesenchymal stem cells (GMSCs) have attracted much attention because they are easily accessible from the oral cavity, the collection procedures do not require invasive procedures, and they have relatively significant self-renewal capacity, multilineage differentiation ability, and anti-inflammatory and immunomodulatory properties.7–9 An increasing number of studies have found that GMSCs can differentiate into chondrocytes, osteoblasts, endothelial cells and smooth muscle cells upon induction by different factors.6,10,11 Most importantly, GMSCs are expected to be safely and effectively applicable to clinical tissue regeneration.
Natural musk has the effects of tranquilizing and allaying excitement, relieving swelling and pain, promoting blood circulation to remove blood stasis and ameliorating infantile convulsions.12 Muscone, muscopyridine, cholesterol, polypeptides, proteins, fatty acids and a few inorganic elements are the main components of natural musk, among which muscone (3-methylcyclopentadecanone, Figure 3A) is the main effective component.12 At present, musk is used in the treatment of cardiovascular diseases, inflammation, and bone injury.13–15 In the 1970s, muscone was used to relieve angina by dilating coronary arteries.16 Some studies found that muscone has a good anti-ischemic effect on the central nervous system and somewhat improves the function of vascular endothelial cells, thereby improving the vascular remodeling of the middle cerebral artery.17,18 It has been reported that muscone exerts cytotoxic effects, induces the apoptosis of cancer cells, and influences the expression of proto-oncogenes and tumor suppressor genes to achieve antitumor effects.19,20 Hou Feiyi demonstrated that muscone promotes the proliferation and osteogenic differentiation of BMSCs.21 Some researchers found that muscone also promotes the migration of exogenous rat BMSCs in a rat model.22
The Wnt/β-catenin signaling pathway is an evolutionarily conserved signal transduction pathway that regulates a wide range of cellular functions during development and adulthood.23 In previous studies, researchers have found that this pathway controls multiple aspects of development, including cell proliferation, cell fate determination, apoptosis, cell migration and cell polarity during development and stem cell maintenance in adults.24 The canonical and noncanonical Wnt signaling pathways regulate the osteogenic and adipogenic differentiation of human MSCs.25,26 Boland showed the upregulation of WNT11, FZD6, SFRP2, and SFRP3 and the downregulation of WNT9A and FZD7 during MSC osteogenesis in vitro.25 Ross found that WNT1, WNT10b and β-catenin inhibited the adipogenic differentiation of fat precursor cells by downregulating the expression of the transcription factors C/EBPα and PPARγ.26
To our knowledge, the effect of muscone on the biological behavior of GMSCs has not been reported. Therefore, the aims of our study were to evaluate the effects of muscone on GMSC proliferation, migration and differentiation and to explore correlations with Wnt/β-catenin signaling. We hope that the results provide a theoretical basis for the application of GMSCs and muscone in tissue engineering and regenerative medicine.
Materials And Methods
Cultivation Of Human GMSCs
Healthy human gingival tissues were obtained as remnants of discarded tissues as approved by the Medical Ethical Committee of School of Stomatology, Shandong University (NO: R 20180401). All procedures were carried out in accordance with the principles of the Declaration of Helsinki. Informed consent was obtained from all participating human subjects for the collection of fresh tissue. The gingival specimens were de-epithelialized to exclude most keratinocytes.13 The cells were routinely cultured in α-MEM (Invitrogen, Carlsbad, CA, USA) supplemented with 10% fetal bovine serum (FBS; BioInd, Kibbutz, Israel), 50 mg/mL streptomycin and 50 U/mL penicillin G. All the cultures were maintained at 37°C in a humidified incubator with 5% CO2. The medium was changed every 3 days. Cells spontaneously migrated from the explant fragments. After reaching 80% confluence, adherent cells were detached with trypsin (Sigma, St Louis, MO, USA). To further isolate and purify stem cells, single cell suspensions of primary cells were cloned with the limiting dilution method as previously described.27 Single cell-derived colonies were used at passages 3–5. For each experiment, GMSCs from the same passage (passages 3–5) were used.
Identification Of Human GMSCs
Cells in the logarithmic growth phase were digested with trypsin into single cell suspensions. Cells were analyzed for the expression of cell surface markers (CD34, CD44, CD45, CD90, and CD105) by flow cytometric analysis (CytoFLEX; Beckman Coulter, Brea, CA, USA). Approximately 106 cells were used to detect each molecule. Labeled cells were thoroughly washed, centrifuged three times and then resuspended in PBS. Flow cytometry was used to determine the fluorescence intensity and number of positive cells. To determine the growth rate of GMSCs in the exponential phase, the population doubling time (PDT) was calculated: PDT = CT/PDN, where CT is the culture time, and PDN = log (N1/N0) ×3.31 (N1, final cell number; N0, initial cell number).28 To examine the multidirectional differentiation potential of GMSCs,29,30 GMSCs were cultured in osteogenesis-inducing medium (α-MEM supplemented with 10% FBS, 1 μM dexamethasone, 50 mg/L ascorbic acid, and 3 mM β-glycerophosphate) (Sigma-Aldrich, St. Louis, MO, USA) or adipogenesis-inducing medium (α-MEM supplemented with 10% FBS, 0.5 mM IBMX, 1 μM dexamethasone, 200 μM indomethacin, and 10 μM insulin) (Sigma-Aldrich, St. Louis, MO, USA). Cells seeded on 6-well plates were cultured for 21 days and then stained and examined under a microscope.
Cell Proliferation And Colony Formation Assays
GMSCs were plated in 96-well plates at 3500 cells per well. After cell synchronization with 1% FBS for 24 hrs, the medium was changed to α-MEM supplemented with 0.1% FBS and 0, 3, 6, or 9 mg/L muscone (National Institutes for Food and Drug Control, Beijing, China) for 24, 48, and 72 hrs. The muscone concentrations were selected based on previous studies.19–22 The muscone concentration for intragastric administration in animals generally ranges from 1 mg/kg to 10 mg/kg.31,32 A Cell Counting Kit-8 (CCK-8, Dojindo Laboratories, Kumamoto, Japan) was used to measure GMSC proliferation. Solutions of α-MEM and CCK-8 were generated at a 9:1 ratio, 100 μL of this solution was added to each well, and the plates were incubated for 2.5 hrs at 37°C. The absorbance was measured at 490 nm using a SPECTROstar Nano microplate reader (BMG Labtech, Ortenberg, Germany).
GMSCs were seeded in culture dishes at 500 cells/dish and stimulated with 0, 3, 6, and 9 mg/L muscone. After 10 days, the cells were fixed in 4% paraformaldehyde for approximately 30 mins. Cell colonies were counted after staining with crystal violet (Solarbio, Beijing, China). The ability of the cells to form colonies (groups of 50 or more adhering cells derived from the same mother cell) was evaluated.
Wound Healing And Cell Migration Assays
GMSCs were cultured in 6-well plates to at least 90% confluence for wound healing assays. After starvation for approximately 24 hrs, the monolayer of GMSCs was scratched by a sterile pipette tip. Then, medium containing 0.1% FBS with 0, 3, 6, and 9 mg/L muscone was added to each well. The same migration position was photographed by a digital camera under an inverted microscope (Olympus) at 0 and 24 hrs. ImageJ software (NIH, Bethesda, MD, USA) was used to measure the migration area and to calculate the number of migrated cells: migration area= [blank area (0 hrs)-blank area (24 hrs)]/blank area (0 hrs).
The effect of muscone on GMSC migration was evaluated using transwell chambers (8.0 μm pore size; Corning, NY, USA) in 24-well plates. The upper chamber contained 105 cells in 200 μl of α-MEM containing 0.1% FBS, and the lower chamber contained 500 μL of α-MEM containing 0.1% FBS with various concentrations of muscone (0, 3, 6, and 9 mg/L). The chambers were incubated for 24 hrs. The nonmigrating cells in the upper chamber were gently removed with a cotton swab. The cells that migrated to the lower chamber were fixed with paraformaldehyde, stained with crystal violet and counted in three randomly selected microscopic fields per chamber in a blinded manner.
ALP Staining And Activity Assays
GMSCs were seeded in 6-well plates at 105 cells/well in osteogenesis-inducing medium with 0, 3, 6, and 9 mg/L muscone. After 7 and 14 days of induction, the cells were fixed. ALP staining was performed according to the instructions of an ALP staining assay kit (Solarbio), and stained plates were photographed and inspected with a light microscope.
After 7 and 14 days of induction, cells were scraped into 1% Triton X-100 (Solarbio) on ice. Then, the cells were sonicated and centrifuged at 12,000 ×g for 15 mins at 4°C. A BCA protein assay kit (Solarbio) was used to measure protein concentration. ALP activity was assayed according to the instructions of an ALP activity assay kit (Nanjing Jiancheng Bioengineering Institute, Nanjing, China), and the absorbance was measured at 520 nm with a spectrophotometer. ALP activity was calculated according to the phenol concentration in the standard well, and the protein concentration was determined according to the bicinchoninic acid (BCA; Solarbio) method.
Alizarin Red And Oil Red O Staining Assays
GMSCs were cultured in 6-well plates in osteogenesis-inducing medium or adipogenesis-inducing medium with 0, 3, 6, and 9 mg/L muscone. After 3 weeks of induction, the cells were fixed with paraformaldehyde and stained with 2% alizarin red (pH 4.2) or oil red O for 15 mins at room temperature. Stained plates were photographed and inspected with a light microscope. To quantify the amount of mineral deposition or the number of lipid droplets, 400 μl of 10% cetylpyridinium chloride (CPC; Sigma-Aldrich) or 700 μl of isopropyl alcohol (Solarbio) was added to the stained dishes, and the absorbance of the extracted dye was measured at 562 nm or 510 nm.
Real-Time Polymerase Chain Reaction (RT-PCR) Analysis
GMSCs were cultured in 6-well plates in osteogenesis-inducing medium or adipogenesis-inducing medium with 0, 3, 6, and 9 mg/L muscone. After 7 and 14 days (osteogenesis) or 3 and 6 days (adipogenesis) of induction, total RNA of cells subjected to different treatments was isolated with Trizol (Takara, Tokyo, Japan). Reverse transcriptase (TaKaRa Biotech) was used for cDNA synthesis according to the manufacturer’s instructions. RT-PCR was performed using SYBR® Premix Ex TaqTM (TaKaRa) with a Roche LightCycler 480 system, and each RNA sample was assayed in triplicate. The ALP, RUNX2, PPARγ, LPL, β-catenin and GAPDH primer sequences were as follows: ALP: 5′-ATGGGATGGGTGTCTCCACA-3′ and 5′-CCACGAAGGGGAACTTGTC-3′; RUNX2: 5′-TCCACACCATTAGGGACCATC-3′ and 5′-TGCTAATGCTTCGTGTTTCCA-3′; PPARγ: 5′-CTCCTATTGACCCAGAAAGC-3′ and 5′-GTAGAGCTGAGTCTTCTCAG-3′; LPL: 5′-AGGACACTTGCCACCTCATTC-3′ and 5′-ACAGCCAGTCCACCACAATG-3′; β-catenin: 5′-GGCTTGGAATGAGACTGCTG-3′ and 5′-GGTCCATACCCAAGGCATCC-3′; and GAPDH: 5′-AGGTCGGTGTGAACGGATTTG-3′ and 5′-TGTAGACCATGTAGTTGAGGTCA-3′. GAPDH was used as an internal control to quantify and normalize the results. The amount of mRNA was calculated.
Western Blot Analysis
GMSCs were seeded in 6-well plates at 105 cells/well. GMSCs were washed with PBS, lysed with RIPA lysis buffer containing 1% PMSF (Solarbio) and then centrifuged at 12,000 ×g for 15 mins at 4°C. After centrifugation, proteins in a supernatant were collected and quantified using a BCA protein assay kit (Solarbio). Total proteins were mixed with 5X sodium dodecyl sulfate polyacrylamide gel electrophoresis (SDS-PAGE) loading buffer and incubated at 100°C for 5 mins. Twenty micrograms of protein per lane was separated by SDS-PAGE and transferred to PVDF membranes (Millipore, Billerica, MA, USA). The membranes were blocked and probed with primary antibodies that recognized ALP and Osterix (1:10,000; Abcam, Cambridge, MA, USA) and β-catenin, GSK3β, p-GSK3β (Ser9), LEF, PPARγ and GAPDH (1:1000; Cell Signaling Technology, Danvers, MA, USA). Secondary antibodies were selected according to the species of origin of the primary antibodies. Blotted proteins were detected using an enhanced chemiluminescent substrate kit (Millipore). The level of each protein was normalized to that of GAPDH before statistical analysis. ImageJ software (NIH, Bethesda) was used to quantify protein expression.
Statistical Analysis
Normally distributed data are presented as the mean ± standard deviation of at least three replicates for each experiment. Either Student's t-tests or one-way analysis of variance (ANOVA) was performed to test the significance of differences between the control group and the experimental groups using SPSS 22.0 software (IBM Corporation, Armonk, NY, USA) and GraphPad Prism 6 (GraphPad Software Inc., La Jolla, CA, USA). P<0.05 was considered statistically significant.
Results
Isolation And Characterization Of GMSCs
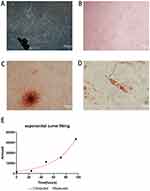
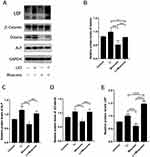
Primary cells were successfully obtained from gingival tissues (Figure 1A), and single cell suspensions of primary cells were cloned with the limiting dilution method (Figure 1B). GMSCs expressed the MSC-associated surface markers CD44 (Figure 2E and F), CD90 (Figure 2A and B), and CD105 (Figure 2C and D) and lacked expression of the hematopoietic markers CD34 (Figure 2I and J) and CD45 (Figure 2G and H). GMSCs were cultured in osteogenesis- or adipogenesis-inducing medium for 21 days. Alizarin red and oil red O staining revealed the formation of mineralized nodules (Figure 1C) and the accumulation of lipid droplets (Figure 1D). We calculated the PDT of GMSCs (Figure 1E) to be 24.12 hours, which is roughly the same as the PDT of other MSCs.
Muscone Enhanced GMSC Proliferation
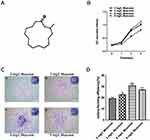
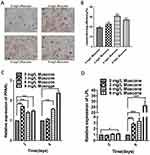
The number of surviving GMSCs in various concentrations of muscone (Figure 3A) was measured by CCK-8 assays on days 0, 1, 3 and 5. All three concentrations of muscone significantly increased the cell yield compared with the control group (P<0.001), especially at 3 and 5 days; at these time points, 6 mg/L muscone showed the strongest effect (Figure 3B). The colony formation assay results showed a significant difference between the control group and the experimental groups (Figure 3C). More and larger colonies were observed in the experimental groups after 10 days (Figure 3D).
Muscone Enhanced The Wound Healing And Migration Capabilities Of GMSCs
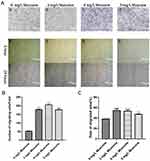
We measured and calculated the area over which the cells had migrated. The results indicated that the migration area was significantly larger for the experimental group than for the control group after 24 hrs (P<0.05) (Figure 4A e, f, g, h; Figure 4C). The ability of muscone to act as a chemotactic for GMSCs was quantified with a transwell migration assay. The experimental groups treated with muscone (3, 6, and 9 mg/L) showed significantly enhanced GMSC migration compared with the control group (0 mg/L) (P<0.05) (Figure 4B); 6 mg/L muscone had the strongest effect (Figure 4A a, b, c, d).
Muscone Inhibited Mineral Deposition And ALP Activity And Downregulated Osteogenesis-Related Gene Expression In GMSCs
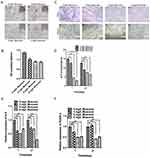
Mineral deposition was analyzed in an alizarin red staining assay, and the relative amount of calcium was quantified. The results showed that 3, 6, and 9 mg/L muscone significantly inhibited mineralization compared with the control (Figure 5B). Fewer and smaller calcified nodules were observed in the muscone group than in the control group (Figure 5A). ALP activity has been widely used as a marker of the early osteogenic differentiation of stem cells. In our study, ALP activity was measured at 7 and 14 days with or without muscone treatment. At days 7 and 14, the experimental groups treated with 3, 6, and 9 mg/L muscone showed significant inhibition of ALP activity compared with the control group (Figure 5C). The same result was observed in the ALP staining assay (Figure 5D). Moreover, ALP and RUNX2 expression levels were measured by qRT-PCR to verify the osteogenic effect of muscone at the molecular level. Treatment of GMSCs with 3, 6, and 9 mg/L muscone significantly decreased ALP and Runx2 expression at days 7 and 14 compared with the control (Figure 5E, F).
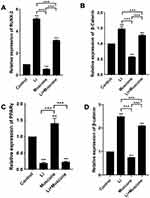
Muscone Enhanced The Formation Of Lipid Droplets And Upregulated Adipogenesis-Related Gene Expression In GMSCs
The formation of lipid droplets was assessed in an oil red O staining assay (Figure 6A), and the relative number of lipid droplets was quantified. The experimental groups treated with 3, 6, and 9 mg/L muscone showed significantly enhanced lipid droplet formation compared with the control group (Figure 6B). Moreover, PPARγ and LPL expression levels were measured by qRT-PCR to verify the adipogenic effect of muscone at the molecular level. Treatment of GMSCs with muscone significantly increased PPARγ expression at days 3 and 6 (Figure 6C). LPL expression was not significantly affected by muscone at day 3 but was significantly increased at day 6 (Figure 6D).
Muscone Promoted The Adipogenic Differentiation And Inhibited The Osteogenic Differentiation Of GMSCs By Inhibiting The Wnt/β-Catenin Signaling Pathway
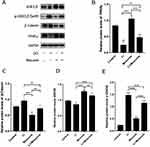
In previous experiments, muscone inhibited the osteogenic differentiation and enhanced the adipogenic differentiation of GMSCs. To characterize the relevant signaling pathway, we treated GMSCs with muscone and lithium chloride (LiCl, an agonist of the Wnt/β-catenin signaling pathway). Then, qRT-PCR and Western blot analyses were performed to investigate mRNA and protein expression levels and to explore the underlying mechanism. After cells were treated with LiCl, the osteogenesis-related genes ALP and Osterix were downregulated, and the signaling pathway-related genes β-catenin and LEF were increased at the protein level (Figure 8A, B, C, D, E). Moreover, the osteogenesis-related gene Runx2 and the signaling pathway-related gene β-catenin were increased at the mRNA level (Figure 7A, B), and increased expression of the adipogenesis-related gene PPARγ and decreased expression of the signaling pathway-related genes β-catenin and GSK3β (Ser9) were observed at the protein level (Figure 9A, B, C, D). Interestingly, the reduced p-GSK3β levels were ameliorated by the Wnt/β-catenin signaling pathway agonist LiCl in GMSCs (Figure 9A, E), and PPARγ and β-catenin mRNA levels were reduced in GMSCs treated with muscone and LiCl (Figure 7C, D). We conclude that muscone inhibits osteogenic differentiation and enhances adipogenic differentiation via the Wnt/β-catenin signaling pathway.
Discussion
GMSCs are easy to isolate from oral mucosa tissues and possess stronger self-renewal and multilineage differentiation abilities and anti-inflammatory and immunomodulatory properties than previously used seed cells.13–15 Previous studies have demonstrated mineralized tissue formation when GMSCs in hydroxyapatite/calcium phosphate scaffolds were subcutaneously implanted into nude mice.13,33 Treves-Manusevitz S found that fibrin/human oral mucosa stem cell (OMSC) constructs were endowed with the constitutive capacity to develop into mineralized tissues.34 An increasing number of recent studies have proposed the use of GMSCs as a novel option in tissue engineering and regenerative therapy.
The drug used in our experiments was muscone, which is an effective component extracted from natural musk. The separation and purification of natural musk components have revealed that muscone is the main effective component of natural musk and the main component that activates blood circulation.12 At present, musk is mainly used in clinical treatment.16–18 Studies have shown that muscone influences the proliferation and differentiation of BMSCs.21,22
Therefore, it would be of considerable research value to ascertain whether muscone affects the biological activity of GMSCs.
In our study, GMSCs were isolated and purified by trypsinization combined with the limiting dilution method, cell passaging, and adherent culture.35,36 Flow cytometry revealed that GMSCs expressed the MSC-associated surface markers CD44, CD90, and CD105 but not the hematopoietic markers CD34 and CD45. GMSCs could be induced to differentiate into osteoblasts and adipocytes in vitro, which indicated their multipotential differentiation capacity. These procedures met the standards for the identification of MSCs formulated by the International Society for Cell & Gene Therapy.37,38 Hou found that serum containing muscone was biologically active and promoted the proliferation of rat BMSCs; moreover, they showed that muscone improved the expression of stem cell factor and fractalkine in rats.21 Stem cell factor is a pluripotent cytokine that stimulates the proliferation of early stem cells39 and is often used in the culture of various stem cells to promote their proliferation, differentiation and migration.40 The present study showed that different concentrations of muscone promoted the proliferation and colony formation ability of GMSCs. These results were consistent with those of previous studies.21,22
Cell migration plays a significant role in some biological processes, such as tissue development and wound healing. The successful migration of GMSCs to the damaged area and their function are of great significance for tissue regeneration. Previous research found that muscone promotes the migration of BMSCs in vivo.22 Jiang Hui found that muscone promotes the expression of SDF-1 and MCP-1 and their corresponding chemokine receptors CXCR4 and CCR2, and the mechanism for promoting exogenous BMSC migration in the skull bone defect rat model was closely related to the sdf-1/CXCR4 and McP-1/CCR2 chemokine signaling axes.41 The results of our study showed that muscone significantly promoted the migration of GMSCs in vitro, which was in accordance with the findings of previous in vivo studies,22,41 but the specific mechanism for promoting GMSC migration in vitro remains to be explored in future studies.
Multidirectional differentiation potential is an important characteristic of GMSCs. ALP can be used as an indicator of early mineralization, RUNX2 is one of the most important transcription factors in osteogenesis, and the formation of calcium nodules is usually regarded as a late marker of osteogenic differentiation.42 Previous studies have shown that muscone promotes the secretion of bone morphogenetic protein (bmp)-4 and bmp-7, which might increase ALP activity and osteocalcin expression in human periodontal ligament cells.43 However, in our study, we found that muscone inhibited ALP activity in GMSCs, decreased the formation of calcium nodules, and downregulated the expression of the osteogenic genes ALP and Osterix; these results were inconsistent with those of previous studies,21,43 perhaps because of the use of different types of stem cells, which have certain differences in multidirectional differentiation capacity. PPARγ is one of the most important adipogenic differentiation transcription factors44 and participates in the regulation of lipid metabolism. LPL is an important gene that encodes lipoprotein lipase, which functions in lipoprotein metabolism. In the present study, we found that muscone increased the formation of lipid droplets and upregulated the expression of the adipogenesis-related genes PPARγ and LPL. Adipogenesis is important for soft tissue reconstruction after trauma or tumor resection.45 Experimental efforts regarding novel soft tissue reconstruction methods have relied on the transplantation of stem cells isolated from adult tissue.46 In a previous study, researchers found that upon stimulation by growth factors, MSCs in scaffold materials can be transplanted into soft tissue defects to promote the repair of defective adipose tissue.47 Cytokine-mediated cell homing may offer an alternative to cell transplantation approaches in the regeneration of various tissues,48 including adipose tissue. We speculate that GMSCs can be transplanted with scaffold materials into soft tissue defects and that muscone is a potential stimulatory factor that may promote the repair of defective adipose tissue. On the other hand, muscone could first promote the migration of GMSCs to local tissues, induce GMSC proliferation and adipogenic differentiation, and finally repair the damaged adipose tissue. Further exploration is needed to confirm these hypotheses.
In tissue engineering and regenerative medicine research, Liu found that muscone can increase the BMSC therapeutic potential for the treatment of AKI.49 Du showed that muscone significantly downregulates the levels of LPS-induced inflammatory cytokines and inhibits inflammasome activation in bone marrow-derived macrophages and suggested that this drug may be a promising and effective therapeutic for postmyocardial infarction.31 A previous study showed the protective effect of muscone against alcohol-induced osteonecrosis of the femoral head both in vitro and in vivo.32 Data indicate that muscone may be a promising and effective promoting factor in the fields of tissue engineering and regenerative medicine.
The Wnt signaling pathway is a conserved signal transduction pathway that is involved in many developmental processes, including embryo formation, and plays an important role in maintaining adult tissue homeostasis.23 Under normal circumstances, Axin, APC, PP2A, GSK3β, and CK1 form a degradation complex in the cytoplasm. When this degradation complex binds to β-catenin, GSK3β promotes the phosphorylation of β-catenin at Ser33 and Ser37, resulting in β-catenin degradation by the proteasome.50 However, when GSK3β is inactivated by increasing Ser9 phosphorylation,51 it can no longer promote β-catenin phosphorylation, leading to β-catenin accumulation in the cytoplasm and, eventually, β-catenin translocation into the nucleus, where it interacts with TCF/LEF to form a transcriptional activation complex that activates WNT‐responsive genes such as c-myc, CD44, Osterix and PPARγ.52 Studies have shown that inhibiting the classical Wnt signaling pathway promotes the adipogenic differentiation of preadipocytes and accelerates the differentiation of adipocytes,53,54 while activating the classical Wnt pathway promotes the osteogenesis of BMSCs by upregulating osteogenic differentiation-related genes.55,56 Therefore, the Wnt/β-catenin signaling pathway is important for the multidirectional differentiation of stem cells. In the present study, we discovered that muscone inhibited osteogenic differentiation by downregulating the expression of the Wnt pathway-related gene β-catenin and the downstream signal LEF in GMSCs. We speculate that muscone inhibits Wnt signaling pathway activation by decreasing β-catenin and LEF levels, being unable to form a transcriptional activation complex, ultimately reducing the expression of the osteogenic genes ALP and Osterix. On the other hand, we observed that muscone decreased β-catenin and p-GSK3β levels, enhanced GSK3β expression, and promoted adipogenic differentiation. However, the effect of muscone on the multidirectional differentiation capacity of GMSCs was significantly reversed by the agonist LiCl (GSK3β inactivator)57,58 through the Wnt/β-catenin signaling pathway. Based on these findings, we speculate that during adipogenic differentiation, muscone reduces Ser9 phosphorylation of GSK3-β, increases GSK3-β activation and, then promotes β-catenin phosphorylation and degradation, decreases β-catenin accumulation in the cytoplasm and blocks Wnt signaling pathway activation, eventually enhancing the expression of the adipogenesis-related gene PPARγ. More researches are needed to confirm our speculation.
In our study, we performed experiments and analyses in vitro at only certain time points and stages. The pharmacological effects of muscone on GMSCs in vivo are still not clear; therefore, animal experiments are required to further explore the physiological and biochemical effects of muscone on GMSCs in vivo. Meanwhile, studies on the effects of muscone on the differentiation of GMSCs towards other lineages, such as chondrogenic and neural differentiation, have not been conducted, and further studies are needed.
Conclusion
In summary, we demonstrated that muscone can effectively inhibit osteogenic differentiation and promote GMSC proliferation, migration and adipogenic differentiation. Moreover, our data clearly show that the Wnt/β-catenin signaling pathway is closely related to the effect of muscone on the osteogenic differentiation and adipogenic differentiation of GMSCs. Muscone promotes the adipogenic differentiation of human GMSCs by inhibiting the Wnt/β-catenin signaling pathway. The results will provide a theoretical basis for the application of GMSCs and muscone in tissue engineering and regenerative medicine.
Abbreviations
GMSCs, gingival mesenchymal stem cells; ALP, alkaline phosphatase; FBS, fetal bovine serum; CCK-8, cell counting kit-8; CPC, cetylpyridinium chloride; RUNX2, runt-related transcription factor 2; PPARγ, peroxisome proliferator-activated receptor γ; LPL, lipoprotein lipase; GAPDH, glyceraldehyde-3-phosphate dehydrogenase; BCA, bicinchoninic acid assay; SDS-PAGE, sodium dodecyl sulfate-polyacrylamide gel electrophoresis; PVDF, polyvinylidenefluoride; PBS, phosphate-buffered Saline.
Highlights
- Muscone promotes the proliferation, chemotaxis in GMSCs and inhibits osteogenic differentiation.
- Muscone promotes adipogenic differentiation in GMSCs.
- Wnt/β-catenin pathway was closely related to the effect of muscone on GMSCs in differentiation.
Acknowledgment
This study was supported by the Province Natural Science Foundation of Shandong Province, China (Grant No. ZR2019MH113).
Disclosure
The authors report no conflicts of interest in this work.
References
1. Heo JS, Choi Y, Kim HS, Kim HO. Comparison of molecular profiles of human mesenchymal stem cells derived from bone marrow, umbilical cord blood, placenta and adipose tissue. Int J Mol Med. 2016;37:115–125. doi:10.3892/ijmm.2015.2413
2. Inzana JA, Olvera D, Fuller SM, et al. 3D printing of composite calcium phosphate and collagen scaffolds for bone regeneration. Biometerial. 2014;35:4026–4034. doi:10.1016/j.biomaterials.2014.01.064
3. Bartold PM, McCulloch CA, Narayanan AS, Pitaru S. Tissue engineering: A new paradigm for periodontal regeneration based on molecular and cell biology. Periodontol. 2000;253–269. doi:10.1034/j.1600-0757.2000.2240113.x
4. Eid AA, Hussein KA, Niu LN, et al. Effects of tricalcium silicate cements on osteogenic differentiation of human bone marrowderived mesenchymal stem cells in vitro. Acta Biomater. 2014;10:3327–3334. doi:10.1016/j.actbio.2014.04.006
5. Açil Y, Zhang X, Nitsche T, et al. Effects of different scaffolds on rat adipose tissue derived stroma cells. J Craniomaxillofac Surg. 2014;42:825–834. doi:10.1016/j.jcms.2013.11.020
6. De Coppi P, Bartsch G
7. Tomar GB, Srivastava RK, Gupta N, et al. Human gingiva-derived mesenchymal stem cells are superior to bone marrow-derived mesenchymal stem cells for cell therapy in regenerative medicine. Biochem Biophys Res Commun. 2010;393:377–383. doi:10.1016/j.bbrc.2010.01.126
8. Zhang Q, Shi S, Liu Y, et al. Mesenchymal stem cells derived from human gingiva are capable of immunomodulatory functions and ameliorate inflammation-related tissue destruction in experimental colitis. J Immunol. 2009;183:7787–7798. doi:10.4049/jimmunol.0900838
9. Zhang QZ, Nguyen AL, Yu WH, Le AD. Human oral mucosa and gingiva: a unique reservoir for mesenchymal stem cells. J Dent Res. 2012;91:1011–1018. doi:10.1177/0022034512461016
10. Mitrano TI, Grob MS, Carrión F, et al. Culture and characterization of mesenchymal stem cells from human gingival tissue. J Periodontol. 2010;81:917–925. doi:10.1902/jop.2010.090566
11. Fawzy El-Sayed KM, Paris S, Becker ST, et al. Periodontal regeneration employing gingival margin-derived stem/progenitor cells: an animal study. J Clin Peridontol. 2012;39:861–870. doi:10.1111/j.1600-051X.2012.01904.x
12. Ye SQ. Chinese medicine gem-musk. Fam Med. 2005;5:55.
13. Zhang S. Comparative study of cytotoxicity of moschus and muscone on rat retinal neurons in vitro. Nat Prod Res Dev. 2007;19:639–641.
14. Zheng LC. Clinical application and curative effect of musk heart protecting pill. Med Info. 2009;22:2964.
15. Cao XH. Research progress on the anti-inflammatory effect of musk. China Pharmacy. 2007;18:1662–1664.
16. Li BY. Effects of musk on brain injury in mice. Int J Traditional Chin Med. 1996;18B:33–38.
17. Jiang ZY, Li CD, Zhou D, Zhou DR. Protective effect of musk on experimental cerebral ischemic neuron injury in rats. Chin J Traditional Med Sci Technol. 2001;8:96–97.
18. Shen Q, Liu YM. The clinical application and pharmacological effect of musk in nervous system diseases. Chin J Integr Med Cardio Cerebrovascul Dis. 2003;1:217–219.
19. Shun MH, Si LQ, Yin XZ. Advances in new formulations of natural antitumor drugs. China Pharmacist. 2004;7:171–173.
20. Li XT. Gene Engineering Expression of Musk Active Polypeptides [D]. Guangdong province, China: Sun yat-sen university; 2007.
21. Hou FY, Xie XW, Xi FQ, Xu SH, Li SH, Song M. Efects of muscone-containing serum on proliferation and diferentiation of rat mesenchymal stem cells. J Xian Jiaotong Univ. 2013;34:110–114.
22. Xie XW, Hou FY, Li N, Li SH, Song M. Effects of musk ketone at different concentrations on in vivo migration of exogenous rat bone marrow mesenchymal stem cells. Chin J Integr Traditional West Med. 2012;32:980–985.
23. Gruber J, Yee Z, Tolwinski NS. Developmental drift and the role of Wnt signaling in aging. Cancers. 2016;8:73. doi:10.3390/cancers8080073
24. Kaur P, Jin H, Lusk J, Tolwinski N. Modeling the role of wnt signaling in human and drosophila stem cells. Genes. 2018;9:101. doi:10.3390/genes9020101
25. Boland GM, Perkins G, Hall DJ, et al. Wnt 3a promotes proliferation and suppresses osteogenic differentiation of adult human mesenchymal stem cells. J Cell Biochem. 2004;93(6):1210–1230. doi:10.1002/jcb.20284
26. Ross SE, Hemati N, Longo KA, et al. Inhibition of adipogenes is by Wnt signaling. Science. 2000;289(5481):950–953. doi:10.1126/science.289.5481.950
27. Du L, Yang P, Ge S. Isolation and characterization of human gingiva-derived mesenchymal stem cells using limiting dilution method. J Dent Sci. 2016;11:304–314. doi:10.1016/j.jds.2016.03.010
28. Fathi E, FarahzadiID R, Valipour B, et al. Cytokines secreted from bone marrow derived mesenchymal stem cells promote apoptosis and change cell cycle distribution of K562 cell line as clinical agent in cell transplantation. PLoS One. 2019;14(4):e0215678. doi:10.1371/journal.pone.0215678
29. Fathia E, Farahzadib R, Sheikhzadehc N. Immunophenotypic characterization, multi-lineage differentiation and aging of zebrafish heart and liver tissue-derived mesenchymal stem cells as a novel approach in stem cell-based therapy. Tissue Cell. 2019;57:15–21. doi:10.1016/j.tice.2019.01.006
30. Farahzadi R, Fathic E, Mesbah-Namin SA, et al. Anti-aging protective effect of L-carnitine as clinical agent in regenerative medicine through increasing telomerase activity and change in the hTERT promoter CpG island methylation status of adipose tissue-derived mesenchymal stem cells. Tissue Cell. 2018;54:105–113. doi:10.1016/j.tice.2018.08.012
31. Du Y, Xin G, Meng H, et al. Muscone improves cardiac function in mice after myocardial infarction by alleviating cardiac macrophage-mediated chronic inflammation through inhibition of NF-κB and NLRP3 inflammasome. Am J Transl Res. 2018;10(12):4235–4246.
32. Guoa Y-J, Luoc S-H, Tanga M-J, et al. Muscone exerts protective roles on alcohol-induced osteonecrosis of the femoral head. Biomed Pharmacother. 2018;97:825–832. doi:10.1016/j.biopha.2017.11.025
33. Fournier BP, Ferre FC, Couty L, et al. Multipotent progenitor cells in gingival connective tissue. Tissue Eng Part A. 2010;16:2891–2899. doi:10.1089/ten.TEA.2009.0796
34. Treves-Manusevitz S, Hoz L, Rachima H, et al. Stem cells of the lamina propria of human oral mucosa and gingiva develop into mineralized tissues in vivo. J Clin Periodontol. 2013;40:73–81. doi:10.1111/jcpe.12016
35. Seo BM, Miura M, Gronthos S, et al. Investigation of multipotent postnatal stem cells from human periodontal ligament. Lancet. 2004;364:149–155. doi:10.1016/S0140-6736(04)16627-0
36. Bm S, Miura M, Sonoyama W, Coppe C, Stanyon R, Shi S. Recovery of stem cells from cryopreserved periodontal ligament. J Dent Res. 2005;84:907–912. doi:10.1177/154405910508401007
37. Dominici M, Le Blanc K, Mueller I, et al. Minimal criteria for defining multipotent mesenchymal stromal cell. The International society for cellular therapy positionstatement. Cytotherapy. 2006;8:315–317. doi:10.1080/14653240600855905
38. Keatinga A. Mesenchymal stromal cells: newdirections. Cell Stem Cell. 2012;10:709–716. doi:10.1016/j.stem.2012.05.015
39. Cho KA, Park M, Kim YH. Th17 cell-mediated immune responses promote mast cell proliferation by triggering stem cell factor in keratinocytes. Biochem Biophys Res Commun. 2017. in press. doi:10.1016/j.bbrc.2017.04.141
40. Zhuo HL, Bai LP, Liu D. Effects of retinol on expressions of epidermal growth factor, stem cell factor, colony-stimulating factor 1 and leukemia inhibitory factor in human umbilical cord-derived mesenchymal stem cells. Nan Fang Yi Ke Da Xue Xue Bao. 2016;37(2):221–225.
41. Yang TY. Correlation of Musk Ketone on Osteogenic Differentiation of Human Periodontal Stem Cells [D]. Jilin province, China: Jilin university; 2015.
42. Aronow MA, Gerstenfeld LC, Owen TA, Tassinari MS, Stein GS, Lian JB. Factors that promote progressive development of the osteoblast phenotype in cultured fetal rat calvaria cell. J Cell Physiol. 1990;143:213–221. doi:10.1002/jcp.1041430203
43. Dereka XE, Markopoulou CE, Mamalis A, Vrotsos IA. Effect of rhBMP-7 combined with two bone grafts on human periodontal ligament cell differentiation. Growth Factor. 2009;27:274–279. doi:10.1080/08977190903112721
44. Garin-Shkolnik T, Rudich A, Hotamisligil GS, Rubinstein M. FABP4 attenuates PPAR-r and adipogenesis and is inversely correlated with PPAR-r in adipose tissues. Diabetes. 2014;63:900–911. doi:10.2337/db13-0436
45. Rosen ED, Spiegelman BM. What we talk about when we talk about fat. Cell. 2014;156(1–2):20–44. doi:10.1016/j.cell.2013.12.012
46. Hu L, Yang G, Hägg D, et al. IGF1 promotes adipogenesis by a lineage bias of endogenous adipose stem/progenitor cells. Stem Cells. 2015;33(8):2483–2495.41. doi:10.1002/stem.2052. Logan C, Nusse R. The Wnt signaling pathway in development and disease. Annu Rev Cell Dev Biol 2004; 20: 781⁃810.
47. Walton RL, Beahm EK, Wu L. Denovo adipose formation in a vascularized engineered construct. Microsurgery. 2004;24(5):378–384. doi:10.1002/micr.20056
48. Lee CH, Cook JL, Mendelson A, Moioli EK, Yao H, Mao JJ. Regeneration of the articular surface of the rabbit synovial joint by cell homing: a proof of concept study. Lancet. 2010;376(9739):440–448. doi:10.1016/S0140-6736(10)60668-X
49. Liu P, Feng Y, Dong C, et al. Administration of BMSCs with muscone in rats with gentamincin-induced AKI improves their therapeutic efficacy. PLoS One. 2013;9:e97123. doi:10.1371/journal.pone.0097123
50. Rao TP, Kuhl M. An updated overview on Wnt signaling pathways: a prelude for more. Circ Res. 1798–1806;106:2010.
51. Bienz M, Clevers H. Armadillo/beta-catenin signals in the nucleus–proof beyond a reasonable doubt? Nat. Cell Biol. 2003;5:179–182.
52. Clevers H, Nusse R. Wnt/beta-catenin signaling and disease. Cell. 2012;149:1192e1205. doi:10.1016/j.cell.2012.05.012
53. Moldes M, Zuo Y, Morrison RF. Peroxisome-proliferator-activated receptor gamma suppresses Wnt/beta-catenin signalling dur-ing adipogenesis. Biochem J. 2003;376:
54. Li PH, Liu YY, Cui L. Regulating effects of Wnt signaling pathway on differentiation of bone marrow mesenchymal stem cells into osteoblasts and adipocytes. Chin J Tiissue Engg Res. 2010;14:
55. Gaur T, Lengner CJ, Hovhannisyan H, et al. Canonical WNT signaling promotes osteogenesis by directly stimulating Runx2 gene expression. J Biol Chem. 2005;280:33132–33140. doi:10.1074/jbc.M500608200
56. Bennett CN, Longo KA, Wright WS, et al. Regulation of osteoblastogenesis and bone mass by Wnt10b. Proc Natl Acad Sci USA. 2005;102:3324–3329. doi:10.1073/pnas.0408742102
57. Bennett CN, Longo KA, Wright WS, et al. Regulation of Wnt signaling during adipogenesis. J Biolchem. 2000;277:30998–31004.
58. Stamboloc V, Ruel L, Woodgett JR. Lithium inhibits glycogen synthase kinase-3 activity and mimics wingless signaling in intact cells. Curr Biol. 1996;6:1664–1668. doi:10.1016/s0960-9822(02)70790-2
 © 2019 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2019 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.