Back to Journals » Drug Design, Development and Therapy » Volume 10
Modulation of neurotrophic signaling pathways by polyphenols
Authors Moosavi F, Hosseini R, Saso L , Firuzi O
Received 23 September 2015
Accepted for publication 12 November 2015
Published 21 December 2015 Volume 2016:10 Pages 23—42
DOI https://doi.org/10.2147/DDDT.S96936
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 4
Editor who approved publication: Prof. Dr. Wei Duan
Fatemeh Moosavi,1,2 Razieh Hosseini,1,2 Luciano Saso,3 Omidreza Firuzi1
1Medicinal and Natural Products Chemistry Research Center, Shiraz University of Medical Sciences, Shiraz, Iran; 2Department of Pharmacology, School of Veterinary Medicine, Shiraz University, Shiraz, Iran; 3Department of Physiology and Pharmacology “Vittorio Erspamer”, Sapienza University of Rome, Rome, Italy
Abstract: Polyphenols are an important class of phytochemicals, and several lines of evidence have demonstrated their beneficial effects in the context of a number of pathologies including neurodegenerative disorders such as Alzheimer’s and Parkinson’s disease. In this report, we review the studies on the effects of polyphenols on neuronal survival, growth, proliferation and differentiation, and the signaling pathways involved in these neurotrophic actions. Several polyphenols including flavonoids such as baicalein, daidzein, luteolin, and nobiletin as well as nonflavonoid polyphenols such as auraptene, carnosic acid, curcuminoids, and hydroxycinnamic acid derivatives including caffeic acid phentyl ester enhance neuronal survival and promote neurite outgrowth in vitro, a hallmark of neuronal differentiation. Assessment of underlying mechanisms, especially in PC12 neuronal-like cells, reveals that direct agonistic effect on tropomyosin receptor kinase (Trk) receptors, the main receptors of neurotrophic factors including nerve growth factor (NGF) and brain-derived neurotrophic factor (BDNF) explains the action of few polyphenols such as 7,8-dihydroxyflavone. However, several other polyphenolic compounds activate extracellular signal-regulated kinase (ERK) and phosphoinositide 3-kinase (PI3K)/Akt pathways. Increased expression of neurotrophic factors in vitro and in vivo is the mechanism of neurotrophic action of flavonoids such as scutellarin, daidzein, genistein, and fisetin, while compounds like apigenin and ferulic acid increase cyclic adenosine monophosphate response element-binding protein (CREB) phosphorylation. Finally, the antioxidant activity of polyphenols reflected in the activation of Nrf2 pathway and the consequent upregulation of detoxification enzymes such as heme oxygenase-1 as well as the contribution of these effects to the neurotrophic activity have also been discussed. In conclusion, a better understanding of the neurotrophic effects of polyphenols and the concomitant modulations of signaling pathways is useful for designing more effective agents for management of neurodegenerative diseases.
Keywords: flavonoids, hydroxycinnamic acids, neuroprotective, neurodegeneration, Trk
Introduction
Polyphenolic compounds
Polyphenols are an important group of phytochemicals that are abundantly present in food sources.1 Several lines of evidence have demonstrated beneficial effects of these compounds in the context of several pathologies,2–8 and it has been repeatedly shown that consumption of foods rich in phenolic compounds can lower the risk of several diseases.9,10
Polyphenols have been reported to be of therapeutic value in neurodegenerative diseases,11–13 hypertension14 and other cardiovascular diseases,15 cancer,16–18 inflammation,19,20 diabetes,21 dyslipidemia,22–24 allergy, and immune system diseases.25,26 They have also been reported to have a role in the prevention of different types of cancer ranging from liver,4 prostate,27 and colorectal28 cancer to lymphoblastic leukemia.29
Neurodegenerative disorders such as Alzheimer’s, Parkinson’s, and Huntington’s disease represent rapidly growing causes of disability and death, which have profound economic and social implications; nonetheless, only few effective disease-modifying therapies are available for these diseases.30–32 Recent research has shown that certain polyphenols may have considerable neuroprotective effects in different brain pathologies including neurodegenerative diseases.8,11,33,34 Polyphenols from grape juice administered to older adult subjects have been shown to ameliorate the mild cognitive impairment.35 Similarly, Witte et al36 have shown that administration of resveratrol, a polyphenol present in wine, in older adults significantly improves memory performance, indicating potential strategies to maintain brain health during aging. Assessment of cognitive performance in middle-aged individuals has indicated that consumption of different polyphenols such as catechins, flavonols, and hydroxybenzoic acids is strongly associated with language and verbal memory.37 Other studies have also shown similar effects of other polyphenols on cognitive function and memory in older individuals, who are at risk for neurodegenerative diseases.38,39 In another human study, epigallocatechin–gallate (EGCG) significantly improved cognitive deficits in individuals with Down syndrome.40
Several studies have demonstrated that different polyphenolics with neuroprotective activity, such as EGCG,41,42 epicatechin,43 curcumin,44 resveratrol,45 quercetin,46 and citrus flavonoids (naringenin and hesperetin),47 are able to cross the blood–brain barrier and therefore indicate that these compounds localize within the brain tissue and may well exert neuroprotective and neuromodulatory actions in the settings of different brain pathologies.
Polyphenolic compounds can be classified into different groups as described in Figure 1.1 Among them, flavonoids constitute a major subgroup, which are highly present in fruits and vegetables and possess several biological activities.1
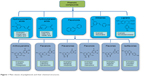  | Figure 1 Main classes of polyphenols and their chemical structures. |
Polyphenols have been extensively studied for their antioxidant action48–52 and some of their beneficial effects, including neuroprotective activity, have been attributed to this capacity.53–55 The neuroprotective effect of polyphenols against the diseases of nervous system has also been addressed in a number of studies.56–59 The aim of this review, however, is to extensively address the alterations of signaling pathways involved in the neurotrophic action of polyphenols that lead to neuronal survival, growth, proliferation, and differentiation. The other aspects of neuroprotective activity of these compounds, which are mainly ascribed to the antioxidant action and mitigation of oxidative stress, have been reviewed elsewhere5,7,8,60–62 and are only briefly mentioned here. It should also be mentioned that in in vitro studies, which are the main focus of this report, supraphysiological doses of polyphenols may have been used and, therefore, caution should be exerted in extrapolation of these data to in vivo conditions.56 The structures of some of the most highly studied neurotrophic polyphenols are depicted in Figures 2 and 3.
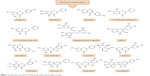  | Figure 2 Chemical structures of flavonoids with neurotrophic activity. |
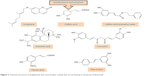  | Figure 3 Chemical structures of polyphenols with neurotrophic activity that do not belong to the group of flavonoids. |
Neurotrophic effects of polyphenols: Enhancement of survival in neuronal cells
Survival signaling is important to suppress the cell death machinery and counterbalance apoptotic signaling in the nervous system.63 Several studies have shown that polyphenolic compounds enhance neuronal survival in serum-deprived conditions. Lin et al64 showed that in neuronal-like PC12 cells, luteolin (3′,4′,5,7-tetrahydroxyflavone, Figure 2) attenuates serum withdrawal-induced cytotoxicity. Other examples of neuroprotectants that exerted prosurvival action in PC12 cells include curcuminoids, the predominant polyphenolic compounds of Curcuma longa Linn.;65 EGCG (Figure 2), the major polyphenol of green tea extract;66 caffeic acid phenethyl ester (CAPE, Figure 3), an active component of propolis;67 5-hydroxy-3,6,7,8,3′,4′-hexamethoxyflavone (HHMF) from the Citrus genus; and nobiletin (Figure 2), the most abundant polymethoxyflavone in orange peel extract.68 Furthermore, other investigators have shown that treatment with genistein and daidzein (Figure 2), isoflavones present in soybeans and soy products, enhances proliferation and survival of the hippocampal neuronal cells.69 In addition, application of 7,8-dihydroxyflavone (7,8-DHF, Figure 2), a flavone, promotes survival of cultured motoneurons, spiral ganglion neurons (SGNs), and hippocampal neuronal cells.70 In another study, the protective ability of quercetin (3,3′,4′,5,7-pentahydroxyflavone, Figure 2) on P19-derived neurons was determined.71 Ferulic acid (4-hydroxy-3-methoxycinnamic acid, Figure 3) was able to significantly increase the survival of neural stem/progenitor cells (NSC/NPCs) cultured from rat embryo, and also increased the number and size of secondary formed neurospheres.72
Promotion of neurite outgrowth in neuronal cells
Neurite outgrowth is a crucial step in the differentiation of neurons, which begins at the cell body and extends outward to form functional synapses. In response to extracellular signals, the growth of neurite processes starts, involving the addition of new plasma membranes, generation of new cytoplasm, and the continued expansion and modification of the cytoskeleton.73 A number of neurotypic proteins have been associated with neurite outgrowth, including growth-associated protein 43 (GAP-43), microtubule-associated protein (MAP) and tau, as well as presynaptic membrane-associated proteins, such as synaptophysin and synapsin.74 Many polyphenolic compounds from natural and synthetic sources have been demonstrated to induce neurite outgrowth in various primary neuronal cultures and neuronal cell lines (Table 1).
Several studies have shown that different polyphenols including flavonoids such as genistein,75 quercetin, liquiritin from Glycyrrhizae radix plant (Figure 2),76 isorhamnetin (a flavonolaglycone from Ginkgo biloba plant, Figure 2),77 and acetylated flavonoid glycosides from Scopariadulcis78 as well as the stilbenoid compound resveratrol (a polyphenol present in grapes and red wine, Figure 3)79 cause a significant enhancement of neurotrophin (nerve growth factor [NGF] and brain-derived neurotrophic factor [BDNF])-mediated neurite outgrowth in PC12 cells.
Some of the phenolic compounds are capable of enhancing the expression levels of the differentiation markers (GAP-43, neurofilament light subunit, synaptophysin, synapsin, etc). Flavonoids that are capable of causing such an effect include luteolin,64 daidzein,80 7,8-DHF,81 citrus HHMF,68 puerarin (an isoflavone),82 CAPE,67 curcuminoids,65 tectoridin (an isoflavone from Belamcandachinensis plant), hesperidin (a flavanone glycoside from the genus Citrus of plants), and also flavonols including kaempferol, quercetin, and isorhamnetin.77
The neuropathy induced by chemotherapeutic agents such as cisplatin is an important side effect of these drugs.83,84 Two polyphenolic compounds, in separate studies, have been tested to block or reverse the cisplatin-induced neurite toxicity in PC12 cells. Phenoxodiol (2H-1-benzopyran-7-0,1,3-[4-hydroxyphenyl]), a compound related to genistein, showed significant neurite-protective effects against cisplatin at 1 μM, a concentration that was not toxic to the PC12 cells.85,86 Curcumin (Figure 3) has also shown similar protective effects against cisplatin-induced neurite toxicity in PC12 cells. Moreover, curcumin did not interfere with the cisplatin’s antitumor mode of action as assessed in vitro in HepG2 cells.87
Activation of tropomyosin receptor kinases
Mammalian neurotrophins including NGF, BDNF, neurotrophin 3 (NT3), and neurotrophin 4 (NT4) play major roles in development, maintenance, repair, and survival of specific neuronal populations.88–90 Several lines of evidence indicate that decreased functioning of neurotrophins and their receptors can lead to neuronal injury and contribute to the pathogenesis of neurodegenerative diseases.30,91,92 Thus, neurotrophins and bioactive compounds capable of activation of neurotrophin receptors have great potential for management of neurodegenerative diseases and other neurological disorders.93,94
Neurotrophins interact with two principal receptor types: p75NTR and the tropomyosin receptor kinase (Trk) receptors consisting of three receptors of TrkA, TrkB, and TrkC in mammals (also known as Ntrk1, Ntrk2, and Ntrk3).95 Different patterns of Trk receptors’ expression exist throughout the mammalian brain and peripheral nervous system. TrkA is highly expressed in cholinergic neurons in the basal forebrain and also the peripheral nervous system, while TrkB and TrkC are highly expressed in the hippocampus.96 These transmembrane receptors belonging to the group of receptor tyrosine kinases (RTKs) and the larger family of catalytic receptors include an extracellular neurotrophin-binding domain and an intracellular tyrosine kinase domain.92,97 NGF has a higher binding rate with TrkA, while BDNF and NT-4/5 bind with TrkB, and finally, NT-3 is the main ligand for TrkC receptor, with a lower affinity for TrkA and TrkB.98 The neurotrophins-induced dimerization of the Trk receptors leads to activation through transphosphorylation of the cytoplasmic domain kinases and stimulates three major signaling pathways: phoshpatidyloinositol-3-kinase (PI3K)/Akt, mitogen-activated protein kinase (MAPK), and phospholipase C-γ1.92 Downstream signaling principally promotes survival, growth, and neuronal differentiation and mediates neurogenesis and plasticity in many neuronal populations.99–101
There are only few polyphenolic compounds that act as direct agonists of Trk receptors and mimic the binding of neurotrophins, while several others stimulate the more downstream pathways leading to the neurotrophic effect (discussed in the following sections). 7,8-DHF provokes TrkB dimerization and tyrosine phosphorylation and activates downstream Akt and extracellular signal-regulated kinase (ERK) as potently as BDNF. 7,8-DHF also inhibits neuronal death in T48, a stably transfected TrkB murine cell line, and hippocampal neurons, and its activity can be inhibited by K252a, a Trk receptor antagonist.102 In another study, 7,8-DHF strongly activated TrkB receptor and its downstream Akt and ERK1/2 pathways, prevented cell death, and promoted neuritogenesis in the retinal ganglion cells.103 It was also shown in a later report that 7,8-DHF as a BDNF agonist causes phosphorylation of TrkB receptors and stimulates survival and neurite growth of cultured motoneurons: PI3K/Akt but not MAPK pathway was responsible for the survival and growth promoting effects of 7,8-DHF,70 which is apparently different from MAPK activation mediated by 7,8-DHF in hippocampal neurons.102
Furthermore, 7,8,3′-trihydroxyflavone, a compound related to 7,8-DHF, has shown similar effects on the survival of SGNs, phosphorylation of TrkB receptor, and ERK.104 Diosmetin, another polyphenol compound belonging to flavonoids class of polyphenols, has also shown antiapoptotic effects, but it induces only weak phosphorylation of TrkB. Diosmetin also increased the phosphorylation of Akt and ERK.102 Another flavonoid, epicatechin, restores TrkA phosphorylation in diabetic animals and reduces diabetes-induced neuronal cell death. It also blocks diabetes-induced p75NTR expression and p75NTR apoptotic pathway in vivo and in Müller cells.105
Modulation of expression of neurotrophic factors and Trk receptors
Several polyphenols increase the expression levels of neurotrophins and Trk receptors (Table 2). Scutellarin, isolated from the traditional Chinese herb, Erigeron breviscapus (Figure 2), alpinetin of the genus Alpinia, and also luteolin, calycosin, genistein, and isorhamnetin effectively upregulate the synthesis and release of NGF, glial cell line-derived neurotrophic factor (GDNF), and BDNF.106,107 The capacity of luteolin, genistein, calycosin, and isorhamnetin in increasing the expression of neurotrophins was mediated through estrogen signaling pathways. In the estrogen receptor (ER)-dependent pathway, the phosphorylated ER dimer was demonstrated to elevate the BDNF messenger RNA levels.108 Scutellarin induced the expression of neurotrophins’ messenger RNAs and proteins through cyclic adenosine monophosphate response element-binding protein (P-CREB) and p-Akt signaling in primary rat astrocytes.106
Several polyphenolic compounds have been reported to increase BDNF levels; different flavonoids such as baicalein (5,6,7-trihydroxyflavone, Figure 2),109 butein and fisetin of Rhusverniciflua (Figure 2),110 chrysin (5,7-dihydroxyflavone, Figure 2),111 daidzein and genistein,112 3,5,6,7,8,3′,4′-heptamethoxyflavone (HMF),113 oroxylin A (5,7-dihidrixy-6-methoxyflavone, Figure 2),114 puerarin,115 and quercetin,116 as well as other polyphenols such as CAPE,117 curcumin,118 and resveratrol,79,119–122 have been reported to possess this property.
The aforementioned studies have examined BDNF levels under various experimental conditions. Some of the polyphenolic compounds, including fisetin and baicalin, one of the major flavonoids isolated from the roots of Scutellaria Baicalensis Georgi could activate the ERK-CREB pathway and exhibited memory-enhancing effects through the activation of CREB-BDNF pathway in the hippocampus of a mouse model of amnesia induced by scopolamine.109,110 In another study, chrysin was shown to rescue memory impairment and BDNF reduction caused by aging in mice.111
Other studies have demonstrated the increased BDNF expression and neuroprotective effects of polyphenolic compounds in the settings of in vitro and in vivo models of ischaemia-, 6-hydroxydopamine (6-OHDA)-, or amyloid-β (Aβ)-mediated injury.113,115,118,123,124 For example, HMF enhances BDNF production in astrocytes and induces neurogenesis in the hippocampus after brain ischemia, which is mediated by the activation of ERK1/2 and CREB pathways.113 Puerarin has also a protective effect in 6-OHDA-lesioned rats by modulating BDNF expression and activation of the nuclear factor E2-related factor 2/antioxidant response element (Nrf2/ARE) signaling pathway.115 Curcumin, on the other hand, was able to block BDNF reduction in the Aβ-infused rats through Akt/GSK-3β signaling pathway.118 Antiamnesic and protective effects of luteolin against Aβ toxicity in mice was associated with the increase of BNDF as well as TrkB expression in the cerebral cortex of mice.123
Many polyphenolic compounds have antidepressant effects that are associated with increased BDNF levels.125,126 Xiaochaihutang, a mixture of seven Chinese herbs that is traditionally used in People’s Republic of China for treatment of depressive-like symptoms, considerably increased BDNF, NGF, TrkB, and TrkA expressions in the hippocampus and also improved depression-like behaviors in chronic unpredictable mild stress (CUMS) model in rats.127 A flavanonol compound, astilbin, reverses depressive-like behaviors in a mouse model of depression and its effect is mediated by the upregulation of BDNF and activation of ERK and Akt pathways.128 Antidepressant effects of baicalein, resveratrol, and rosmarinic acid (a caffeic acid ester) are also mediated by BDNF-ERK-mediated neurotrophic action.129–131 Another polyphenol, ferulic acid, not only increases the proliferation of NSC/NPCs in vitro and in vivo but also produces an additive antidepressant-like effect in corticosterone-treated mice, mediated by CREB-BDNF signaling pathway.72 Also, chrysin improves age-related memory decline in mice, an effect that is probably related to the modulation of BDNF production and free radical scavenging action of this compound.111
ERK pathway activation
Modulation of neuronal survival signaling pathways may represent a promising approach to the management of central nervous system diseases. Several pathways including ERK1/2 and PI3K promote cell survival in the nervous system as well as other tissues.132,133 Recently, the ERK pathway, a part of MAPKs, has been implicated in several physiological functions of neurons, including proliferation, differentiation, survival, and regulation of response to various growth factors.134–138 The activation of ERK1/2 requires phosphorylation of threonine and tyrosine residues that is carried out by the upstream activator kinase, mitogen-activated protein kinase kinase (MEK). Activated ERK1/2 then changes its localization and phosphorylates different target molecules, including transcription regulators and cytoskeletal proteins (Figure 4).139,140
ERK1/2 activation by some of the polyphenolic compounds promotes survival and antiapoptotic signaling in various cell lines (Table 3). It has been shown that ERK1/2 activation by luteolin protects PC12 cells against apoptosis. Furthermore, the cell viability of PC12 cells that were pretreated with U0126, a specific inhibitor of ERK1/2 kinase, was significantly reduced.64
Activation of the ERK1/2 pathway has mediated the neuroprotective activity against damaging insults.132 Aβ peptide can cause a significant decrease in cell viability and neurite outgrowth and can induce apoptosis in neuronal cells. Liquiritin’s protection against Aβ-induced neuronal apoptosis and its effect on the differentiation of rats’ primary cultured hippocampal neurons were inhibited with a MAPK inhibitor.141 Similarly, in another study, the decreased cell viability induced by Aβ25–35 was reported to be blocked by icaritin (a prenyl flavonoid derivative from Chinese tonic herb Epimedium). A blocker of ERK/MAPK pathway weakened this protective effect, which implied that ERK1/2 pathway is involved in the neuroprotective action of icaritin.142 Likewise, rutin (3,3′,4,5,7-pentahydroxyflavone-3-rhamnoglucoside) has also shown beneficial effects against Aβ-induced neurotoxicity in rats through the activation of MAPK and BDNF.143
As already mentioned, ERK1/2 signaling is crucial for neuronal differentiation and activation of associated cytoskeletal and synaptic proteins. It has been found that luteolin increases neurite outgrowth and expression of GAP-43 protein, a neuronal differentiation biomarker, and also heme oxygenase-1 (HO-1) expression in PC12 cells. All these effects could be blocked by pharmacological inhibition of ERK1/2.64 In another report, it was established that luteolin induced microRNA-132 expression in PC12 cells and induced neurite outgrowth, while these effects were suppressed by protein kinase A (PKA) and MEK1/2 inhibitors, but not by protein kinase C (PKC) inhibitors.144
Lin et al64 have shown the involvement of both ERK and PKC signaling pathways in PC12 neurite outgrowth. Similarly, the involvement of the ERK and PKC signaling pathways in the process of neuritogenesis in PC12 cells was demonstrated in response to curcuminoids.65 In another study, artepillin C-induced neurite outgrowth of PC12m3 cells has been shown to be inhibited by the ERK and p38 MAPK inhibitors. On the other hand, inhibition of ERK by U0126 entirely blocked artepillin C-mediated p38 MAPK phosphorylation of PC12m3 cells. It was suggested that the activation of p38 MAPK through the ERK signaling pathway is responsible for the artepillin C-induced neurite outgrowth of PC12m3 cells.145 Finally, fisetin has been very effective in induction of PC12 cell differentiation, while MEK inhibitors considerably decreased fisetin-induced ERK activation and neurite outgrowth.146 The different neurotrophic effects of polyphenols involving the ERK pathway are listed in Tables 1, 3–5.
PI3K pathway activation
A number of studies have demonstrated that PI3K and its downstream effector Akt are involved in neuronal survival and increased neurite outgrowth as well as other neurotrophic effects of polyphenols82,106,118,128,147 (Tables 1, 3–5). In this regard, Hoppe et al118 showed that PI3K/Akt pathway is involved in curcumin-mediated neuroprotection in Aβ-induced cognitive impairment in rats. Similarly, scutellarin is reported to protect neurons against hypoxia by increasing neurotrophins through pCREB and pAkt signaling, but not MAPKs.106 In cultured primary cortical neurons, methyl 3,4-dihydroxybenzoate, a phenolic acid derivative, promoted neuronal survival and neurite outgrowth via PI3K/Akt signaling pathway, and these effects could be inhibited by a PI3K-specific inhibitor.147 In another report, evidence was provided that oroxylin A increased BDNF production and neuronal differentiation in primary cortical neurons by activation of the Akt pathway. Puerarin also protected dopaminergic cells and potentiated the effect of NGF on neuritogenesis in PC12 cells via activation of the ERK1/2 and PI3K/Akt pathways.82 Finally, astilbin, a natural flavonoid, has been demonstrated to reduce depressive-like behaviors in mice models of depression by activation of the MAPK/ERK and PI3K/Akt pathways that are the downstream signaling pathways of BDNF.128
Phosphorylation of CREB
Cyclic AMP response element (CRE) sequence is found in the regulatory region of several genes. CREB is a transcription regulator that recognizes CRE sequence and activates gene transcription, a process believed to play an important role in learning and memory in the brain. In neuronal cells, activation of the CREB pathway by neurotrophic factors such as NGF leads to the expression of genes that regulate survival, growth, synaptic plasticity, differentiation, dendritic spine formation, and long-term memory.148,149
Mantamadiotis et al150 provided evidence that mice lacking CREB function in the brain showed neurodegenerative process in the hippocampus and dorsolateral striatum. Considering the importance of this pathway in the nervous system, we hereby summarize the studies on some phenolic compounds that activate CREB pathway and discuss their possible therapeutic effects in neurodegenerative diseases (Figure 4).
Luteolin,144 auraptene (a coumarin derivative, Figure 3),151 curcumin and demethoxycurcumin,65 nobiletin,152 hesperetin (a flavonoid),153 and citrus HHMF68 have all been shown to increase CREB phosphorylation in PC12 cells. Chai et al106 have also suggested that one of the signaling pathways related to neuroprotective effect of scutellarin is pCREB, which stimulates the production and release of neurotrophic factors in primary rat astrocyte cultures.
Resveratrol has shown neurotrophic effect in dopaminergic neurons79 and antidepressant-like effects in rats via activation of CREB in the hippocampus and amygdala.130 In a study by Li et al,154 green tea catechins-treated mice showed significantly higher CREB phosphorylation than the aged control mice. Ferulic acid has also been reported to increase CREB phosphorylation in the hippocampus of corticosterone-treated mice.72 In an animal model of traumatic brain injury, 7,8-DHF restores the levels of significantly reduced CREB phosphorylation.81 Baicalein increased pCREB in the hippocampus of mice109 and also in NPC.155 Administration of baicalein to rats with cognitive impairment induced by corticosterone significantly improved memory-associated decrease in the expression levels of BDNF and CREB proteins in the hippocampus of these animals.156 Activation of CREB by apigenin (4′,5,7-trihydroxyflavone, Figure 2)157 and pinocembrin (5,7-dihydroxyflavanone)158 was seen in transgenic Alzheimer’s disease mouse model. Finally, rutin and fustin flavonoids have been demonstrated to increase the expression of CREB in the hippocampi of rats and to attenuate Aβ-induced learning impairment in mice, respectively.143,159
Antioxidant activity
The CNS is unique in its high susceptibility to oxidative stress, which is a result of its high oxygen consumption and also the presence of large amounts of fatty acids and metals.160 Several lines of evidence suggest that oxidative stress is involved in the pathogenesis of neurodegenerative disorders such as Alzheimer’s and Parkinson’s disease.61,161 The antioxidant effect of polyphenols has long been studied as a mechanism of their neuroprotection against neurodegenerative disorders61,162 and other neurological diseases.163 Although the direct interaction of polyphenols with reactive oxygen species (ROS) does not seem to be very likely to happen in vivo as a main mechanism of action, their antioxidant effects are most probably exerted through other mechanisms such as activation of Nrf2 pathway, upregulation of antioxidant enzymes, induction of hypoxia signal transduction (HIF-1-α pathway), and interaction with metal ions as sources of ROS.12,161,164
For example, EGCG165 and resveratrol166 have been reported to exert their neuroprotective action through activation of the HIF-1 pathway. Mangiferin and morin (3,5,7,2′,4′-pentahydroxyflavone) revealed considerable antioxidant and antiapoptotic properties through the activation of antioxidant enzymes.167
Protection against damage induced by neurotoxins
Cellular, biochemical, and animal studies have shown that Aβ is a crucial factor in the pathogenesis of Alzheimer’s disease.168,169 There are several reports suggesting that some polyphenols protect neuronal cells against Aβ-induced neuronal cell death or other forms of neuronal injury (Table 5). For instance, icaritin was shown to protect primary rat cortical neuronal cells against apoptosis induced by Aβ25–35 insult.142 Similarly, Ushikubo et al170 demonstrated that 3,3′,4′,5,5′-pentahydroxyflavone prevents Aβ fibril formation and that lowering fibril formation decreases Aβ-induced cell death in rat hippocampal neuronal cells. In another study, ursolic acid, p-coumaric acid, and gallic acid extracted from Cornifructus plant were shown to attenuate apoptotic features such as morphological nuclear changes, DNA fragmentation, and cell blebbing induced by Aβ peptide in PC12 cells.171 The major flavonoids of cocoa, epicatechin and catechin, protect PC12 cells from Aβ-induced neurotoxicity.172 Carnosic acid (Figure 3), a highly bioactive phenolic compound found in rosemary (Rosmarinus officinalis), and liquiritin have shown a protective effect against Aβ in SH-SY5Y human neuroblastoma cells and primary cultured hippocampal neurons, respectively.141,173
6-OHDA is a compound used to induce a Parkinson-like pathology in vitro and in vivo. It has been reported that baicalein174 and also ferulic acid and caffeic acid derivatives, both belonging to hydroxycinnamic acid family (Figure 1),60 protect neuronal SH-SY5Y cells against 6-OHDA.
Hydrogen peroxide is an ROS that has been shown to induce neuronal cell damage in experimental models.175 It has been shown that several polyphenols such as quercetin in cultured neuronal precursor cells,176 7,8-DHF in retinal ganglion and RGC-5 cells,103 and caffeic acid esters in PC12 cells53 are able to exert protective effects against ROS. In addition, other authors suggest that the neuroprotective effects of 7,8-DHF are mediated by its ability to scavenge ROS and increase cellular glutathione levels.55
Other neurotoxins have also been applied to produce experimental models to assess the neuroprotective capacity of polyphenolic compounds. CAPE protects PC12 cells against dopaminergic neurotoxin MPP+ (1-methyl-4-phenylpyridinium).67 Administration of 7,8-DHF prevented neuronal death in mouse brain induced by kainic acid.102 Icariin is another polyphenol compound that protects primary cultured rat hippocampal neuronal cells against corticosterone-induced apoptosis.177 In addition, baicalein was also shown to inhibit necrotic cell death damage in NPCs and to attenuate impairment of hippocampal neurogenesis caused by irradiation.155
Polyphenols have also shown therapeutic potential in animal models of neurodegenerative diseases induced by various neurotoxins. In an Aβ-induced amnesia model in mice, oral administration of luteolin mitigated learning and memory impairment.123 Curcumin has also been shown to be effective in preventing neuroinflammation, tau hyperphosphorylation, and behavioral impairments, triggered by Aβ in vivo.118
Activation of the Nrf2 signaling pathway
One of the elegant mechanisms that neuronal cells have adapted to protect themselves against oxidative stress and other insults is Nrf2 pathway and the binding of this master transcriptional regulator with ARE in the regulatory region of many genes, which leads to the expression of several enzymes with antioxidant and detoxification capacities (Figure 5).178 The main enzymes that are transcribed under the control of ARE include γ-glutamylcysteine synthetase, glutathione peroxidase, glutathione S-transferase, HO-1, NADPH quinine oxidoreductase 1, peroxiredoxin, sulfiredoxin, thioredoxin, and thioredoxin reductase all of which have important roles in the protection of cells.179–181 The pivotal finding that Nrf2-knockout mice exhibit a severe deficiency in the coordinated regulation of gene expression and their susceptibility to oxidative damage indicate the crucial role of Nrf2 in maintaining intracellular redox homeostasis and antioxidant defense mechanism.182 The role of this pathway and the detoxification enzymes that it regulates has been especially emphasized in CNS diseases.181
Some phenolic compounds such as luteolin64 and puerarin82 in PC12 cells and also CAPE in dopaminergic neurons117 enhance the binding of Nrf2 to ARE and increase the expression of HO-1. In cultured neurons, EGCG was able to protect cells against oxidative stress by increasing HO-1 expression via activation of the transcription factor Nrf2.183 Li et al184 evaluated the neuroprotective effect of puerarin on lesioned substantia nigra induced by 6-OHDA. Their findings showed that puerarin effectively protects neurons in substantia nigra by modulating brain-derived neurotrophic factor (BDNF) expression and also by activating the Nrf2/ARE pathway.115 Carnosic acid and common sage (Salvia officinalis) plants also showed neuroprotective effects by activating Nrf2 in PC12h cells.184
Polyphenols and activation of other neurotrophic pathways
Polyphenols having multiple beneficial effects on the nervous system could provide an important resource for the development of new drugs for management of neurodegenerative diseases. In addition to the aforementioned signaling pathways involved in the neurotrophic action of polyphenols, other mechanisms may also be involved. Daidzein has caused significant axonal outgrowth via upregulation of GAP-43 expression in hippocampal neurons in culture. Interestingly, daidzein-promoted phosphorylation of PKC, and GAP-43 was abolished by pretreatment with ER and PKC antagonist. These results suggest that ER-mediated PKC phosphorylation of GAP-43 may play a role in daidzein-mediated axonal outgrowth.80 Hesperetin can also exhibit multiple neurotrophic effects via ER- and TrkA-mediated parallel pathways.153
The Na+/K+/2Cl cotransporter (NKCC) is a member of the cation–chloride cotransporter family and is involved in the transport of chloride ion(s) coupled with cation(s) across the plasma membrane.185 A previous study has shown that NGF treatment of PC12D cells increased the expression of NKCC1 protein.186 In another report, it has been demonstrated that knockdown of NKCC1 strongly inhibits NGF induced-neurite outgrowth in PC12 cells. Interestingly, quercetin also promoted NGF-induced neurite outgrowth by increasing Cl−, and knockdown of NKCC1 inhibited this stimulatory effect. In these cells, intracellular Cl− affects microtubule polymerization via modulation of intrinsic GTPase activity of tubulin.187
A2A subtype of adenosine receptors has been reported to elevate the expression of BDNF as well as synaptic actions of BDNF.188,189 This receptor also activates TrkB receptor and Akt signaling pathway, which induces neuronal survival and modulates neurite outgrowth in several different cell types.190–192 Jeon et al114 have recently shown that oroxylin A could induce BDNF production in cortical neurons via activation of A2A receptor, which induced cellular survival, synapse formation, and neurite outgrowth. In another report, the adenosine A2A receptor inhibitor could block methyl 3,4-dihydroxybenzoate-induced neuronal survival and neurite outgrowth in cultured primary cortical neurons.147
Conclusion
Polyphenols with multiple beneficial effects in the nervous system could provide an important resource for the discovery of new neurotrophic agents. Agonistic action on Trk receptors, activation of the ERK, PI3Kinase/Akt and CREB pathways, activation of the Nrf2 pathway and upregulation of antioxidant and detoxification enzymes, as well as several other mechanisms underlie the neurotrophic action of different polyphenols. A better understanding of the neurotrophic effects and the molecular mechanisms of action of these compounds could help design better agents for management of neurodegenerative diseases and other disorders of the nervous system.
Acknowledgment
The authors thank the support provided by the vice chancellor for research, Shiraz University of Medical Sciences.
Disclosure
The authors report no conflict of interest in this work.
References
Manach C, Scalbert A, Morand C, Rémésy C, Jiménez L. Polyphenols: food sources and bioavailability. Am J Clin Nutr. 2004;79(5):727–747. | ||
Link A, Balaguer F, Goel A. Cancer chemoprevention by dietary polyphenols: promising role for epigenetics. Biochem Pharmacol. 2010;80(12):1771–1792. | ||
Weng CJ, Yen GC. Chemopreventive effects of dietary phytochemicals against cancer invasion and metastasis: phenolic acids, monophenol, polyphenol, and their derivatives. Cancer Treat Rev. 2012;38(1):76–87. | ||
Darvesh AS, Bishayee A. Chemopreventive and therapeutic potential of tea polyphenols in hepatocellular cancer. Nutr Cancer. 2013;65(3):329–344. | ||
Annuzzi G, Bozzetto L, Costabile G, et al. Diets naturally rich in polyphenols improve fasting and postprandial dyslipidemia and reduce oxidative stress: a randomized controlled trial. Am J Clin Nutr. 2014;99(3):463–471. | ||
Shay J, Elbaz HA, Lee I, Zielske SP, Malek MH, Huttemann M. Molecular mechanisms and therapeutic effects of (-)-epicatechin and other polyphenols in cancer, inflammation, diabetes, and neurodegeneration. Oxid Med Cell Longev. 2015;2015:181260. | ||
Dai J, Mumper RJ. Plant phenolics: extraction, analysis and their antioxidant and anticancer properties. Molecules. 2010;15(10):7313–7352. | ||
Pocernich CB, Lange ML, Sultana R, Butterfield DA. Nutritional approaches to modulate oxidative stress in Alzheimer’s disease. Curr Alzheimer Res. 2011;8(5):452–469. | ||
Vauzour D, Vafeiadou K, Rodriguez-Mateos A, Rendeiro C, Spencer JP. The neuroprotective potential of flavonoids: a multiplicity of effects. Genes Nutr. 2008;3(3–4):115–126. | ||
Scalbert A, Manach C, Morand C, Rémésy C, Jiménez L. Dietary polyphenols and the prevention of diseases. Crit Rev Food Sci Nutr. 2005;45(4):287–306. | ||
Bhullar KS, Rupasinghe H. Polyphenols: multipotent therapeutic agents in neurodegenerative diseases. Oxid Med Cell Longev. 2013;2013. | ||
Ebrahimi A, Schluesener H. Natural polyphenols against neurodegenerative disorders: potentials and pitfalls. Ageing Res Rev. 2012;11(2):329–345. | ||
Li H, Zhang Y, Cao L, et al. Curcumin could reduce the monomer of TTR with Tyr114Cys mutation via autophagy in cell model of familial amyloid polyneuropathy. Drug Des Devel Ther. 2014;8:2121–2128. | ||
Larson AJ, Symons JD, Jalili T. Therapeutic potential of quercetin to decrease blood pressure: review of efficacy and mechanisms. Adv Nutr. 2012;3(1):39–46. | ||
Quiñones M, Miguel M, Aleixandre A. Beneficial effects of polyphenols on cardiovascular disease. Pharmacol Res. 2013;68(1):125–131. | ||
Fresco P, Borges F, Marques M, Diniz C. The anticancer properties of dietary polyphenols and its relation with apoptosis. Curr Pharm Des. 2010;16(1):114–134. | ||
Nishiumi S, Miyamoto S, Kawabata K, et al. Dietary flavonoids as cancer-preventive and therapeutic biofactors. Front Biosci. 2010;3: 1332–1362. | ||
Martin MA, Goya L, Ramos S. Potential for preventive effects of cocoa and cocoa polyphenols in cancer. Food Chem Toxicol. 2013;56:336–351. | ||
Conte E, Fagone E, Fruciano M, Gili E, Iemmolo M, Vancheri C. Anti-inflammatory and antifibrotic effects of resveratrol in the lung. Histol Histopathol. 2014;30(5):523–529. | ||
Gonzalez R, Ballester I, Lopez-Posadas R, et al. Effects of flavonoids and other polyphenols on inflammation. Crit Rev Food Sci Nutr. 2011;51(4):331–362. | ||
Babu PVA, Liu D, Gilbert ER. Recent advances in understanding the anti-diabetic actions of dietary flavonoids. J Nutr Biochem. 2013;24(11):1777–1789. | ||
Assini JM, Mulvihill EE, Huff MW. Citrus flavonoids and lipid metabolism. Curr Opin Lipidol. 2013;24(1):34–40. | ||
Yao Z, Zhang L, Ji G. Efficacy of polyphenolic ingredients of Chinese herbs in treating dyslipidemia of metabolic syndromes. Eur J Integr Med. 2014;12(3):135–146. | ||
Islam B, Sharma C, Adem A, Aburawi E, Ojha S. Insight into the mechanism of polyphenols on the activity of HMGR by molecular docking. Drug Des Devel Ther. 2015;9:4943–4951. | ||
Chirumbolo S. Dietary assumption of plant polyphenols and prevention of allergy. Curr Pharm Des. 2014;20(6):811–839. | ||
Cuevas A, Saavedra N, Salazar LA, Abdalla DS. Modulation of immune function by polyphenols: possible contribution of epigenetic factors. Nutrients. 2013;5(7):2314–2332. | ||
Cimino S, Sortino G, Favilla V, et al. Polyphenols: key issues involved in chemoprevention of prostate cancer. Oxid Med Cell Longev. 2012;2012. | ||
Jin H, Leng Q, Li C. Dietary flavonoid for preventing colorectal neoplasms. Cochrane Libr. 2012;8:CD009350. | ||
Zand H. Chemopreventive and chemosensitization potential of flavonoids in acute lymphoblastic leukemia. J Pediatr Biochem. 2012;2(1):15–21. | ||
Querfurth HW, LaFerla FM. Alzheimer’s disease. New Engl J Med. 2010;362(4):329–344. | ||
Citron M. Alzheimer’s disease: strategies for disease modification. Nat Rev Drug Discov. 2010;9(5):387–398. | ||
Mangialasche F, Solomon A, Winblad B, Mecocci P, Kivipelto M. Alzheimer’s disease: clinical trials and drug development. Lancet Neurol. 2010;9(7):702–716. | ||
Shay J, Elbaz HA, Lee I, Zielske SP, Malek MH, Hüttemann M. Molecular mechanisms and therapeutic effects of (−)-epicatechin and other polyphenols in cancer, inflammation, diabetes, and neurodegeneration. Oxid Med Cell Longev. 2015;2015. | ||
Albarracin SL, Stab B, Casas Z, et al. Effects of natural antioxidants in neurodegenerative disease. Nutr Neurosci. 2012;15(1):1–9. | ||
Krikorian R, Boespflug EL, Fleck DE, et al. Concord grape juice supplementation and neurocognitive function in human aging. J Agric Food Chem. 2012;60(23):5736–5742. | ||
Witte AV, Kerti L, Margulies DS, Flöel A. Effects of resveratrol on memory performance, hippocampal functional connectivity, and glucose metabolism in healthy older adults. J Neurosci. 2014;34(23): 7862–7870. | ||
Kesse-Guyot E, Fezeu L, Andreeva VA, et al. Total and specific polyphenol intakes in midlife are associated with cognitive function measured 13 years later. J Nutr. 2012;142(1):76–83. | ||
Letenneur L, Proust-Lima C, Le Gouge A, Dartigues J-F, Barberger-Gateau P. Flavonoid intake and cognitive decline over a 10-year period. Am J Epidemiol. 2007;165(12):1364–1371. | ||
Cimrová B, Budáč S, Melicherová U, Jergelová M, Jagla F. Electrophysiological evidence of the effect of natural polyphenols upon the human higher brain functions. Neuro Endocrinol Lett. 2011;32(4):464–468. | ||
Torre R, Sola S, Pons M, et al. Epigallocatechin-3-gallate, a DYRK1A inhibitor, rescues cognitive deficits in Down syndrome mouse models and in humans. Mol Nutr Food Res. 2014;58(2):278–288. | ||
Gundimeda U, McNeill TH, Schiffman JE, Hinton DR, Gopalakrishna R. Green tea polyphenols potentiate the action of nerve growth factor to induce neuritogenesis: possible role of reactive oxygen species. J Neurosci Res. 2010;88(16):3644–3655. | ||
Suganuma M, Okabe S, Oniyama M, Tada Y, Ito H, Fujiki H. Wide distribution of [3H](-)-epigallocatechin gallate, a cancer preventive tea polyphenol, in mouse tissue. Carcinogenesis. 1998;19(10):1771–1776. | ||
El Mohsen MMA, Kuhnle G, Rechner AR, et al. Uptake and metabolism of epicatechin and its access to the brain after oral ingestion. Free Radic Biol Med. 2002;33(12):1693–1702. | ||
Rossi L, Mazzitelli S, Arciello M, Capo C, Rotilio G. Benefits from dietary polyphenols for brain aging and Alzheimer’s disease. Neurochem Res. 2008;33(12):2390–2400. | ||
Vingtdeux V, Giliberto L, Zhao H, et al. AMP-activated protein kinase signaling activation by resveratrol modulates amyloid-β peptide metabolism. J Biol Chem. 2010;285(12):9100–9113. | ||
Youdim KA, Qaiser MZ, Begley DJ, Rice-Evans CA, Abbott NJ. Flavonoid permeability across an in situ model of the blood–brain barrier. Free Radic Biol Med. 2004;36(5):592–604. | ||
Youdim KA, Dobbie MS, Kuhnle G, Proteggente AR, Abbott NJ, Rice-Evans C. Interaction between flavonoids and the blood–brain barrier: in vitro studies. J Neurochem. 2003;85(1):180–192. | ||
Firuzi O, Lacanna A, Petrucci R, Marrosu G, Saso L. Evaluation of the antioxidant activity of flavonoids by “ferric reducing antioxidant power” assay and cyclic voltammetry. Biochim Biophys Acta. 2005;1721(1–3):174–184. | ||
Firuzi O, Mladěnka P, Petrucci R, Marrosu G, Saso L. Hypochlorite scavenging activity of flavonoids. J Pharm Pharmacol. 2004;56(6): 801–807. | ||
Dueñas M, González-Manzano S, González-Paramás A, Santos-Buelga C. Antioxidant evaluation of O-methylated metabolites of catechin, epicatechin and quercetin. J Pharm Biomed Anal. 2010;51(2):443–449. | ||
Lemmens KJ, van de Wier B, Vaes N, et al. The flavonoid 7-mono-O-(β-hydroxyethyl)-rutoside is able to protect endothelial cells by a direct antioxidant effect. Toxicol in Vitro. 2014;28(4):538–543. | ||
Martín MÁ, Fernández-Millán E, Ramos S, Bravo L, Goya L. Cocoa flavonoid epicatechin protects pancreatic beta cell viability and function against oxidative stress. Mol Nutr Food Res. 2014;58(3):447–456. | ||
Garrido J, Gaspar A, Garrido EM, et al. Alkyl esters of hydroxycinnamic acids with improved antioxidant activity and lipophilicity protect PC12 cells against oxidative stress. Biochimie. 2012;94(4):961–967. | ||
Gaspar A, Martins M, Silva P, et al. Dietary phenolic acids and derivatives. Evaluation of the antioxidant activity of sinapic acid and its alkyl esters. J Agric Food Chem. 2010;58(21):11273–11280. | ||
Chen J, Chua K-W, Chua CC, et al. Antioxidant activity of 7,8-dihydroxyflavone provides neuroprotection against glutamate-induced toxicity. Neurosci Lett. 2011;499(3):181–185. | ||
Vauzour D. Dietary polyphenols as modulators of brain functions: biological actions and molecular mechanisms underpinning their beneficial effects. Oxid Med Cell Longev. 2012;2012:914273. | ||
Gutierrez-Merino C, Lopez-Sanchez C, Lagoa R, Samhan-Arias AK, Bueno C, Garcia-Martinez V. Neuroprotective actions of flavonoids. Curr Med Chem. 2011;18(8):1195–1212. | ||
Hwang S-L, Shih P-H, Yen G-C. Neuroprotective effects of citrus flavonoids. J Agric Food Chem. 2012;60(4):877–885. | ||
Venkatesan R, Ji E, Kim SY. Phytochemicals that regulate neurodegenerative disease by targeting neurotrophins: a comprehensive review. Biomed Res Int. 2015;2015:814068. | ||
Silva T, Bravo J, Summavielle T, et al. Biology-oriented development of novel lipophilic antioxidants with neuroprotective activity. RSC Adv. 2015;5(21):15800–15811. | ||
Darvesh AS, Carroll RT, Bishayee A, Geldenhuys WJ, Van der Schyf CJ. Oxidative stress and Alzheimer’s disease: dietary polyphenols as potential therapeutic agents. Expert Rev Neurother. 2010;10(5):729–745. | ||
Firuzi O, Miri R, Tavakkoli M, Saso L. Antioxidant therapy: current status and future prospects. Curr Med Chem. 2011;18(25):3871–3888. | ||
Yuan J, Yankner BA. Apoptosis in the nervous system. Nature. 2000;407(6805):802–809. | ||
Lin C-W, Wu M-J, Liu IY-C, Su J-D, Yen J-H. Neurotrophic and cytoprotective action of luteolin in PC12 cells through ERK-dependent induction of Nrf2-driven HO-1 expression. J Agric Food Chem. 2010;58(7):4477–4486. | ||
Liao K-K, Wu M-J, Chen P-Y, et al. Curcuminoids promote neurite outgrowth in PC12 cells through MAPK/ERK-and PKC-dependent pathways. J Agric Food Chem. 2011;60(1):433–443. | ||
Reznichenko L, Amit T, Youdim M, Mandel S. Green tea polyphenol (−)-epigallocatechin-3-gallate induces neurorescue of long-term serum-deprived PC12 cells and promotes neurite outgrowth. J Neurochem. 2005;93(5):1157–1167. | ||
dos Santos NAG, Martins NM, de Barros Silva R, Ferreira RS, Sisti FM, dos Santos AC. Caffeic acid phenethyl ester (CAPE) protects PC12 cells from MPP+ toxicity by inducing the expression of neuron-typical proteins. NeuroToxicology. 2014;45:131–138. | ||
Lai H-C, Wu M-J, Chen P-Y, et al. Neurotrophic effect of citrus 5-hydroxy-3, 6, 7, 8, 3′, 4′-hexamethoxyflavone: promotion of neurite outgrowth via cAMP/PKA/CREB pathway in PC12 cells. PLoS One. 2011;6(11):e28280. | ||
Yang S-H, Liao C-C, Chen Y, Syu J-P, Jeng C-J, Wang S-M. Daidzein induces neuritogenesis in DRG neuronal cultures. J Biomed Sci. 2012;19:80. | ||
Tsai T, Klausmeyer A, Conrad R, et al. 7,8-Dihydroxyflavone leads to survival of cultured embryonic motoneurons by activating intracellular signaling pathways. Mol Cell Neurosci. 2013;56:18–28. | ||
Tangsaengvit N, Kitphati W, Tadtong S, Bunyapraphatsara N, Nukoolkarn V. Neurite outgrowth and neuroprotective effects of quercetin from Caesalpinia mimosoides Lamk. on cultured P19-derived neurons. Evid Based Complement Alternat Med. 2013;2013:838051. | ||
Yabe T, Hirahara H, Harada N, et al. Ferulic acid induces neural progenitor cell proliferation in vitro and in vivo. Neuroscience. 2010;165(2):515–524. | ||
Tang BL. Protein trafficking mechanisms associated with neurite outgrowth and polarized sorting in neurons. J Neurochem. 2001;79(5):923–930. | ||
Das KP, Freudenrich TM, Mundy WR. Assessment of PC12 cell differentiation and neurite growth: a comparison of morphological and neurochemical measures. Neurotoxicol Teratol. 2004;26(3):397–406. | ||
Nakajima K-I, Niisato N, Marunaka Y. Genistein enhances the NGF-induced neurite outgrowth. Biomed Res. 2011;32(5):351–356. | ||
Chen Z-A, Wang J-L, Liu R-T, et al. Liquiritin potentiate neurite outgrowth induced by nerve growth factor in PC12 cells. Cytotechnology. 2009;60(1–3):125–132. | ||
Xu SL, Choi RC, Zhu KY, et al. Isorhamnetin, a flavonol aglycone from Ginkgo biloba L., induces neuronal differentiation of cultured PC12 cells: potentiating the effect of nerve growth factor. Evid Based Complement Alternat Med. 2012;2012:278273. | ||
Li Y, Chen X, Satake M, Oshima Y, Ohizumi Y. Acetylated flavonoid glycosides potentiating NGF action from scoparia dulcis. J Nat Prod. 2004;67(4):725–727. | ||
Zhang F, Wang Y-Y, Liu H, et al. Resveratrol produces neurotrophic effects on cultured dopaminergic neurons through prompting astroglial BDNF and GDNF release. Evid Based Complement Alternat Med. 2012;2012:937605. | ||
Wang P, Jeng C-J, Chien C-L, Wang S-M. Signaling mechanisms of daidzein-induced axonal outgrowth in hippocampal neurons. Biochem Biophys Res Commun. 2008;366(2):393–400. | ||
Agrawal R, Noble E, Tyagi E, Zhuang Y, Ying Z, Gomez-Pinilla F. Flavonoid derivative 7,8-DHF attenuates TBI pathology via TrkB activation. Biochim Biophys Acta. 2015;1852(5):862–872. | ||
Zhao J, Cheng YY, Fan W, et al. Botanical drug puerarin coordinates with nerve growth factor in the regulation of neuronal survival and neuritogenesis via activating ERK1/2 and PI3K/Akt signaling pathways in the neurite extension process. CNS Neurosci Ther. 2015;21(1):61–70. | ||
Ta LE, Espeset L, Podratz J, Windebank AJ. Neurotoxicity of oxaliplatin and cisplatin for dorsal root ganglion neurons correlates with platinum–DNA binding. Neurotoxicology. 2006;27(6):992–1002. | ||
Quasthoff S, Hartung HP. Chemotherapy-induced peripheral neuropathy. J Neurol. 2002;249(1):9–17. | ||
Klein R, Brown D, Turnley AM. Phenoxodiol protects against Cisplatin induced neurite toxicity in a PC-12 cell model. BMC Neurosci. 2007;8(1):61. | ||
Kamsteeg M, Rutherford T, Sapi E, et al. Phenoxodiol – an isoflavone analog – induces apoptosis in chemoresistant ovarian cancer cells. Oncogene. 2003;22(17):2611–2620. | ||
Dikshit P, Goswami A, Mishra A, Catterjee M, Jana NR. Curcumin induces stress response, neurite outgrowth and prevent NF-κB activation by inhibiting the proteasome function. Neurotox Res. 2006;9(1):29–37. | ||
Levy YS, Gilgun-Sherki Y, Melamed E, Offen D. Therapeutic potential of neurotrophic factors in neurodegenerative diseases. Bio Drugs. 2005;19(2):97–127. | ||
Hyman C, Hofer M, Barde YA, et al. BDNF is a neurotrophic factor for dopaminergic neurons of the substantia nigra. Nature. 1991;350(6315):230–232. | ||
Maisonpierre PC, Belluscio L, Friedman B, et al. NT-3, BDNF, and NGF in the developing rat nervous system: parallel as well as reciprocal patterns of expression. Neuron. 1990;5(4):501–509. | ||
Schindowski K, Belarbi K, Buee L. Neurotrophic factors in Alzheimer’s disease: role of axonal transport. Genes Brain Behav. 2008;7(s1):43–56. | ||
Longo FM, Massa SM. Small-molecule modulation of neurotrophin receptors: a strategy for the treatment of neurological disease. Nat Rev Drug Discov. 2013;12(7):507–525. | ||
Nagahara AH, Tuszynski MH. Potential therapeutic uses of BDNF in neurological and psychiatric disorders. Nat Rev Drug Discov. 2011;10(3):209–219. | ||
Allen SJ, Watson JJ, Shoemark DK, Barua NU, Patel NK. GDNF, NGF and BDNF as therapeutic options for neurodegeneration. Pharmacol Ther. 2013;138(2):155–175. | ||
Huang EJ, Reichardt LF. Trk receptors: Roles in neuronal signal transduction. Annu Rev Biochem. 2012;722003:609–642. | ||
Webster NJ, Pirrung MC. Small molecule activators of the Trk receptors for neuroprotection. BMC Neuroscience. 2008;9 (Suppl 2):S1. | ||
Chao MV. Neurotrophins and their receptors: a convergence point for many signalling pathways. Nat Rev Neurosci. 2003;4(4):299–309. | ||
Ultsch MH, Wiesmann C, Simmons LC, et al. Crystal structures of the neurotrophin-binding domain of TrkA, TrkB and TrkC. J Mol Biol. 1999;290(1):149–159. | ||
Huang EJ, Reichardt LF. Trk receptors: roles in neuronal signal transduction. Annu Rev Biochem. 2003;72(1):609–642. | ||
Kaplan DR, Miller FD. Neurotrophin signal transduction in the nervous system. Curr Opin Neurobiol. 2000;10(3):381–391. | ||
Patapoutian A, Reichardt LF. Trk receptors: mediators of neurotrophin action. Curr Opin Neurobiol. 2001;11(3):272–280. | ||
Jang S-W, Liu X, Yepes M, et al. A selective TrkB agonist with potent neurotrophic activities by 7,8-dihydroxyflavone. Proc Natl Acad Sci U S A. 2010;107(6):2687–2692. | ||
Gupta VK, You Y, Li JC, Klistorner A, Graham SL. Protective effects of 7,8-dihydroxyflavone on retinal ganglion and RGC-5 cells against excitotoxic and oxidative stress. J Mol Neurosci. 2013;49(1):96–104. | ||
Yu Q, Chang Q, Liu X, et al. Protection of spiral ganglion neurons from degeneration using small-molecule TrkB receptor agonists. J Neurosci. 2013;33(32):13042–13052. | ||
Al-Gayyar M, Matragoon S, Pillai B, Ali T, Abdelsaid M, El-Remessy A. Epicatechin blocks pro-nerve growth factor (proNGF)-mediated retinal neurodegeneration via inhibition of p75 neurotrophin receptor proNGF expression in a rat model of diabetes. Diabetologia. 2011;54(3):669–680. | ||
Chai L, Guo H, Li H, et al. Scutellarin and caffeic acid ester fraction, active components of Dengzhanxixin injection, upregulate neurotrophins synthesis and release in hypoxia/reoxygenation rat astrocytes. J Ethnopharmacol. 2013;150(1):100–107. | ||
Xu SL, Bi CW, Choi RC, et al. Flavonoids induce the synthesis and secretion of neurotrophic factors in cultured rat astrocytes: a signaling response mediated by estrogen receptor. Evid Based Complement Alternat Med. 2013;2013:127075. | ||
Sohrabji F, Miranda R, Toran-Allerand CD. Identification of a putative estrogen response element in the gene encoding brain-derived neurotrophic factor. Proc Natl Acad Sci U S A. 1995;92(24):11110–11114. | ||
Park SJ, Kim DH, Kim JM, et al. Mismatch between changes in baicalein-induced memory-related biochemical parameters and behavioral consequences in mouse. Brain Res. 2010;1355:141–150. | ||
Cho N, Lee KY, Huh J, et al. Cognitive-enhancing effects of Rhus verniciflua bark extract and its active flavonoids with neuroprotective and anti-inflammatory activities. Food Chem Toxicol. 2013;58:355–361. | ||
Souza LC, Antunes MS, Borges Filho C, et al. Flavonoid Chrysin prevents age-related cognitive decline via attenuation of oxidative stress and modulation of BDNF levels in aged mouse brain. Pharmacol Biochem Behav. 2015;134:22–30. | ||
Pan M, Han H, Zhong C, Geng Q. Effects of genistein and daidzein on hippocampus neuronal cell proliferation and BDNF expression in H19-7 neural cell line. J Nutr Health Aging. 2012;16(4):389–394. | ||
Okuyama S, Shimada N, Kaji M, et al. Heptamethoxyflavone, a citrus flavonoid, enhances brain-derived neurotrophic factor production and neurogenesis in the hippocampus following cerebral global ischemia in mice. Neurosci Lett. 2012;528(2):190–195. | ||
Jeon SJ, Bak H, Seo J, et al. Oroxylin A induces BDNF expression on cortical neurons through adenosine A2A receptor stimulation: a possible role in neuroprotection. Biomol Ther. 2012;20(1):27. | ||
Li R, Liang T, Xu L, Zheng N, Zhang K, Duan X. Puerarin attenuates neuronal degeneration in the substantia nigra of 6-OHDA-lesioned rats through regulating BDNF expression and activating the Nrf2/ARE signaling pathway. Brain Res. 2013;1523:1–9. | ||
Liu P, Zou D, Yi L, et al. Quercetin ameliorates hypobaric hypoxia-induced memory impairment through mitochondrial and neuron function adaptation via the PGC-1α pathway. Restor Neurol Neurosci. 2015;33(2):143–157. | ||
Kurauchi Y, Hisatsune A, Isohama Y, Mishima S, Katsuki H. Caffeic acid phenethyl ester protects nigral dopaminergic neurons via dual mechanisms involving haem oxygenase-1 and brain-derived neurotrophic factor. Br J Pharmacol. 2012;166(3):1151–1168. | ||
Hoppe JB, Coradini K, Frozza RL, et al. Free and nanoencapsulated curcumin suppress β-amyloid-induced cognitive impairments in rats: involvement of BDNF and Akt/GSK-3β signaling pathway. Neurobiol Learn Mem. 2013;106:134–144. | ||
Zhang F, Lu Y-F, Wu Q, Liu J, Shi J-S. Resveratrol promotes neurotrophic factor release from astroglia. Exp Biol Med. 2012;237(8):943–948. | ||
Rahvar M, Nikseresht M, Shafiee SM, et al. Effect of oral resveratrol on the BDNF gene expression in the hippocampus of the rat brain. Neurochem Res. 2011;36(5):761–765. | ||
Yuan H, Zhang J, Liu H, Li Z. The protective effects of resveratrol on Schwann cells with toxicity induced by ethanol in vitro. Neurochem Int. 2013;63(3):146–153. | ||
Moriya J, Chen R, Yamakawa J-I, Sasaki K, Ishigaki Y, Takahashi T. Resveratrol improves hippocampal atrophy in chronic fatigue mice by enhancing neurogenesis and inhibiting apoptosis of granular cells. Biol Pharm Bull. 2011;34(3):354–359. | ||
Liu R, Gao M, Qiang G-F, et al. The anti-amnesic effects of luteolin against amyloid β 25–35 peptide-induced toxicity in mice involve the protection of neurovascular unit. Neuroscience. 2009;162(4):1232–1243. | ||
Lee YW, Kim DH, Jeon SJ, et al. Neuroprotective effects of salvianolic acid B on an Aβ 25–35 peptide-induced mouse model of Alzheimer’s disease. Eur J Pharmacol. 2013;704(1):70–77. | ||
Donato F, de Gomes MG, Goes ATR, et al. Hesperidin exerts antidepressant-like effects in acute and chronic treatments in mice: Possible role of L-arginine-NO-cGMP pathway and BDNF levels. Brain Res Bull. 2014;104:19–26. | ||
Wang Z, Gu J, Wang X, et al. Antidepressant-like activity of resveratrol treatment in the forced swim test and tail suspension test in mice: the HPA axis, BDNF expression and phosphorylation of ERK. Pharmacol Biochem Behav. 2013;112:104–110. | ||
Su GY, Yang JY, Wang F, et al. Antidepressant-like effects of Xiaochaihutang in a rat model of chronic unpredictable mild stress. J Ethnopharmacol. 2014;152(1):217–226. | ||
Lv Q-Q, Wu W-J, Guo X-L, et al. Antidepressant activity of astilbin: involvement of monoaminergic neurotransmitters and BDNF signal pathway. Biol Pharm Bull. 2014;37(6):987–995. | ||
Xiong Z, Jiang B, Wu P-F, et al. Antidepressant effects of a plant-derived flavonoid baicalein involving extracellular signal-regulated kinases cascade. Biol Pharm Bull. 2011;34(2):253–259. | ||
Liu D, Xie K, Yang X, et al. Resveratrol reverses the effects of chronic unpredictable mild stress on behavior, serum corticosterone levels and BDNF expression in rats. Behav Brain Res. 2014;264:9–16. | ||
Jin X, Liu P, Yang F, Zhang Y-H, Miao D. Rosmarinic acid ameliorates depressive-like behaviors in a rat model of CUS and up-regulates BDNF levels in the hippocampus and hippocampal-derived astrocytes. Neurochem Res. 2013;38(9):1828–1837. | ||
Hetman M, Gozdz A. Role of extracellular signal regulated kinases 1 and 2 in neuronal survival. Eur J Biochem. 2004;271(11):2050–2055. | ||
Hetman M, Xia Z. Signaling pathways mediating anti-apoptotic action of neurotrophins. Acta Neurobiol Exp. 2000;60(4):531–546. | ||
Satoh Y, Kobayashi Y, Takeuchi A, Pagès G, Pouysségur J, Kazama T. Deletion of ERK1 and ERK2 in the CNS causes cortical abnormalities and neonatal lethality: Erk1 deficiency enhances the impairment of neurogenesis in Erk2-deficient mice. J Neurosci. 2011;31(3):1149–1155. | ||
Parmar MS, Jaumotte JD, Wyrostek SL, Zigmond MJ, Cavanaugh JE. The role of ERK1, 2, and 5 in dopamine neuron survival during aging. Neurobiol Aging. 2014;35(3):669–679. | ||
Li Z, Theus MH, Wei L. Role of ERK 1/2 signaling in neuronal differentiation of cultured embryonic stem cells. Dev Growth Differ. 2006;48(8):513–523. | ||
Li Q, Chen M, Liu H, Yang L, Yang T, He G. The dual role of ERK signaling in the apoptosis of neurons. Front Biosci. 2013;19:1411–1417. | ||
Munshi A, Ramesh R. Mitogen-activated protein kinases and their role in radiation response. Genes Cancer. 2013;4(9–10):401–408. | ||
Moustafa K, AbuQamar S, Jarrar M, Al-Rajab AJ, Trémouillaux-Guiller J. MAPK cascades and major abiotic stresses. Plant Cell Rep. 2014;33(8):1217–1225. | ||
Komis G, Illés P, Beck M, Šamaj J. Microtubules and mitogen-activated protein kinase signalling. Curr Opin Plant Biol. 2011;14(6): 650–657. | ||
Yang Y, Bian G, Lu Q. Neuroprotection and neurotrophism effects of liquiritin on primary cultured hippocampal cells. J Chin Mater Med. 2008;33(8):931–935. | ||
Wang Z, Zhang X, Wang H, Qi L, Lou Y. Neuroprotective effects of icaritin against beta amyloid-induced neurotoxicity in primary cultured rat neuronal cells via estrogen-dependent pathway. Neuroscience. 2007;145(3):911–922. | ||
Moghbelinejad S, Nassiri-Asl M, Farivar TN, et al. Rutin activates the MAPK pathway and BDNF gene expression on beta-amyloid induced neurotoxicity in rats. Toxicol Lett. 2014;224(1):108–113. | ||
Lin L-F, Chiu S-P, Wu M-J, Chen P-Y, Yen J-H. Luteolin induces microRNA-132 expression and modulates neurite outgrowth in PC12 cells. PloS One. 2012;7(8):e43304. | ||
Kano Y, Horie N, Doi S, et al. Artepillin C derived from propolis induces neurite outgrowth in PC12m3 cells via ERK and p38 MAPK pathways. Neurochem Res. 2008;33(9):1795–1803. | ||
Sagara Y, Vanhnasy J, Maher P. Induction of PC12 cell differentiation by flavonoids is dependent upon extracellular signal-regulated kinase activation. J Neurochem. 2004;90(5):1144–1155. | ||
Zhang Z, Cai L, Zhou X, et al. Methyl 3, 4-dihydroxybenzoate promote rat cortical neurons survival and neurite outgrowth through the adenosine A2a receptor/PI3K/Akt signaling pathway. NeuroReport. 2015;26(6):367–373. | ||
Lonze BE, Ginty DD. Function and regulation of CREB family transcription factors in the nervous system. Neuron. 2002;35(4):605–623. | ||
Boss V, Roback JD, Young AN, et al. Nerve growth factor, but not epidermal growth factor, increases Fra-2 expression and alters Fra-2/JunD binding to AP-1 and CREB binding elements in pheochromocytoma (PC12) cells. J Neurosci. 2001;21(1):18–26. | ||
Mantamadiotis T, Lemberger T, Bleckmann SC, et al. Disruption of CREB function in brain leads to neurodegeneration. Nat Genet. 2002;31(1):47–54. | ||
Furukawa Y, Watanabe S, Okuyama S, Nakajima M. Neurotrophic effect of Citrus auraptene: neuritogenic activity in PC12 cells. Int J Mol Sci. 2012;13(5):5338–5347. | ||
Nagase H, Omae N, Omori A, et al. Nobiletin and its related flavonoids with CRE-dependent transcription-stimulating and neuritegenic activities. Biochem Biophys Res Commun. 2005;337(4):1330–1336. | ||
Hwang S-L, Lin J-A, Shih P-H, Yeh C-T, Yen G-C. Pro-cellular survival and neuroprotection of citrus flavonoid: the actions of hesperetin in PC12 cells. Food Funct. 2012;3(10):1082–1090. | ||
Li Q, Zhao H, Zhang Z, et al. Long-term administration of green tea catechins prevents age-related spatial learning and memory decline in C57BL/6 J mice by regulating hippocampal cyclic amp-response element binding protein signaling cascade. Neuroscience. 2009;159(4):1208–1215. | ||
Oh SB, Park HR, Jang YJ, Choi SY, Son TG, Lee J. Baicalein attenuates impaired hippocampal neurogenesis and the neurocognitive deficits induced by γ-ray radiation. Br J Pharmacol. 2013;168(2):421–431. | ||
Lee B, Sur B, Shim I, Lee H, Hahm D-H. Baicalin improves chronic corticosterone-induced learning and memory deficits via the enhancement of impaired hippocampal brain-derived neurotrophic factor and cAMP response element-binding protein expression in the rat. J Nat Med. 2014;68(1):132–143. | ||
Zhao L, Wang J-L, Liu R, Li X-X, Li J-F, Zhang L. Neuroprotective, anti-amyloidogenic and neurotrophic effects of apigenin in an Alzheimer’s disease mouse model. Molecules. 2013;18(8):9949–9965. | ||
Liu R, Li J-Z, Song J-K, et al. Pinocembrin improves cognition and protects the neurovascular unit in Alzheimer related deficits. Neurobiol Aging. 2014;35(6):1275–1285. | ||
Jin CH, Shin EJ, Park JB, et al. Fustin flavonoid attenuates β-amyloid (1–42)-induced learning impairment. J Neurosci Res. 2009;87(16):3658–3670. | ||
Basli A, Soulet S, Chaher N, et al. Wine polyphenols: potential agents in neuroprotection. Oxid Med Cell Longev. 2012;2012. | ||
Saso L, Firuzi O. Pharmacological applications of antioxidants: lights and shadows. Curr Drug Targets. 2014;15(13):1177–1199. | ||
Amara F, Berbenni M, Fragni M, et al. Neuroprotection by cocktails of dietary antioxidants under conditions of nerve growth factor deprivation. Oxid Med Cell Longev. 2015;2015:217258. | ||
Panickar KS, Anderson RA. Effect of polyphenols on oxidative stress and mitochondrial dysfunction in neuronal death and brain edema in cerebral ischemia. Int J Mol Sci. 2011;12(11):8181–8207. | ||
Razzaghi-Asl N, Garrido J, Khazraei H, Borges F, Firuzi O. Antioxidant properties of hydroxycinnamic acids: A review of structure-activity relationships. Curr Med Chem. 2013;20(36):4436–4450. | ||
Weinreb O, Amit T, Youdim MB. A novel approach of proteomics and transcriptomics to study the mechanism of action of the antioxidant–iron chelator green tea polyphenol (-)-epigallocatechin-3-gallate. Free Radic Biol Med. 2007;43(4):546–556. | ||
Harikumar KB, Aggarwal BB. Resveratrol: a multitargeted agent for age-associated chronic diseases. Cell Cycle. 2008;7(8):1020–1035. | ||
Campos-Esparza MR, Sanchez-Gomez MV, Matute C. Molecular mechanisms of neuroprotection by two natural antioxidant polyphenols. Cell Calcium. 2009;45(4):358–368. | ||
Selkoe DJ. Clearing the brain’s amyloid cobwebs. Neuron. 2001;32(2):177–180. | ||
Villemagne VL, Burnham S, Bourgeat P, et al. Amyloid β deposition, neurodegeneration, and cognitive decline in sporadic Alzheimer’s disease: a prospective cohort study. Lancet Neurol. 2013;12(4):357–367. | ||
Ushikubo H, Watanabe S, Tanimoto Y, et al. 3,3′,4′,5, 5′-Pentahydroxyflavone is a potent inhibitor of amyloid β fibril formation. Neurosci Lett. 2012;513(1):51–56. | ||
Hong S-Y, Jeong W-S, Jun M. Protective effects of the key compounds isolated from Corni fructus against β-amyloid-induced neurotoxicity in PC12 cells. Molecules. 2012;17(9):10831–10845. | ||
Heo HJ, Lee CY. Epicatechin and catechin in cocoa inhibit amyloid β protein induced apoptosis. J Agric Food Chem. 2005;53(5):1445–1448. | ||
Meng P, Yoshida H, Tanji K, et al. Carnosic acid attenuates apoptosis induced by amyloid-1–42 or 1–43 in SH-SY5Y human neuroblastoma cells. Neurosci Res. 2015;94:1–9. | ||
Mu X, He G, Cheng Y, Li X, Xu B, Du G. Baicalein exerts neuroprotective effects in 6-hydroxydopamine-induced experimental parkinsonism in vivo and in vitro. Pharmacol Biochem Behav. 2009;92(4):642–648. | ||
Tavakkoli M, Miri R, Jassbi AR, et al. Carthamus, Salvia and Stachys species protect neuronal cells against oxidative stress-induced apoptosis. Pharm Biol. 2014;52(12):1550–1557. | ||
Sajad M, Zargan J, Zargar MA, et al. Quercetin prevents protein nitration and glycolytic block of proliferation in hydrogen peroxide insulted cultured neuronal precursor cells (NPCs): Implications on CNS regeneration. Neurotoxicology. 2013;36:24–33. | ||
Liu B, Zhang H, Xu C, et al. Neuroprotective effects of icariin on corticosterone-induced apoptosis in primary cultured rat hippocampal neurons. Brain Res. 2011;1375:59–67. | ||
Kensler TW, Wakabayashi N, Biswal S. Cell survival responses to environmental stresses via the Keap1-Nrf2-ARE pathway. Annu Rev Pharmacol Toxicol. 2007;47:89–116. | ||
Chung SW, Liu X, Macias AA, Baron RM, Perrella MA. Heme oxygenase-1–derived carbon monoxide enhances the host defense response to microbial sepsis in mice. J Clin Invest. 2008;118(1):239. | ||
Zhang DD. Mechanistic studies of the Nrf2-Keap1 signaling pathway. Drug Metab Rev. 2006;38(4):769–789. | ||
Zhang M, An C, Gao Y, Leak RK, Chen J, Zhang F. Emerging roles of Nrf2 and phase II antioxidant enzymes in neuroprotection. Prog Neurobiol. 2013;100:30–47. | ||
Cho H-Y, Jedlicka AE, Reddy SP, et al. Role of NRF2 in protection against hyperoxic lung injury in mice. Am J Respir Cell Mol Biol. 2002;26(2):175–182. | ||
Romeo L, Intrieri M, D’Agata V, et al. The major green tea polyphenol, (−)-epigallocatechin-3-gallate, induces heme oxygenase in rat neurons and acts as an effective neuroprotective agent against oxidative stress. J Am Coll Nutr. 2009;28(sup4):492S–499S. | ||
Kosaka K, Mimura J, Itoh K, et al. Role of Nrf2 and p62/ZIP in the neurite outgrowth by carnosic acid in PC12h cells. J Biochem. 2010;147(1):73–81. | ||
Russell JM. Sodium-potassium-chloride cotransport. Physiol Rev. 2000;80(1):211–276. | ||
Nakajima K-I, Miyazaki H, Niisato N, Marunaka Y. Essential role of NKCC1 in NGF-induced neurite outgrowth. Biochem Biophys Res Commun. 2007;359(3):604–610. | ||
Nakajima K-I, Niisato N, Marunaka Y. Quercetin stimulates NGF-induced neurite outgrowth in PC12 cells via activation of Na+/K+/2Cl-cotransporter. Cell Physiol Biochem. 2011;28(1):147–156. | ||
Diógenes MJ, Fernandes CC, Sebastiao AM, Ribeiro JA. Activation of adenosine A2A receptor facilitates brain-derived neurotrophic factor modulation of synaptic transmission in hippocampal slices. J Neurosci. 2004;24(12):2905–2913. | ||
Tebano M, Martire A, Potenza R, et al. Adenosine A2A receptors are required for normal BDNF levels and BDNF-induced potentiation of synaptic transmission in the mouse hippocampus. J Neurochem. 2008;104(1):279–286. | ||
Wiese S, Jablonka S, Holtmann B, et al. Adenosine receptor A2A-R contributes to motoneuron survival by transactivating the tyrosine kinase receptor TrkB. Proc Natl Acad Sci U S A. 2007;104(43):17210–17215. | ||
Cheng H-C, Shih H-M, Chern Y. Essential role of cAMP-response element-binding protein activation by A2A adenosine receptors in rescuing the nerve growth factor-induced neurite outgrowth impaired by blockage of the MAPK cascade. J Biol Chem. 2002;277(37):33930–33942. | ||
Flajolet M, Wang Z, Futter M, et al. FGF acts as a co-transmitter through adenosine A2A receptor to regulate synaptic plasticity. Nat Neurosci. 2008;11(12):1402–1409. | ||
Palazzolo G, Horvath P, Zenobi-Wong M. The flavonoid isoquercitrin promotes neurite elongation by reducing RhoA activity. PLoS One. 2012;7(11):e49979. | ||
Bora-Tatar G, Erdem-Yurter H. Investigations of curcumin and resveratrol on neurite outgrowth: perspectives on spinal muscular atrophy. Biomed Res Int. 2014;2014:709108. | ||
Dasgupta B, Milbrandt J. Resveratrol stimulates AMP kinase activity in neurons. Proc Natl Acad Sci U S A. 2007;104(17):7217–7222. | ||
Li M, Tsang KS, Choi ST, Li K, Shaw PC, Lau KF. Neuronal differentiation of C17. 2 neural stem cells induced by a natural flavonoid, baicalin. Chembiochem. 2011;12(3):449–456. | ||
Woo KW, Kwon OW, Kim SY, et al. Phenolic derivatives from the rhizomes of Dioscorea nipponica and their anti-neuroinflammatory and neuroprotective activities. J Ethnopharmacol. 2014;155(2):1164–1170. | ||
Guan S, Zhang X-L, Ge D, Liu T-Q, Ma X-H, Cui Z-F. Protocatechuic acid promotes the neuronal differentiation and facilitates survival of phenotypes differentiated from cultured neural stem and progenitor cells. Eur J Pharmacol. 2011;670(2):471–478. | ||
Lin C-Y, Ni C-C, Yin M-C, Lii C-K. Flavonoids protect pancreatic beta-cells from cytokines mediated apoptosis through the activation of PI3-kinase pathway. Cytokine. 2012;59(1):65–71. | ||
El Omri A, Han J, Yamada P, Kawada K, Abdrabbah MB, Isoda H. Rosmarinus officinalis polyphenols activate cholinergic activities in PC12 cells through phosphorylation of ERK1/2. J Ethnopharmacol. 2010;131(2):451–458. | ||
Nishina A, Kimura H, Tsukagoshi H, et al. Neurite outgrowth of PC12 cells by 4′-O-β-d-glucopyranosyl-3′,4-dimethoxychalcone from Brassica rapa L. ‘hidabeni’ was enhanced by pretreatment with p38MAPK inhibitor. Neurochem Res. 2013;38(11):2397–2407. | ||
Zeni ALB, Zomkowski ADE, Maraschin M, Rodrigues ALS, Tasca CI. Involvement of PKA, CaMKII, PKC, MAPK/ERK and PI3K in the acute antidepressant-like effect of ferulic acid in the tail suspension test. Pharmacol Biochem Behav. 2012;103(2):181–186. | ||
Maher P, Akaishi T, Abe K. Flavonoid fisetin promotes ERK-dependent long-term potentiation and enhances memory. Proc Natl Acad Sci U S A. 2006;103(44):16568–16573. | ||
Mendonca LM, da Silva Machado C, Teixeira CCC, de Freitas LAP, Bianchi MdLP, Antunes LMG. Curcumin reduces cisplatin-induced neurotoxicity in NGF-differentiated PC12 cells. Neurotoxicology. 2013;34:205–211. | ||
Levites Y, Amit T, Mandel S, Youdim MB. Neuroprotection and neurorescue against Aβ toxicity and PKC-dependent release of nonamyloidogenic soluble precursor protein by green tea polyphenol (−)-epigallocatechin-3-gallate. FASEB J. 2003;17(8):952–954. | ||
Levites Y, Amit T, Youdim MB, Mandel S. Involvement of protein kinase C activation and cell survival/cell cycle genes in green tea polyphenol (−)-epigallocatechin 3-gallate neuroprotective action. J Biol Chem. 2002;277(34):30574–30580. |
 © 2015 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2015 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.