Back to Journals » International Journal of Nanomedicine » Volume 13
Microfluidization trends in the development of nanodelivery systems and applications in chronic disease treatments
Authors Ganesan P , Karthivashan G, Park S, Kim J , Choi DK
Received 23 June 2018
Accepted for publication 14 August 2018
Published 9 October 2018 Volume 2018:13 Pages 6109—6121
DOI https://doi.org/10.2147/IJN.S178077
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 3
Editor who approved publication: Dr Thomas Webster
Palanivel Ganesan,1 Govindarajan Karthivashan,2 Shin Young Park,2 Joonsoo Kim,2 Dong-Kug Choi1,2
1Department of Integrated Bio Science and Biotechnology, College of Biomedical and Health Science, Nanotechnology Research Center, Konkuk University, Chungju 27478, Republic of Korea; 2Department of Applied Life Sciences, Graduate School of Konkuk University, Research Institute of Inflammatory Diseases, Chungju 27478, Republic of Korea
Abstract: Plant bioactive compounds are known for their extensive health benefits and therefore have been used for generations in traditional and modern medicine to improve the health of humans. Processing and storage instabilities of the plant bioactive compounds, however, limit their bioavailability and bioaccessibility and thus lead researchers in search of novel encapsulation systems with enhanced stability, bioavailability, and bioaccessibility of encapsulated plant bioactive compounds. Recently many varieties of encapsulation methods have been used; among them, microfluidization has emerged as a novel method used for the development of delivery systems including solid lipid nanocarriers, nanoemulsions, liposomes, and so on with enhanced stability and bioavailability of encapsulated plant bioactive compounds. Therefore, the nanodelivery systems developed using microfluidization techniques have received much attention from the medical industry for their ability to facilitate controlled delivery with enhanced health benefits in the treatment of various chronic diseases. Many researchers have focused on plant bioactive compound-based delivery systems using microfluidization to enhance the bioavailability and bioaccessibility of encapsulated bioactive compounds in the treatment of various chronic diseases. This review focuses on various nanodelivery systems developed using microfluidization techniques and applications in various chronic disease treatments.
Keywords: bioavailability, solid lipid nanoparticles, plant bioactive compounds, nanoemulsions
Introduction
Currently there is an increasing demand for natural plant bioactive compounds in the treatment of various chronic diseases like cancer and diabetes, and neurological and other age-related chronic diseases owing to lower side effects.1–5 This demand has led to inter-collaboration across multiple research areas including medicinal, functional food, pharmaceuticals, and nutraceuticals. Owing to various process parameters during extraction, poor stability, oral environmental conditions, inaccessibility, and bioavailability, the application and development of various plant bioactive compound-based treatments for chronic diseases have been limited.6–12 Therefore, an innovative approach that can protect bioactivity during oral treatments as well as provide enhanced bioavailability of those plant bioactive compounds for the successive treatment of chronic diseases is necessary.
Nanoencapsulation has been an efficient method of encapsulation of plant bioactive compounds to enhance the protection, stability, and bioavailability of plant bioactive compounds.4,13,14 Various nanodelivery systems including solid lipid nanocarriers, nano-structured lipid carriers, nanoemulsions, and nanoliposomes have been efficiently used in the development of encapsulation of the plant bioactive compounds with their own merits and demerits for the treatment of chronic diseases.2–5,13,15–17 Several techniques like emulsification, supercritical fluidization, high-pressure homogenization, and ultrasonication are most commonly used in the development of those nanodelivery systems.18–21 However, the stability of these nanodelivery systems when loaded with plant bioactive compounds was not acceptable. This in turn affects the bioavailability and bioaccessibility of those plant bioactive compounds developed through nanodelivery systems using conventional techniques. Microfluidization techniques have been recently applied in the development of nanodelivery systems with enhanced stability and bioavailability of plant-based bioactive compounds.22–24
Novel stable nanodelivery systems have been developed with microfluidization techniques with enhanced stability of encapsulated compounds.25 The major advantages of the techniques include higher stability with a smaller particle size, higher scale production of nanodelivery systems with higher reproducibility, no aggregation of developed nanodelivery systems along with lower fusibility, and higher encapsulation efficacy with lower usages of other solvents.26–30 Further microfluidized nanodelivery systems with reduced particle size and higher bioaccessibility31,32 can be effectively achieved using food grade biopolymer along with non-toxic and highly biodegradable carriers, which can broaden its application in nutraceutical and functional food development using plant-based bioactive compounds for chronic disease treatments. Recently, many different types of food grade polymer carriers including polysaccharides and proteins have been effectively used in the production of mini emulsion production for its uniformity in production and higher reproducibility using the microfluidization process. To the best of our knowledge, no review paper on the application of microfluidization in nanodelivery systems development for the effective delivery of plant bioactive compounds and its application in chronic disease treatment has been published.
Microfluidization
Plant bioactive compound-based nanodelivery systems development using microfluidization is an emerging technique to enhance the stability and bioavailability of the incorporated plant bioactive compounds.26,33 Development of stable nanodelivery systems using plant bioactive compounds has been a research area of emerging delivery systems in the medical field, thereby facilitating oral delivery without much loss in activity.33–35 Microfluidization mechanism is very essential to understand the development of stable nanodelivery systems, thereby enhancing the production of those systems with broader applications through the interdisciplinary approach of nutraceuticals and medicine.36,37 The microfluidization process is a type of high energy process which works on the dynamics of the specially designed microchannels. The generated turbulence and momentum makes the lipid carrier overcome its barrier. The pump driven by the compressed air mixe the lipids and active compounds at very high velocities in the designed microchannels, thereby forming stable delivery systems of a nano size.34,35 In the development of nanodelivery systems, two types of microfluidization are currently practiced. One is two-step, single-channel microfluidization and the other is single-step, dual-channel microfluidization, with their own advantages and disadvantages. In the case of the nanoemulsion-based delivery system, microfluidization-based nanoemulsion developed using a two-step single channel has many disadvantages like additional energy and more expensive wastage of lipid and oil for making coarse emulsion initially to be fed into the microfluidizer.38 However, single-step dual-channel microfluidization overcomes the above disadvantages and thereby prepares the stable nanoemulsion with higher loading abilities, thereby having broader application in the medical, food, and nutraceutical sectors. Microfluidizing types are shown in Figure 1.
  | Figure 1 Microfluidization process for the preparation of nanodelivery systems. |
Microfluidization has several advantages over the development of nanodelivery systems. For example, microfluidization mechanism eases the development of the stable nanoemulsion with the particle size <160 nm.39 The mechanism involves forcing the coarse emulsion through microchannels to the particular area by pneumatically powered pump by pressurizing compressed air up to about 150 MPa which results in nanoemulsion,35,40 and the different passes lead to different sizes. It is also an easy-to-use and effective method for the development of other stable nanodelivery systems. Those developed nanodelivery systems show enhanced stability of the incorporated bioactive compounds, uniformity, and greater reproducibility, and food grade delivery systems can be effectively developed for greater application and development of functional food.28,34,41–44 Recently, weighted orange oil terpenes used with different food grade polymers like modified gum arabic and modified starch to prepare nanoemulsion with the particle size of about 77 nm showed enhanced stability for the clear beverage development using microfluidization.45 In addition, it has greater advantages in the medical field for its oral delivery with greater bioavailability and the sustained release of incorporated bioactive compounds. For example, orange oil nanoemulsion developed using ester gum incorporated in oil phase and Quillaja saponins in continuous phase has higher stability of 2 weeks with the particle size of about 69 nm.46
Microfluidization-based nanoencapsulation of plant bioactive compounds
Plant bioactive compounds play a critical role in the prevention and treatment of various chronic diseases including cancers, type 2 diabetes, hypertension, obesity, and neurological diseases. Traditionally, food-based medicine or phytomedicine is followed generally throughout the world, and some bioactive compounds are documented in several countries.6,10,11,47,48 In general practice, plant bioactive compounds are orally consumed in the form of either extracts or nutraceuticals, which benefit mankind by inhibiting or slowing down the occurrence of diseases by anticancer, anti-inflammation, antidiabetic, antiobesity, and antioxidation mechanisms. However, during oral consumption of plant bioactive compounds, their activity and mechanisms are fully achieved due to numerous factors in the gastrointestinal tracts. In order to overcome those factors, encapsulation of those bioactive compounds is a very efficient alternative, thereby enhancing activity and disease prevention. Several researchers have recently studied the development of various nanoencapsulation systems for higher encapsulation and greater efficacy in nanoencapsulated bioactive compounds using microfluidization. Nanoencapsulated plant bioactive compounds using microfluidization have greater stability of the developed systems and can also be repeatable in bulk production. In addition, a microfluidized delivery system can be produced in a uniform size, and there is less breakage and release of encapsulated bioactive compounds in comparison with other systems.28,29,49–51 Furthermore, lower usage of the organic solvents is required, and a food grade carrier can be used to develop highly biodegradable, lower toxic delivery systems. Therefore, the current work focused on providing a detailed review of the developed nanodelivery systems for plant bioactive compounds using microfluidization and its application in various chronic disease treatments.
Development of microfluidization-based nanodelivery systems
Nanodelivery systems play a key role in the delivery of plant bioactive compounds in enhanced oral delivery mostly due to the smaller size and higher surface exposure of those bioactive compounds. However, based on the size and preparation of those delivery systems using different equipment, different characteristic effects were shown in the functional properties of developed nanodelivery systems.6,10,11,47,48 In a recent study, solid lipid nanoparticles (SLNs) developed using microfluidization showed a smaller particle size of about 36–136 nm along with enhanced stability when generated using microfluidizing techniques.52 Similarly, pickering nanoemulsions with very high stability were also developed using microfluidization by preventing droplet coalescence with surface coverage and achieving a higher bridging effect between the droplet. Based on the abovementioned few studies, the microfluidizing effect showed higher stability in the development of nanodelivery systems.53 Therefore, it can enhance the bioavailability of those encapsulated compounds and can be helpful in the remedies for various chronic diseases. Microfluidized nanodelivery systems are shown in Figure 2. Briefly, some of the nanodelivery systems developed using microfluidization and their properties are discussed in this section.
  | Figure 2 Applications of microfluidization process in the development of various nanodelivery systems. |
SLNs
SLNs are among the lipid nanocarriers developed for transporting the hydrophobic bioactive compounds with higher loading capacity along with the enhanced stability of the loaded bioactive compounds.54–60 SLNs showed higher loading efficacy and bioavailability of various plant bioactive compounds like curcumin, resveratrol, quercetin, and catechin. These plant-derived bioactive compound–loaded SLNs showed higher potential in the prevention and cure of various chronic diseases.61–66 Curcumin-loaded SLNs with some modification have been recently developed, showing a higher anticancer effect with enhanced loading efficacy. SLNs have been developed using different methods including high-speed homogenization, spray drying, cold homogenization, hot homogenization, ultrasonication, double emulsion, and supercritical technology. Usage of all those techniques includes various advantages and disadvantages in the development of SLNs.67–69 The major disadvantages of the above systems in the development of SLNs include partitioning of the lipids, lower stability, and higher usage of the organic solvents, which limit its development in novel SLN development for chronic disease treatment. Recently, microfluidization was used for the development of SLNs with very high loading efficacy and higher bioavailability of those encapsulated compounds in the SLNs.70–75 Microalgae oil contains a higher amount of docosahexaenoic acid (DHA; 22:6), which is among the essential fatty acids required in healthy brain development and for various body functions. Consumption of the microalgae oil rich in DHA showed various beneficial activities in humans, including anticancer and antineurological properties and enhanced heart function. However, due to lesser stability and bioactivity loss during oral consumptions, alternatively SLNs were developed using microalgae oil. Higher encapsulation efficacy and lower particle size were highly achieved through the microfluidization techniques in the microalgae-loaded SLNs. Microalgae oil-rich DHA-loaded SLNs developed with the particle size of about 300–350 nm with uniform distribution of oil in the SLNs could be potentially applicable in functional food development76 with prevention or treatment of chronic diseases. Similarly, transparent and stable SLNs can be developed using microfluidization techniques and could be highly applicable in the development of various plant bioactive compound–loaded SLNs.
Nanoemulsions
Nanoemulsions are one among the nanodelivery systems that play a key role in the delivery of plant bioactive compounds like curcumin, resveratrol, and quercetin for its enhanced application in the prevention and treatment of chronic diseases.77–80 The advantages of incorporating bioactive compounds in nanoemulsion are smaller particle size, higher stability, and transparent emulsion, where the scattering effect of the light is very low compared with that of normal emulsions. The coalescence and flocculation effect of the plant bioactive compound–loaded nanoemulsion was much lower due to the small particle size, and thus the attractive forces between the droplets will be greatly reduced. Nanoemulsion can be effectively prepared using various methods like low energy methods including spontaneous formation by mixing or phase inversion and a high energy method including high-pressure homogenizer or sonication. Every method has its own advantages and disadvantages in the preparation of the nanoemulsion-loaded plant bioactive compounds.81–85 The most common disadvantages of these methods are usage of synthetic solvents, emulsifiers or oils, bioavailability and potential toxicity of the solvents, and stability during oral delivery. To overcome the above disadvantages, a microfluidizer has recently been used in the development of the nanoemulsion with a smaller particle size, higher stability, and higher encapsulation efficacy of incorporated bioactive compounds.35,38,45,86–89 The application of microfluidized nanoemulsions is shown in Figure 3. Curcumin is among the top bioactive compounds extensively used in traditional medicine for generations owing to its greater potential effects including anticancer, antihypertension, antidiabetic, and antineuroinflammatory effects. Owing to the lower solubility and lesser bioavailability of those compounds, many studies on the development of curcumin-loaded nanoemulsion development have found higher beneficial effects in the treatment of various chronic diseases. In order to enhance its efficacy, various approaches have used curcumin-loaded nanoemulsion for the enhanced bioavailability of the curcumin. Microfluidized nanoemulsion can be obtained with a particle size of about 275 nm90 with higher stability, which can scatter light weakly, and it could be highly applicable in food grade bioactive compounds or nutraceutical development.
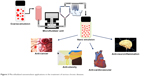  | Figure 3 Microfluidized nanoemulsion applications in the treatment of various chronic diseases. |
Nanoliposomes
Nanoliposomes are yet another delivery vehicle made up of phospholipid bilayers, which contain aqueous compartments that can encapsulate various plant bioactive compounds for the controlled and sustained delivery of the encapsulated active compounds. Owing to the lower particle size and controlled delivery, it has a wide range of applications in medicine, pharmaceuticals, nutraceuticals, and functional foods.91–95 Various methods are involved in the preparation and development of nanoliposomes for enhanced stability like ultrasonic injection, ethanol injection, and homogenizer methods.96–102 The above methods are able to produce plant bioactive compound–loaded nanoliposomes, but the encapsulation efficacy and bioavailability of those encapsulated bioactive compounds vary with the methods.103–108 Recently, the microfluidization method has been very effectively used for plant bioactive compound–loaded nanoliposome development, overcoming the above disadvantages in the preparation method and enhancing the sustained release of those bioactive compounds. Tea polyphenol–loaded nanoliposome was effectively prepared using the microfluidization method with a particle size of about 66 nm along with enhanced stability of those developed nanoliposomes.107 The same research group also developed nanoliposome with different production technologies including a high-pressure homogenizer and ultrasonication methods with a particle size >100 nm. Higher stability and sustained release of the tea polyphenol–loaded nanoliposomes were observed in the microfluidized nanoliposomes, and it could be applicable in the development of food grade nutraceuticals and medicine. In a recent study, black carrot extract rich in anthocyanin-loaded nanoliposome was also developed with a particle size of <50 nm, and it could be helpful in the development of nutraceuticals.109 Similarly, vitamin C–loaded nanophytosomes were also developed with a lower particle size and higher stability, and sustained release of vitamin C was achieved through the microfluidization method.109 The particle size of the nanophytosomes developed by microfluidized method was about 92 nm, which was much lower than the traditional method.110 The skin permeation study of vitamin C–loaded nanoliposomes developed using the microfluidization method was very high in comparison with liposomes and vitamin C during 24 hours. Similarly, curcumin-loaded nanoliposomes were also developed using microfluidization techniques with a lower particle size of about 68 nm and higher stability than the liposomes. The stability of the curcumin-loaded nanoliposomes was also enhanced against alkaline pH and metal ions. Refrigerated storage temperature also enhances the stability of microfluidized nanoliposomes along with the sustained release of the encapsulated curcumin.111 Overall, the microfluidization method could be effectively used in the preparation of nanoliposome loaded with plant bioactive compounds for its enhanced application in the nutraceutical, functional food, pharmaceutical, and medicine industries.
Nanosuspensions
Microfluidized nanosuspensions are among the emerging techniques in the development of low soluble bioactive compound-based nanosuspension. Microfluidization helps to increase the bioavailability of those compounds by reducing the particle size and thereby increasing the surface area. Microfluidization-based nanosuspensions have several advantages over the traditional suspensions including lower particle size, higher stability, a simple process, and higher dissolution rate.112–118 Several drug-based nanosuspensions were developed with higher efficiency in the bioavailability of those drugs in various chronic disease treatments. Recently, budesonide nanosuspension was developed using the microfluidization method with a smaller particle size of about 122 nm. The pulmonary delivery and distribution of the drug in the lung were higher than that of the normal-sized particles.119 Similarly, another drug named ritonavir suspension was developed using the microfluidization process with a uniform lower particle size and higher efficacy of about 3.5-fold. In another study, the plant bioactive compound gambogenic acid nanosuspensions were developed using the solvent precipitation method with the particle size of about 183 nm with higher anticancer efficacy than gambogenic acid.120 However, microfluidization-based nanosuspension will be an alternative approach in the delivery of many plant bioactive compound-based nanosuspensions with higher efficacy in the bioavailability of those compounds against various chronic diseases.
Poly(lactic-co-glycolic acid) (PLGA)-based nanoparticles
PLGA-based nanoparticles are highly used in the delivery of various drugs and bioactive compounds as carriers for their sustained release and target-specific delivery. PLGA was widely accepted by the FDA owing to the lower toxicity; after hydrolysis, it can produce monomers without any harmful effects.121–127 Various methods were used in the preparation of PLGA-based nanoparticles including emulsification, solvent precipitation, nanoprecipitation, and interfacial polymerization methods. Different methods have advantages and disadvantages in PLGA-based nanoparticle development and drug loading efficacy while the major limitations in most methods involve no uniformity and large-scale production limitations. This leads researchers to search for low energy, higher uniform bulk production, and microfluidization overcomes the limitation in the development of PLGA-based nanoparticles in the entrapment of various drugs128–130 and plant bioactive compounds. Recently, efavirenz-loaded PLGA nanoparticles were also developed using the microfluidization method with a particle size of about 73 nm along with the higher permeability of about 1.3-fold higher than the normal drug, thus showing a higher anti-HIV effect. It makes the researchers in use of plant bioactive compound-based PLGA nanoparticles for the efficient delivery using microfluidization methods.131 Recently, curcumin-loaded PLGA nanoparticles were developed using microfluidization methods with a particle size of about 30–70 nm, controlled delivery, and lower degradation of the curcumin. Higher anticancer efficacy of the developed nanoparticle was also observed against cancer cell lines.
Role of microfluidized nanodelivery systems loaded with plant bioactive compounds in chronic diseases
Microfluidization techniques help to produce various nanodelivery systems including SLNs, nanoemulsions, nanoliposomes, and PLGA nanoparticles loaded with drugs or plant bioactive compounds with enhanced stability and bioavailability of those loaded compounds.132–139 Various in vitro or in vivo studies have confirmed that these microfluidized nanodelivery systems loaded with bioactive compounds showed enhanced protection in the treatment of chronic diseases including cancer, obesity, neurological diseases, and diabetes. A few of those studies are discussed in the following sections.
Anticancer effect
Cancer is among the major chronic diseases that cause major human death throughout the world, and scientists work diligently to produce various drugs for its treatment.140–142 General medical practice includes radiation and chemotherapy, which lead to various other complications leading the patients in much stress.143–145 Recently, nanomedicine has played a vital role in the treatment of cancer overcoming several side effects of traditional medicines, although nanodelivery systems loaded with drugs or plant-based bioactive compounds face critical challenges in the delivery of the bioactive compounds to the target sites and through the delivery systems.146–149 Microfluidization techniques try to solve some disadvantages during the production of those nanodelivery systems developed using anticancer drugs or plant-based bioactive compounds. Curcumin, resveratrol, quercetin, and catechin are the most active compounds showing extensive benefits in anticancer activities. Recently curcumin-loaded palm oil–based nanoemulsion was developed with a smaller particle size, and it could be used in future food-based nanomedicine against various cancer treatments. Similarly, curcumin nanoliposome also produced using curcumin as an active compound by using microfluidization techniques with the particle size of about 68 nm showed sustained release of the curcumin, which could be useful for chronic diseases including cancer.111 Recently, a plant-based bioactive compound known as camptothecin, a compound from Chinese tree bark, was used in the development of target-specific nanolipospheres owing to the potential toxicity of the active compound to the natural cells. Researchers developed target-specific nanolipospheres of <20 nm size by using those active compounds,150 and further research is necessary in terms of their toxicity effects on normal cells during treatments.
Antiobesity effects
Obesity is yet another major cause linked to various chronic diseases including hypertension, diabetes, and cardiovascular diseases. Consuming lipid-rich foods and sedentary lifestyle link to obesity, and it is a big burden to the well-being of mankind.151,152 Treating obesity with plant-based bioactive compounds in the form of food, nutraceuticals, or drugs is practiced.9,10,153–158 However, the bioavailability of those compounds through oral delivery faces many challenges. Recently, nanomedicine develops antiobesity bioactive compound–loaded nanodelivery systems, which has enhanced the delivery potential over traditional medicines. Recently, microfluidized nanomedicines developed using antiobesity bioactive compounds have enhanced the stability of nanodelivery systems. Capsaicin is among the major plant bioactive compounds extensively used in the treatment of obesity. Owing to higher pungency, odor, and low solubility, its usage and its bioavailability of the bioactive compounds in the treatment of obesity are limited.159–162 Recently, microfluidization techniques have been extensively used in the development of food grade nanodelivery systems or nanomedicines for the enhanced bioavailability of the capsaicin and its related compounds.163 Recently, oleoresin capsicum–loaded nanoemulsion was developed using microfluidization techniques with the particle size of about 50 nm showing enhanced antiobesity effects in a high-fat-induced rat. Similarly conjugated linoleic acid–loaded nanoemulsion was developed using microfluidizing techniques, which also showed an enhanced antiobesity effect.164 Higher efficacy of the antiobesity plant-based bioactive compounds like zeaxanthin was also studied using microfluidization techniques by reducing its particle size. Further development of various nanodelivery systems using those antiobesity bioactive compounds is necessary to enhance its application.
Cardiovascular effect
Plant-derived bioactive compounds showed higher potential in the prevention of cardiovascular diseases.10,165,166 Consumption of the plant bioactive compound–rich food showed higher prevention either by the prevention of the oxidation of lipoprotein or by the prevention of atherosclerotic lesion development. A diet rich in plant bioactive compounds showed an active preventive role in various mechanisms against the atherosclerotic effect.167–171 However, the bioavailability of those bioactive compounds against atherosclerosis is very low and development of novel nanodelivery systems is currently playing a key role. Recently various nanodelivery systems were developed using plant bioactive compounds like nanoemulsion or nanoparticles for their effective preventive role against various chronic diseases including cardiovascular effects.172–175 Recently, Baicalein-loaded nanoemulsion was developed with a particle size of about 91 nm, showing excellent bioavailability of these compounds in rats,176 and it could be possibly used in anticardiovascular studies. Another potential anticardiovascular compound β-carotene was studied using the microfluidization technique, and are able to produce food grade nanoemulsion along with higher stability with a particle size of <200 nm.176 However, the enhanced stability of those active compound loaded nanodelivery systems developed using microfluidization techniques are still limited in their protective role in the anticardiovascular effects.
Antineuroinflammation effect
Plant bioactive compounds like curcumin, resveratrol, and piperine play a significant role in antineuroinflammation and neuroprotection activity; they thereby can prevent various neuroinflammatory diseases including Parkinson’s, Alzheimer’s, and other brain diseases.177–183 However, the delivery of those bioactive compounds is playing a key role in the prevention of the above neuroinflammatory diseases. Novel nanodelivery systems like SLNs, nanoemulsions, and nanoliposomes are successful in the delivery of various plant-based bioactive compounds in the treatment of neuroinflammatory diseases.184–191 However, the stability and bioavailability of bioactive compound–loaded nanodelivery systems were greatly enhanced through microfluidization techniques. Curcumin, a potential antineuroinflammatory compound, was successfully loaded in zein nanoparticles using microfluidization techniques with a lower particle size and showed higher bioaccessibility.192
Conclusion
Microfluidization-based nanodelivery systems using plant bioactive compounds is a technology that is expected to make tremendous progress in producing various nanodelivery systems including SLNs, nanoemulsions, nanoliposomes, and PLGA nanoparticles. These techniques are able to produce stable and highly reproducible nanosystems of certain drugs with a lower particle size with the higher possibility of industrial scale production and application in the treatment of various chronic diseases. Stable nanodelivery systems developed using microfluidization techniques also showed higher bioavailability and bioaccessibility of those encapsulated plant-based bioactive compounds. Several microfluidized drugs are in the commercial market which are used in the treatment of various chronic diseases. Further research studies are also necessary in the design of microfluidization processing parameters for the development of particular nanodelivery systems using plant bioactive compounds in determining the stability and in the treatment of certain diseases. This will lead to the development of novel plant bioactive compound-based nanodelivery systems using microfluidization techniques with higher beneficial effects in the treatment of many chronic diseases.
Acknowledgments
This research was supported by the Basic Science Research Program through the National Research Foundation of Korea (NRF) funded by the Ministry of Science and ICT (NRF-2017R1C1B2010276 and 2017R1A2A2A07001035).
Disclosure
The authors report no conflicts of interest in this work.
References
Dias MI, Ferreira IC, Barreiro MF. Microencapsulation of bioactives for food applications. Food Funct. 2015;6(4):1035–1052. | ||
Jia Z, Dumont M-J, Orsat V. Encapsulation of phenolic compounds present in plants using protein matrices. Food Biosci. 2016;15:87–104. | ||
Lacatusu I, Badea N, Niculae G, Bordei N, Stan R, Meghea A. Lipid nanocarriers based on natural compounds: An evolving role in plant extract delivery. Eur J Lipid Sci Technol. 2014;116(12):1708–1717. | ||
Soukoulis C, Bohn T. A comprehensive overview on the micro- and nano-technological encapsulation advances for enhancing the chemical stability and bioavailability of carotenoids. Crit Rev Food Sci Nutr. 2018;58(1):1–36. | ||
Wang L, Gulati P, Santra D, Rose D, Zhang Y. Nanoparticles prepared by proso millet protein as novel curcumin delivery system. Food Chem. 2018;240:1039–1046. | ||
Bahri S, Ben Ali R, Abidi A, Jameleddine S. The efficacy of plant extract and bioactive compounds approaches in the treatment of pulmonary fibrosis: A systematic review. Biomed Pharmacother. 2017;93:666–673. | ||
Boniface PK, Baptista Ferreira S, Roland Kaiser C, Ferreira SB, Kaiser CR. Current state of knowledge on the traditional uses, phytochemistry, and pharmacology of the genus Hymenaea. J Ethnopharmacol. 2017;206:193–223. | ||
Edwards CA, Havlik J, Cong W, et al. Polyphenols and health: Interactions between fibre, plant polyphenols and the gut microbiota. Nutr Bull. 2017;42(4):356–360. | ||
Inada AC, Figueiredo PS, Santos-Eichler RAD, et al. Morinda citrifolia Linn. (Noni) and Its Potential in Obesity-Related Metabolic Dysfunction. Nutrients. 2017;9(6):540. | ||
Raiola A, Errico A, Petruk G, Monti DM, Barone A, Rigano MM. Bioactive Compounds in Brassicaceae Vegetables with a Role in the Prevention of Chronic Diseases. Molecules. 2018;23(1):15. | ||
Sharma A, Kashyap D, Sak K, Tuli HS, Sharma AK. Therapeutic charm of quercetin and its derivatives: a review of research and patents. Pharm Pat Anal. 2018;7(1):15–32. | ||
Torres-Contreras AM, Nair V, Cisneros-Zevallos L, Jacobo-Velázquez DA. Stability of Bioactive Compounds in Broccoli as Affected by Cutting Styles and Storage Time. Molecules. 2017;22(4):636. | ||
García-Moreno PJ, Özdemir N, Stephansen K, et al. Development of carbohydrate-based nano-microstructures loaded with fish oil by using electrohydrodynamic processing. Food Hydrocoll. 2017;69:273–285. | ||
Yang R, Zhou Z, Sun G, Gao Y, Xu J, Ferritin XJJ. Ferritin, a novel vehicle for iron supplementation and food nutritional factors encapsulation. Trend Food Sci Technol. 2015;44(2):189–200. | ||
Prakash B, Kujur A, Yadav A, Kumar A, Singh PP, Dubey NK. Nanoencapsulation: An efficient technology to boost the antimicrobial potential of plant essential oils in food system. Food Control. 2018;89:1–11. | ||
Yousuf B, Gul K, Wani AA, Singh P. Health Benefits of Anthocyanins and Their Encapsulation for Potential Use in Food Systems: A Review. Crit Rev Food Sci Nutr. 2016;56(13):2223–2230. | ||
Yucel C, Seker-Karatoprak G. Development and Evaluation of the Antioxidant Activity of Liposomes and Nanospheres Containing Rosmarinic Acid. Farmacia. 2017;65(1):40–45. | ||
Kesharwani P, Xie L, Banerjee S, et al. Hyaluronic acid-conjugated polyamidoamine dendrimers for targeted delivery of 3,4-difluorobenzylidene curcumin to CD44 overexpressing pancreatic cancer cells. Colloids Surf B Biointerfaces. 2015;136:413–423. | ||
Malik P, Singh M. Study of curcumin antioxidant activities in robust oil–water nanoemulsions. New J Chem. 2017;41(21):12506–12519. | ||
Moghaddasi F, Housaindokht MR, Darroudi M, Bozorgmehr MR, Sadeghi A. Synthesis of nano curcumin using black pepper oil by O/W Nanoemulsion Technique and investigation of their biological activities. LWT. 2018;92:92–100. | ||
Shin K, Choi H, Song SK, et al. Nanoemulsion Vehicles as Carriers for Follicular Delivery of Luteolin. Acs Biomater Sci Eng. 2018;4(5):1723–1729. | ||
Takahashi M, Kitamoto D, Imura T, Oku H, Takara K, Wada K. Characterization and bioavailability of liposomes containing a ukon extract. Biosci Biotechnol Biochem. 2008;72(5):1199–1205. | ||
Zeeb B, Saberi AH, Weiss J, Mcclements DJ. Formation and characterization of filled hydrogel beads based on calcium alginate: Factors influencing nanoemulsion retention and release. Food Hydrocoll. 2015;50:27–36. | ||
Zou L, Zheng B, Zhang R, et al. Enhancing the bioaccessibility of hydrophobic bioactive agents using mixed colloidal dispersions: Curcumin-loaded zein nanoparticles plus digestible lipid nanoparticles. Food Res Int. 2016;81:74–82. | ||
Shamsi M, Zahedi P. On-Chip Preparation of Streptokinase Entrapped in Chitosan Nanoparticles Used in Thrombolytic Therapy Potentially. J Pharm Sci. 2017;106(12):3623–3630. | ||
Dag D, Oztop MH. Formation and Characterization of Green Tea Extract Loaded Liposomes. J Food Sci. 2017;82(2):463–470. | ||
Dammak I, do Amaral Sobral PJ, Sobral PJD. Formulation and Stability Characterization of Rutin-Loaded Oil-in-Water Emulsions. Food Bioproc Tech. 2017;10(5):926–939. | ||
Liu X, Liu Y-Y, Guo J, Yin S-W, Yang X-Q. Microfluidization initiated cross-linking of gliadin particles for structured algal oil emulsions. Food Hydrocoll. 2017;73:153–161. | ||
Yan B, Park SH, Balasubramaniam VM. Influence of high pressure homogenization with and without lecithin on particle size and physicochemical properties of whey protein-based emulsions. J Food Process Eng. 2017;40(6):e12578. | ||
Yildirim ST, Oztop MH, Soyer Y. Cinnamon oil nanoemulsions by spontaneous emulsification: Formulation, characterization and antimicrobial activity. LWT. 2017;84:122–128. | ||
Cha KH, Koo SY, Song DG, Pan CH. Effect of microfluidization on bioaccessibility of carotenoids from Chlorella ellipsoidea during simulated digestion. J Agric Food Chem. 2012;60(37):9437–9442. | ||
Silva HD, Cerqueira MA, Souza BWS, et al. Nanoemulsions of β-carotene using a high-energy emulsification–evaporation technique. J Food Eng. 2011;102(2):130–135. | ||
Goh PS, Ng MH, Choo YM, Amru NB, Chuah CH. Production of Nanoemulsions from Palm-Based Tocotrienol Rich Fraction by Microfluidization. Molecules. 2015;20(11):19936–19946. | ||
Guerra-Rosas MI, Morales-Castro J, Ochoa-Martínez LA, Salvia-Trujillo L, Martín-Belloso O. Long-term stability of food-grade nanoemulsions from high methoxyl pectin containing essential oils. Food Hydrocoll. 2016;52:438–446. | ||
Uluata S, Decker EA, Mcclements DJ. Optimization of Nanoemulsion Fabrication Using Microfluidization: Role of Surfactant Concentration on Formation and Stability. Food Biophys. 2016;11(1):52–59. | ||
Bartheldyová E, Effenberg R, Mašek J, et al. Hyaluronic Acid Surface Modified Liposomes Prepared via Orthogonal Aminoxy Coupling: Synthesis of Nontoxic Aminoxylipids Based on Symmetrically α-Branched Fatty Acids, Preparation of Liposomes by Microfluidic Mixing, and Targeting to Cancer Cells Expressing CD44. Bioconjug Chem. 2018;29(7):2343–2356. | ||
Oktay AN, Karakucuk A, Ilbasmis-Tamer S, Celebi N. Dermal flurbiprofen nanosuspensions: Optimization with design of experiment approach and in vitro evaluation. Eur J Pharm Sci. 2018;122:254–263. | ||
Bai L, Mcclements DJ. Development of microfluidization methods for efficient production of concentrated nanoemulsions: Comparison of single- and dual-channel microfluidizers. J Colloid Interface Sci. 2016;466:206–212. | ||
Teng J, Xiaoqian H, Wang MF, Tao NP. Fabrication of chia (Salvia hispanica L.) seed oil nanoemulsions using different emulsifiers. J Food Process Pres. 2018;42(1):e13416. | ||
Doost AS, Devlieghere F, Dirckx A, van der Meeren P. Fabrication of Origanum compactum essential oil nanoemulsions stabilized using Quillaja Saponin biosurfactant. J Food Process Pres. 2018;42(7):e13668. | ||
Jing S, Wang S, Li Q, et al. Dynamic high pressure microfluidization-assisted extraction and bioactivities of Cyperus esculentus (C. esculentus L.) leaves flavonoids. Food Chem. 2016;192:319–327. | ||
Leidy R, de Jesus PFM, Anamaria M, Ximena QCM. Production of high-oleic palm oil nanoemulsions by high-shear homogenization (microfluidization). Innov Food Sci Emerg. 2016;35:75–85. | ||
Liu C-M, Liang R-H, Dai T-T, et al. Effect of dynamic high pressure microfluidization modified insoluble dietary fiber on gelatinization and rheology of rice starch. Food Hydrocolloid. 2016;57:55–61. | ||
Liu F, Zhu Z, Ma C, et al. Fabrication of Concentrated Fish Oil Emulsions Using Dual-Channel Microfluidization: Impact of Droplet Concentration on Physical Properties and Lipid Oxidation. J Agric Food Chem. 2016;64(50):9532–9541. | ||
Zhang J, Peppard TL, Reineccius GA. Preparation and characterization of nanoemulsions stabilized by food biopolymers using microfluidization. Flavour Fragr J. 2015;30(4):288–294. | ||
Zhang J, Bing L, Reineccius GA. Comparison of modified starch and Quillaja saponins in the formation and stabilization of flavor nanoemulsions. Food Chem. 2016;192:53–59. | ||
Gómez-Favela MA, Gutiérrez-Dorado R, Cuevas-Rodríguez EO, et al. Improvement of Chia Seeds with Antioxidant Activity, GABA, Essential Amino Acids, and Dietary Fiber by Controlled Germination Bioprocess. Plant Foods Hum Nutr. 2017;72(4):345–352. | ||
Leong HY, Show PL, Lim MH, Ooi CW, Ling TC. Natural red pigments from plants and their health benefits: A review. Food Rev Int. 2018;34(5):463–482. | ||
Komaiko J, Sastrosubroto A, Mcclements DJ. Encapsulation of ω-3 fatty acids in nanoemulsion-based delivery systems fabricated from natural emulsifiers: Sunflower phospholipids. Food Chem. 2016;203:331–339. | ||
Li Y, Arranz E, Guri A, Corredig M. Mucus interactions with liposomes encapsulating bioactives: Interfacial tensiometry and cellular uptake on Caco-2 and cocultures of Caco-2/HT29-MTX. Food Res Int. 2017;92:128–137. | ||
Luo X, Zhou Y, Bai L, Liu F, Deng Y, Mcclements DJ. Fabrication of β-carotene nanoemulsion-based delivery systems using dual-channel microfluidization: Physical and chemical stability. J Colloid Interface Sci. 2017;490:328–335. | ||
Helgason T, Salminen H, Kristbergsson K, Mcclements DJ, Weiss J. Formation of transparent solid lipid nanoparticles by microfluidization: influence of lipid physical state on appearance. J Colloid Interface Sci. 2015;448:114–122. | ||
Schröder A, Sprakel J, Schroën K, Spaen JN, Berton-Carabin CC. Coalescence stability of Pickering emulsions produced with lipid particles: A microfluidic study. J Food Eng. 2018;234:63–72. | ||
Aditya NP, Ko S. Solid lipid nanoparticles (SLNs): delivery vehicles for food bioactives. Rsc Adv. 2015;5(39):30902–30911. | ||
Ambwani S, Tandon R, Ambwani TK, Malik YS. Division of Biological Standardization, ICAR-Indian Veterinary Research Institute, Izatnagar 243122, Uttar Pradesh, India. Current Knowledge on Nanodelivery Systems and Their Beneficial Applications in Enhancing the Efficacy of Herbal Drugs. J Exp Biol Agricult Sci. 2018;6(1):87–107. | ||
Amore E, Ferraro M, Manca ML, et al. Mucoadhesive solid lipid microparticles for controlled release of a corticosteroid in the chronic obstructive pulmonary disease treatment. Nanomedicine. 2017;12(19):2287–2302. | ||
Gungor S, Rezigue M. Nanocarriers Mediated Topical Drug Delivery for Psoriasis Treatment. Curr Drug Metab. 2017;18(5):454–468. | ||
Jain S, Patel N, Shah MK, Khatri P, Vora N. Recent Advances in Lipid-Based Vesicles and Particulate Carriers for Topical and Transdermal Application. J Pharm Sci. 2017;106(2):423–445. | ||
Saporito F, Sandri G, Bonferoni MC, et al. Essential oil-loaded lipid nanoparticles for wound healing. Int J Nanomed. 2018;13:175–186. | ||
Zhou M, Hou J, Zhong Z, Hao N, Lin Y, Li C. Targeted delivery of hyaluronic acid-coated solid lipid nanoparticles for rheumatoid arthritis therapy. Drug Deliv. 2018;25(1):716–722. | ||
Arora R, Kuhad A, Kaur IP, Chopra K. Curcumin loaded solid lipid nanoparticles ameliorate adjuvant-induced arthritis in rats. Eur J Pain. 2015;19(7):940–952. | ||
Cui G-J, Xu L-M, Zhou Y, Zhang J-J, Wang J-X, Chen J-F. Microfluidic fabrication of silybin nanodispersion with high dissolution rate and tunable sizes. Chem Eng J. 2013;222:512–519. | ||
He Y, Yue Y, Zheng X, Zhang K, Chen S, Du, Z. Curcumin, Inflammation, and Chronic Diseases: How Are They Linked? Molecules. 2015;20(5):9183–9213. | ||
Singh N, Khullar N, Kakkar V, Kaur IP. Hepatoprotective effects of sesamol loaded solid lipid nanoparticles in carbon tetrachloride induced sub-chronic hepatotoxicity in rats. Environ Toxicol. 2016;31(5):520–532. | ||
Watkins R, Wu L, Zhang CM, Davis RM, Xu B. Natural product-based nanomedicine: recent advances and issues. Int J Nanomed. 2015;10:6055–6074. | ||
Yadav VR, Suresh S, Devi K, Yadav S. Novel formulation of solid lipid microparticles of curcumin for anti-angiogenic and anti-inflammatory activity for optimization of therapy of inflammatory bowel disease. J Pharm Pharmacol. 2009;61(3):311–321. | ||
Behbahani ES, Ghaedi M, Abbaspour M, Rostamizadeh K. Optimization and characterization of ultrasound assisted preparation of curcumin-loaded solid lipid nanoparticles: Application of central composite design, thermal analysis and X-ray diffraction techniques. Ultrason Sonochem. 2017;38:271–280. | ||
Cortés-Rojas DF, Souza CRF, Oliveira WP. Encapsulation of eugenol rich clove extract in solid lipid carriers. J Food Eng. 2014;127:34–42. | ||
Kim JH, Baek JS, Park JK, et al. Development of Houttuynia cordata Extract-Loaded Solid Lipid Nanoparticles for Oral Delivery: High Drug Loading Efficiency and Controlled Release. Molecules. 2017;22(12):2215. | ||
Helgason T, Awad TS, Kristbergsson K, Mcclements DJ, Weiss J. Effect of surfactant surface coverage on formation of solid lipid nanoparticles (SLN). J Colloid Interface Sci. 2009;334(1):75–81. | ||
Kim JE, Park YJ. Paclitaxel-loaded hyaluronan solid nanoemulsions for enhanced treatment efficacy in ovarian cancer. Int J Nanomedicine. 2017;12:645–658. | ||
Salminen H, Helgason T, Kristinsson B, Kristbergsson K, Weiss J. Formation of nanostructured colloidosomes using electrostatic deposition of solid lipid nanoparticles onto an oil droplet interface. Food Res Int. 2016;79:11–18. | ||
Suter F, Schmid D, Wandrey F, Zülli F. Heptapeptide-loaded solid lipid nanoparticles for cosmetic anti-aging applications. Eur J Pharm Biopharm. 2016;108:304–309. | ||
Trujillo CC, Wright AJ. Properties and Stability of Solid Lipid Particle Dispersions Based on Canola Stearin and Poloxamer 188. J Am Oil Chem Soc. 2010;87(7):715–730. | ||
Wang R, Tian Z, Chen L. Nano-encapsulations liberated from barley protein microparticles for oral delivery of bioactive compounds. Int J Pharm. 2011;406(1–2):153–162. | ||
Wang JL, Dong XY, Wei F, et al. Preparation and characterization of novel lipid carriers containing microalgae oil for food applications. J Food Sci. 2014;79(2):E169–E177. | ||
Harwansh RK, Mukherjee PK, Bahadur S, Biswas R. Enhanced permeability of ferulic acid loaded nanoemulsion based gel through skin against UVA mediated oxidative stress. Life Sci. 2015;141:202–211. | ||
Harwansh RK, Mukherjee PK, Biswas S. Nanoemulsion as a novel carrier system for improvement of betulinic acid oral bioavailability and hepatoprotective activity. J Mol Liq. 2017;237:361–371. | ||
Karthik P, Ezhilarasi PN, Anandharamakrishnan C. Challenges associated in stability of food grade nanoemulsions. Crit Rev Food Sci Nutr. 2017;57(7):1435–1450. | ||
Lohith Kumar DH, Sarkar P. Encapsulation of bioactive compounds using nanoemulsions. Environ Chem Lett. 2018;16(1):59–70. | ||
Li Y, Zheng J, Xiao H, Mcclements DJ. Nanoemulsion-based delivery systems for poorly water-soluble bioactive compounds: Influence of formulation parameters on Polymethoxyflavone crystallization. Food Hydrocoll. 2012;27(2):517–528. | ||
Ochoa AA, Hernandez-Becerra JA, Cavazos-Garduno A, Vernon-Carter EJ, Garcia HS. Preparation and Characterization of Curcumin Nanoemulsions Obtained by Thin-Film Hydration Emulsification and Ultrasonication Methods. Rev Mex Ing Quim. 2016;15(1):79–90. | ||
Oliveira AEMFM, Duarte JL, Cruz RAS, Conceição ECDA, Carvalho JCT, Fernandes CP. Utilization of dynamic light scattering to evaluate Pterodon emarginatus oleoresin-based nanoemulsion formation by non-heating and solvent-free method. Revista Brasileira de Farmacognosia. 2017;27(3):401–406. | ||
Yi J, Zhang Y, Liang R, Zhong F, Ma J, Jg M. Beta-carotene chemical stability in Nanoemulsions was improved by stabilized with beta-lactoglobulin-catechin conjugates through free radical method. J Agric Food Chem. 2015;63(1):297–303. | ||
Zhong J, Liu X, Wang Y, Qin X, Li Z. γ-Oryzanol nanoemulsions produced by a low-energy emulsification method: an evaluation of process parameters and physicochemical stability. Food Funct. 2017;8(6):2202–2211. | ||
Kotta S, Khan AW, Ansari SH, Sharma RK, Ali J. Formulation of nanoemulsion: a comparison between phase inversion composition method and high-pressure homogenization method. Drug Deliv. 2015;22(4):455–466. | ||
Qian C, Mcclements DJ. Formation of nanoemulsions stabilized by model food-grade emulsifiers using high-pressure homogenization: Factors affecting particle size. Food Hydrocoll. 2011;25(5):1000–1008. | ||
Salvia-Trujillo L, Rojas-Graü MA, Soliva-Fortuny R, Martín-Belloso O. Effect of processing parameters on physicochemical characteristics of microfluidized lemongrass essential oil-alginate nanoemulsions. Food Hydrocoll. 2013;30(1):401–407. | ||
Walker RM, Decker EA, Mcclements DJ. Physical and oxidative stability of fish oil nanoemulsions produced by spontaneous emulsification: Effect of surfactant concentration and particle size. J Food Eng. 2015;164:10–20. | ||
Raviadaran R, Chandran D, Shin LH, Manickam S. Optimization of palm oil in water nano-emulsion with curcumin using microfluidizer and response surface methodology. LWT. 2018;96:58–65. | ||
Akhavan S, Assadpour E, Katouzian I, Jafari SM. Lipid nano scale cargos for the protection and delivery of food bioactive ingredients and nutraceuticals. Trend Food Sci Technol. 2018;74:132–146. | ||
Faezizadeh Z, Gharib A, Godarzeeb M. In-vitro and In-vivo Evaluation of Silymarin Nanoliposomes against Isolated Methicillin-resistant Staphylococcus aureus. Iran J Pharm Res Spr. 2015;14(2):627–633. | ||
Haghighi M, Yarmand MS, Emam-Djomeh Z, Mcclements DJ, Saboury AA, Rafiee-Tehrani M. Design and fabrication of pectin-coated nanoliposomal delivery systems for a bioactive polyphenolic: Phloridzin. Int J Biol Macromol. 2018;112:626–637. | ||
Liang T, Guan R, Wang Z, Shen H, Xia Q, Liu M. Comparison of anticancer activity and antioxidant activity between cyanidin-3-O-glucoside liposomes and cyanidin-3-O-glucoside in Caco-2 cells in vitro. RSC Adv. 2017;7(59):37359–37368. | ||
Ramezanzade L, Hosseini SF, Nikkhah M. Biopolymer-coated nanoliposomes as carriers of rainbow trout skin-derived antioxidant peptides. Food Chem. 2017;234:220–229. | ||
Arabi MH, Mirzapour A, Chabok H, Ardestani MS, Saffari M. Preparation methods of nanoliposomes containing Zataria multiflora essential oil: A comparative study. Biosci Biotechno Res. 2017;10(1):151–160. | ||
Bochicchio S, Barba AA, Grassi G, Lamberti G. Vitamin delivery: Carriers based on nanoliposomes produced via ultrasonic irradiation. LWT Food Sci Technol. 2016;69:9–16. | ||
Chay SY, Tan WK, Saari N. Preparation and characterisation of nanoliposomes containing winged bean seeds bioactive peptides. J Microencapsul. 2015;32(5):488–495. | ||
Gong KJ, Shi AM, Liu HZ, et al. Preparation of nanoliposome loaded with peanut peptide fraction: stability and bioavailability. Food Funct. 2016;7(4):2034–2042. | ||
Kt L, Duan QQ, Chen Q, et al. The effect of aloe emodin-encapsulated nanoliposome-mediated r-caspase-3 gene transfection and photodynamic therapy on human gastric cancer cells. Cancer Med-Us. 2016;5(2):361–369. | ||
Lu Q, Li DC, Jiang JG. Preparation of a tea polyphenol nanoliposome system and its physicochemical properties. J Agric Food Chem. 2011;59(24):13004–13011. | ||
Mozafari MR, Khosravi-Darani K, Borazan GG, Cui J, Pardakhty A, Yurdugul S. Encapsulation of Food Ingredients Using Nanoliposome Technology. Int J Food Prop. 2008;11(4):833–844. | ||
Peng S, Zou L, Liu W, et al. Storage stability and antibacterial activity of eugenol nanoliposomes prepared by an ethanol injection-dynamic high-pressure microfluidization method. J Food Prot. 2015;78(1):22–30. | ||
Pezeshky A, Ghanbarzadeh B, Hamishehkar H, Moghadam M, Babazadeh A. Vitamin A palmitate-bearing nanoliposomes: Preparation and characterization. Food Biosci. 2016;13:49–55. | ||
Rafiee Z, Barzegar M, Sahari MA, Maherani B. Nanoliposomal carriers for improvement the bioavailability of high – valued phenolic compounds of pistachio green hull extract. Food Chem. 2017;220:115–122. | ||
Tuan AN, Quan DT, Duc D, Mau CD. Micro and nano liposome vesicles containing curcumin for a drug delivery system. Adv Nat Sci-Nanosci. 2016;7(3):035003. | ||
Zou LQ, Liu W, Liu WL, et al. Characterization and bioavailability of tea polyphenol nanoliposome prepared by combining an ethanol injection method with dynamic high-pressure microfluidization. J Agric Food Chem. 2014;62(4):934–941. | ||
Zou LQ, Peng SF, Liu W, et al. Improved in vitro digestion stability of (-)-epigallocatechin gallate through nanoliposome encapsulation. Food Res Int. 2014;64:492–499. | ||
Guldiken B, Gibis M, Boyacioglu D, Capanoglu E, Weiss J. Physical and chemical stability of anthocyanin-rich black carrot extract-loaded liposomes during storage. Food Res Int. 2018;108:491–497. | ||
Li T, Yang S, Liu W, et al. Preparation and Characterization of Nanoscale Complex Liposomes Containing Medium-Chain Fatty Acids and Vitamin C. Int J Food Prop. 2015;18(1):113–124. | ||
Chen X, Zou LQ, Niu J, Liu W, Peng SF, Liu CM. The Stability, Sustained Release and Cellular Antioxidant Activity of Curcumin Nanoliposomes. Molecules. 2015;20(8):14293–14311. | ||
Fung HW, Mikasa TJ, Vergara J, et al. Optimizing manufacturing and composition of a TLR4 nanosuspension: physicochemical stability and vaccine adjuvant activity. J Nanobiotechnology. 2013;11:43. | ||
Hao L, Luan J, Zhang D, et al. Research on the in vitro anticancer activity and in vivo tissue distribution of Amoitone B nanocrystals. Colloids Surf B Biointerfaces. 2014;117:258–266. | ||
Karakucuk A, Celebi N, Teksin ZS. Preparation of ritonavir nanosuspensions by microfluidization using polymeric stabilizers: I. A Design of Experiment approach. Eur J Pharm Sci. 2016;95:111–121. | ||
Ren F, Fu J, Xiong H, et al. Complexes of Felodipine Nanoparticles With Zein Prepared Using a Dual Shift Technique. J Pharm Sci. 2018;107(1):239–249. | ||
Seok SH, Kang SY, Seo JW, Kim SH, Hwang KM, Park ES. Formulation of Nanoparticle Containing Everolimus Using Microfluidization and Freeze-Drying. Chem Pharm Bull. 2016;64(10):1445–1449. | ||
Tian X, Li H, Zhang D, et al. Nanosuspension for parenteral delivery of a p-terphenyl derivative: preparation, characteristics and pharmacokinetic studies. Colloids Surf B Biointerfaces. 2013;108:29–33. | ||
Tian X, Li H, Zhang D, et al. Parenteral nanosuspension of a novel synthesized antitumor candidate: Investigation of tissue biodistributions and plasma pharmacokinetics. Colloids Surf A Physicochem Eng Asp. 2013;436:868–872. | ||
Zhang Y, Zhang J. Preparation of budesonide nanosuspensions for pulmonary delivery: Characterization, in vitro release and in vivo lung distribution studies. Artif Cells Nanomed Biotechnol. 2016;44(1):285–289. | ||
Yuan H, Li X, Zhang C, et al. Nanosuspensions as delivery system for gambogenic acid: characterization and in vitro/in vivo evaluation. Drug Deliv. 2016;23(8):2772–2779. | ||
Ganesan P, Ko HM, Kim IS, Choi DK. Recent trends in the development of nanophytobioactive compounds and delivery systems for their possible role in reducing oxidative stress in Parkinson’s disease models. Int J Nanomedicine. 2015;10:6757–6772. | ||
Pereira MC, Hill LE, Zambiazi RC, Mertens-Talcott S, Talcott S, Gomes CL. Nanoencapsulation of hydrophobic phytochemicals using poly (dl-lactide-co-glycolide) (PLGA) for antioxidant and antimicrobial delivery applications: Guabiroba fruit (Campomanesia xanthocarpa O. Berg) study. LWT Food Sci Technol. 2015;63(1):100–107. | ||
Shirode AB, Bharali DJ, Nallanthighal S, Coon JK, Mousa SA, Reliene R. Nanoencapsulation of pomegranate bioactive compounds for breast cancer chemoprevention. Int J Nanomedicine. 2015;10:475–484. | ||
Siddiqui IA, Sanna V. Impact of nanotechnology on the delivery of natural products for cancer prevention and therapy. Mol Nutr Food Res. 2016;60(6):1330–1341. | ||
Abd-Rabou AA, Ahmed HH. CS-PEG decorated PLGA nano-prototype for delivery of bioactive compounds: A novel approach for induction of apoptosis in HepG2 cell line. Adv Med Sci. 2017;62(2):357–367. | ||
Andrade KS, Poncelet D, Ferreira SRS. Sustainable extraction and encapsulation of pink pepper oil. J Food Eng. 2017;204:38–45. | ||
Oliveira DA, Angonese M, Ferreira SRS, Gomes CL. Nanoencapsulation of passion fruit by-products extracts for enhanced antimicrobial activity. Food Bioprod Process. 2017;104:137–146. | ||
Lamprecht A, Ubrich N, Hombreiro Pérez M, Lehr C, Hoffman M, Maincent P. Biodegradable monodispersed nanoparticles prepared by pressure homogenization-emulsification. Int J Pharm. 1999;184(1):97–105. | ||
Lamprecht A, Ubrich N, Hombreiro Pérez M, Lehr C, Hoffman M, Maincent P. Influences of process parameters on nanoparticle preparation performed by a double emulsion pressure homogenization technique. Int J Pharm. 2000;196(2):177–182. | ||
Sani SN, das NG, das SK. Effect of microfluidization parameters on the physical properties of PEG-PLGA nanoparticles prepared using high pressure microfluidization. J Microencapsul. 2009;26(6):556–561. | ||
Martins C, Araújo F, Gomes MJ, et al. Using microfluidic platforms to develop CNS-targeted polymeric nanoparticles for HIV therapy. Eur J Pharm Biopharm. Epub 2018 Jan 31. | ||
Bai L, Mcclements DJ. Development of microfluidization methods for efficient production of concentrated nanoemulsions: Comparison of single- and dual-channel microfluidizers. J Colloid Interface Sci. 2016;466:206–212. | ||
Chung C, Sher A, Rousset P, Mcclements DJ. Influence of homogenization on physical properties of model coffee creamers stabilized by quillaja saponin. Food Res Int. 2017;99(Pt 1):770–777. | ||
Liu F, Zhu Z, Ma C, et al. Fabrication of Concentrated Fish Oil Emulsions Using Dual-Channel Microfluidization: Impact of Droplet Concentration on Physical Properties and Lipid Oxidation. J Agric Food Chem. 2016;64(50):9532–9541. | ||
Qiu D, Yang L, Shi YC. Formation of vitamin E emulsion stabilized by octenylsuccinic starch: factors affecting particle size and oil load. J Food Sci. 2015;80(4):C680–C686. | ||
Santos J, Calero N, Muñoz J, Cidade MT. Development of food emulsions containing an advanced performance xanthan gum by microfluidization technique. Food Sci Technol Int. 2018;24(5):373–381. | ||
Stupak R, Makauskas N, Radzevičius K, Valančius Z. Optimization of intracellular product release from Neisseria denitrificans using microfluidizer. Prep Biochem Biotechnol. 2015;45(7):667–683. | ||
Wang FC, Acevedo N, Marangoni AG. Encapsulation of phytosterols and phytosterol esters in liposomes made with soy phospholipids by high pressure homogenization. Food Funct. 2017;8(11):3964–3969. | ||
Zhang Y, Zhang J. Preparation of budesonide nanosuspensions for pulmonary delivery: Characterization, in vitro release and in vivo lung distribution studies. Artif Cells Nanomed Biotechnol. 2016;44(1):285–289. | ||
Chen MJ, Wu IC, Chen YJ, et al. Nutrition therapy in esophageal cancer-Consensus statement of the Gastroenterological Society of Taiwan. Dis Esophagus. 2018;31(8):doy016. | ||
Jafari E, Alavi M, Zal F. The evaluation of protective and mitigating effects of vitamin C against side effects induced by radioiodine therapy. Radiat Environ Biophys. 2018;57(3):233–240. | ||
Lasalvia-Prisco E, Dau C, Vázquez J, Goldschmidt P, Galmarini F. Geroprotection in cancer prevention. Adv Gerontol. 2018;31(1):21–24. | ||
Lee JW, Cho CJ, Kim DH, et al. Long-Term Survival and Tumor Recurrence in Patients with Superficial Esophageal Cancer after Complete Non-Curative Endoscopic Resection: A Single-Center Case Series. Clin Endosc. Epub 2018 Jun 1. | ||
Xiao S, Yang J. Preclinical study of the antitumor effect of sphingosine-1-phosphate receptor1 antibody (S1PR1-antibody) against human breast cancer cells. Invest New Drugs. Epub 2018 Jun 2. | ||
Zhao HD, Xie HJ, Li J, Ren CP, Chen YX. Research Progress on Reversing Multidrug Resistance in Tumors by Using Chinese Medicine. Chin J Integr Med. 2018;24(6):474–480. | ||
Conte R, Luca ID, Luise AD, Petillo O, Calarco A, Peluso G. New Therapeutic Potentials of Nanosized Phytomedicine. J Nanosci Nanotechnol. 2016;16(8):8176–8187. | ||
Davatgaran-Taghipour Y, Masoomzadeh S, Farzaei MH, et al. Polyphenol nanoformulations for cancer therapy: experimental evidence and clinical perspective. Int J Nanomedicine. 2017;12:2689–2702. | ||
Namdari M, Eatemadi A, Soleimaninejad M, Hammed AT. A brief review on the application of nanoparticle enclosed herbal medicine for the treatment of infective endocarditis. Biomed Pharmacother. 2017;87:321–331. | ||
Wang Y, Zhang L, Wang Q, Zhang D. Recent advances in the nanotechnology-based drug delivery of Silybin. J Biomed Nanotechnol. 2014;10(4):543–558. | ||
Loredo-Tovias M, Duran-Meza AL, Villagrana-Escareño MV, et al. Encapsidated ultrasmall nanolipospheres as novel nanocarriers for highly hydrophobic anticancer drugs. Nanoscale. 2017;9(32):11625–11631. | ||
Meng Y, Eirin A, Zhu XY, et al. Obesity-induced mitochondrial dysfunction in porcine adipose tissue-derived mesenchymal stem cells. J Cell Physiol. 2018;233(8):5926–5936. | ||
Mohareri F, Asl SN, Behdani F, Ghaemi N. Evaluating of Psychiatric Behavior in Obese Children and Adolescents. Iran J Child Neurol. 2018;12(1):26–36. | ||
Donado-Pestana CM, Moura MHC, de Araujo RL, et al. Polyphenols from Brazilian native Myrtaceae fruits and their potential health benefits against obesity and its associated complications. Curr Opin Food Sci. 2018;19:42–49. | ||
Hasan MM, Ahmed QU, Soad SZM, et al. Flavonoids from Tetracera indica Merr. induce adipogenesis and exert glucose uptake activities in 3T3-L1 adipocyte cells. BMC Complement Altern Med. 2017;17(1):431. | ||
Mopuri R, Islam MS. Medicinal plants and phytochemicals with anti-obesogenic potentials: A review. Biomed Pharmacother. 2017;89:1442–1452. | ||
Sido A, Radhakrishnan S, Kim SW, et al. A food-based approach that targets interleukin-6, a key regulator of chronic intestinal inflammation and colon carcinogenesis. J Nutr Biochem. 2017;43:11–17. | ||
Somtimuang C, Olatunji OJ, Ovatlarnporn C. Evaluation of In Vitro α-Amylase and α-Glucosidase Inhibitory Potentials of 14 Medicinal Plants Constituted in Thai Folk Antidiabetic Formularies. Chem Biodivers. 2018;15(4):e1800025. | ||
Udechukwu MC, Abbey L, Nwodo U, Udenigwe CC. Potential of Moringa oleifera seeds and leaves as functional food ingredients for human health promotion. J Food Nutr Res-Slov. 2018;57(1):1–14. | ||
Arent SM, Walker AJ, Pellegrino JK, et al. The Combined Effects of Exercise, Diet, and a Multi-Ingredient Dietary Supplement on Body Composition and Adipokine Changes in Overweight Adults. J Am Coll Nutr. 2018;37(2):111–120. | ||
Hirotani Y, Fukamachi J, Ueyama R, Urashima Y, Ikeda K. Effects of Capsaicin Coadministered with Eicosapentaenoic Acid on Obesity-Related Dysregulation in High-Fat-Fed Mice. Biol Pharm Bull. 2017;40(9):1581–1585. | ||
Son HK, Shin HW, Jang ES, Moon BS, Lee CH, Lee JJ. Comparison of Antiobesity Effects Between Gochujangs Produced Using Different Koji Products and Tabasco Hot Sauce in Rats Fed a High-Fat Diet. J Med Food. 2018;21(3):233–243. | ||
Sun L, Camps SG, Goh HJ, et al. Capsinoids activate brown adipose tissue (BAT) with increased energy expenditure associated with subthreshold 18-fluorine fluorodeoxyglucose uptake in BAT-positive humans confirmed by positron emission tomography scan. Am J Clin Nutr. 2018;107(1):62–70. | ||
Surassmo S, Min S-G, Bejrapha P, Choi M-J. Effects of surfactants on the physical properties of capsicum oleoresin-loaded nanocapsules formulated through the emulsion–diffusion method. Food Res Int. 2010;43(1):8–17. | ||
Kim JY, Lee MS, Jung S, et al. Anti-obesity efficacy of nanoemulsion oleoresin capsicum in obese rats fed a high-fat diet. Int J Nanomedicine. 2014;9:301–310. | ||
Scolaro B, Soo Jin Kim H, de Castro IA. Bioactive compounds as an alternative for drug co-therapy: Overcoming challenges in cardiovascular disease prevention. Crit Rev Food Sci Nutr. 2018;58(6):958–971. | ||
Tam DNH, Truong DH, Nguyen TTH, et al. Ginsenoside Rh1: A Systematic Review of Its Pharmacological Properties. Planta Med. 2018;84(3):139–152. | ||
Banjari I, Misir A, Šavikin K, et al. Antidiabetic Effects of Aronia melanocarpa and Its Other Therapeutic Properties. Front Nutr. 2017;4:53. | ||
Braga ARC, Murador DC, de Souza Mesquita LM, de Rosso VV. Bioavailability of anthocyanins: Gaps in knowledge, challenges and future research. J Food Composit Analy. 2018;68:31–40. | ||
Fang J, Little PJ, Xu S, Sw X. Atheroprotective Effects and Molecular Targets of Tanshinones Derived From Herbal Medicine Danshen. Med Res Rev. 2018;38(1):201–228. | ||
Morais R, Morais A, Dammak I, et al. Functional Dehydrated Foods for Health Preservation. J Food Quality. 2018;2018:1739636. | ||
Olas B. The beneficial health aspects of sea buckthorn (Elaeagnus rhamnoides (L.) A.Nelson) oil. J Ethnopharmacol. 2018;213:183–190. | ||
Huang ZF, Wang HS, Gao CS, Shen HY, Xe F. Drug Loaded Gold Nano-Particulates for Therapeutics of Myocardial Infarction in Rat Model. J Biomater Tiss Eng. 2018;8(2):197–205. | ||
Jiang J, Ao J, He CY, et al. Preparation and characterisation of ginkgolide nanoparticles via the emulsion solvent evaporation method. Micro Nano Lett. 2018;13(5):636–640. | ||
Katsuki S, Matoba T, Koga JI, Nakano K, Egashira K. Anti-inflammatory Nanomedicine for Cardiovascular Disease. Front Cardiovasc Med. 2017;4:87. | ||
Nakhlband A, Eskandani M, Omidi Y, et al. Combating atherosclerosis with targeted nanomedicines: recent advances and future prospective. Bioimpacts. 2018;8(1):59–75. | ||
Yin J, Xiang C, Wang P, Yin Y, Hou Y. Biocompatible nanoemulsions based on hemp oil and less surfactants for oral delivery of baicalein with enhanced bioavailability. Int J Nanomedicine. 2017;12:2923–2931. | ||
Businaro R, Corsi M, Asprino R, et al. Modulation of Inflammation as a Way of Delaying Alzheimer’s Disease Progression: The Diet’s Role. Curr Alzheimer Res. 2018;15(4):363–380. | ||
Cho DY, Ko HM, Kim J, et al. Scoparone Inhibits LPS-Simulated Inflammatory Response by Suppressing IRF3 and ERK in BV-2 Microglial Cells. Molecules. 2016;21(12):E1718:1718. | ||
Jo MJ, Kumar H, Joshi HP, et al. Oral Administration of α-Asarone Promotes Functional Recovery in Rats With Spinal Cord Injury. Front Pharmacol. 2018;9:445. | ||
Lee SY, Suh WS, Cha JM, et al. Anti-neuroinflammatory constituents from Sinomenium acutum rhizomes. Phytochem Lett. 2016;17:79–84. | ||
Li N, Wang Y, Li X, et al. Bioactive phenols as potential neuroinflammation inhibitors from the leaves of Xanthoceras sorbifolia Bunge. Bioorg Med Chem Lett. 2016;26(20):5018–5023. | ||
Liu F, Dong B, Yang X, et al. NO inhibitors function as potential anti-neuroinflammatory agents for AD from the flowers of Inula japonica. Bioorg Chem. 2018;77:168–175. | ||
Park SY, Jin ML, Ko MJ, Park G, Choi YW. Anti-neuroinflammatory Effect of Emodin in LPS-Stimulated Microglia: Involvement of AMPK/Nrf2 Activation. Neurochem Res. 2016;41(11):2981–2992. | ||
Aqil F, Munagala R, Jeyabalan J, Agrawal AK, Gupta R. Exosomes for the Enhanced Tissue Bioavailability and Efficacy of Curcumin. Aaps J. 2017;19(6):1691–1702. | ||
Barbara R, Belletti D, Pederzoli F, et al. Novel Curcumin loaded nanoparticles engineered for Blood-Brain Barrier crossing and able to disrupt Abeta aggregates. Int J Pharm. 2017;526(1–2):413–424. | ||
Chang CZ, Wu SC, Lin CL, Kwan AL. Curcumin, encapsulated in nano-sized PLGA, down-regulates nuclear factor κB (p65) and subarachnoid hemorrhage induced early brain injury in a rat model. Brain Res. 2015;1608:215–224. | ||
di Martino P, Censi R, Gigliobianco MR, et al. Nano-medicine Improving the Bioavailability of Small Molecules for the Prevention of Neurodegenerative Diseases. Curr Pharm Des. 2017;23(13):1897–1908. | ||
Wh J, Xiao ZB, Liu GY, Zhang X. Development and application of nano-flavor-drug carriers in neurodegenerative diseases. Chinese Chem Lett. 2017;28(9):1829–1834. | ||
Kalani A, Chaturvedi P, Kamat PK, et al. Curcumin-loaded embryonic stem cell exosomes restored neurovascular unit following ischemia-reperfusion injury. Int J Biochem Cell Biol. 2016;79:360–369. | ||
Zhang Z. Enhanced therapeutic potential of nano-curcumin against subarachnoid hemorrhage-induced blood-brain barrier disruption through inhibition of inflammatory response and oxidative stress. J Cerebr Blood F Met. 2017;37:449–450. | ||
Zhang ZY, Jiang M, Fang J, et al. Enhanced Therapeutic Potential of Nano-Curcumin Against Subarachnoid Hemorrhage-Induced Blood-Brain Barrier Disruption Through Inhibition of Inflammatory Response and Oxidative Stress. Mol Neurobiol. 2017;54(1):1–14. | ||
Zou L, Zheng B, Zhang R, et al. Enhancing the bioaccessibility of hydrophobic bioactive agents using mixed colloidal dispersions: Curcumin-loaded zein nanoparticles plus digestible lipid nanoparticles. Food Res Int. 2016;81:74–82. |
 © 2018 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2018 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
