Back to Journals » Journal of Blood Medicine » Volume 11
Massive Transfusion Protocols for Pediatric Patients: Current Perspectives
Authors Evangelista ME , Gaffley M , Neff LP
Received 19 February 2020
Accepted for publication 24 April 2020
Published 21 May 2020 Volume 2020:11 Pages 163—172
DOI https://doi.org/10.2147/JBM.S205132
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 2
Editor who approved publication: Dr Martin H Bluth
Meagan E Evangelista,1 Michaela Gaffley,1 Lucas P Neff2
1General Surgery, Wake Forest School of Medicine, Winston-Salem, NC, USA; 2Pediatric Surgery, Wake Forest School of Medicine, Winston-Salem, NC, USA
Correspondence: Michaela Gaffley Email [email protected]
Abstract: In adults, the use of balanced resuscitation and study of massive transfusion protocols have led to improved outcomes for patients and continues to be refined. In children, massive transfusion protocols require further development and study to assess efficacy. Standardization is needed as transfusions and activation of protocols still rely on physician discretion in most pediatric settings. Further research is required to define the pediatric trauma population that will benefit, when to activate these protocols and how to use adjuncts such as tranexamic acid or factor VII in resuscitation. In addition, future implementation of technology such as hemoglobin-based oxygen carriers to increase survival should be studied further in this subset of patients.
Keywords: pediatric trauma, pediatric resuscitation, blood transfusions, pediatric massive transfusion, massive transfusion protocols
Introduction
Injury is the leading cause of death in children and adolescents with falls and motor vehicle accidents as the two leading mechanisms of injury.1,2 Bleeding from these mechanisms can be severe enough to warrant massive transfusion protocol (MTP) activations. Additional etiologies of life-threatening hemorrhage include operative complications, invasive tumors whose removal results in significant blood loss, liver surgery and gastrointestinal bleeding.3–6 Despite these varied reasons for hemorrhage, traumatic injury remains the main driver for activation of a pediatric MTP. This subset of trauma victims is generally older, more hypothermic, with a higher injury severity score when compared with other pediatric trauma patients who may have been transfused but did not meet the criteria for pediatric massive transfusion (MT).7 This review of massive transfusion protocols in pediatric patients consisted of a search of the PubMed and Google Scholar databases. We aim to not only summarize current evidence-based practices in pediatric massive transfusion but also to highlight future directions in this field and present ideas for future study to improve the care and outcomes of pediatric patients.
Definition of Massive Transfusion in Children
Over the last several years, multiple pediatric studies have attempted to define MT in the pediatric population as the initial step towards developing prediction tools and refining protocols using retrospective data sets. This difficulty in a universal definition is apparent in the adult transfusion literature as well, with multiple competing definitions of MT, all seeking to adequately describe this patient population. This attempt to create a standardized definition as the foundation of further inquiry has been limited by the heterogeneity of patient populations studied and the difficulty in acquiring the granularity of detail required to adequately describe each MTP activation.8–12
As such, many pediatric MT definitions are based on the volume of blood products transfused as a function of the estimated total blood volume for the child over a 4 h, 12 h or 24-h period.6,13–15 These rather crude definitions are borne out of the difficulty in accessing large amounts of pooled patient data with the appropriate level of detail. A recent study of children treated in a combat environment indicated that roughly half of the total blood volume transfused within a 24-h period reliably identified critically injured children at risk for death.16 Given this 24-h time frame, the introduction of survival bias may have confounded the assessment in that some children did not survive past the first 24 h or survive long enough to meet the 40mL/kg/24hr threshold. Additionally, the predominance of blast and other penetrating mechanisms did not generally reflect the average pediatric trauma patient who is injured from more common mechanisms. To limit survival bias and define MT in a more “representative” pediatric trauma population, a recent Trauma Quality Improvement Project (TQIP) study of civilian trauma patients with and without traumatic brain injury found that this same mortality threshold existed above 37mL/kg/4hr time point.17 As with many advances in medicine, current transfusion practices have required time to evolve and gain broad clinical acceptance. In many ways, periods of armed conflict tend to accelerate the pace of adoption and refinement, especially with trauma care. As such, the genesis of current practice and the story of MT is told in the context of US military practice during the Global War on Terror.18 As such, there exists an ever-evolving definition of pediatric massive transfusion (Table 1).3,16,17,19–21
 |
Table 1 Definitions of Massive Transfusion in Pediatric Literature3,16,17,19-21 |
Population at Risk
From 2010 to 2012, 13,523 (4%) of the 356,583 pediatric patients in the National Trauma Data Bank required any amount of blood product transfusion within 24 h of injury. Of that group, 173 children (0.04%) required MT.7 While pediatric MTP activation is a fairly rare event, defining the population that will benefit is important to increase their survival. As such, it is important to note changing blood volumes across stages of development. Neonates may require MT by virtue of the relatively small amount of absolute blood loss that they can tolerate before profound shock results when compared to children and adolescents (Figure 1).22
 |
Figure 1 Estimated blood volume in milliliters/kg from preterm infants to childhood. Data from Coté et al.22 |
Patients who suffer traumatic brain injury are a subset of trauma patients who require specific attention in regards to limiting shock as they have a high incidence of coagulopathy.23 Three distinct patterns of coagulation dysregulation: clot strength abnormalities, fibrinolysis abnormalities, and depletion in clotting factors are seen in these patients and all are associated with mortality and disability.24 The use of fresh frozen plasma (FFP) in patients with severe traumatic brain injuries promotes sustained fibrinolysis and worsens the prognosis for these children. As such the use of FFP should be judicious and not used solely as a means to achieve a specific International Normalized Ratio (INR). Rather, FFP should be used to treat bleeding in the setting of prolonged prothrombin time (PT) or prolonged “R time” on thromboelastography.6,25
Triggers for Massive Transfusion and Predictors of Massive Transfusion
Massive transfusion protocol activation often occurs in a time-sensitive, emergent fashion. Given that blood product delivery and transfusion is a highly regulated and coordinated process, it requires some lead time. Thus, the ability to rapidly predict MTP activation ahead of its need is an area of great interest in the trauma community. Multiple rapid scoring systems have been devised in the adult transfusion literature to predict the need for MT protocol activation. These rapid prognostic systems rely on admission physiologic parameters, lab values, associated injuries and etiology of hemorrhage. Moreover, these scoring systems were developed in the context of hemorrhage from traumatic injury, and in all cases, these predictions scoring systems are validated by multiple follow-up studies. For children, no such tool exists that takes into account the differences in physiology and common injury patterns that predominate in pediatric trauma.26 However, the ideal pediatric MT prognostic tool would capture prehospital or admission hemodynamic variables, be simple, and would integrate automatically within the workflow of the resuscitation team. At present, the only validated scoring systems for children relay mortality prediction capabilities rather than signaling the need for MT.27
For the majority of pediatric hospitals, the decision to activate the MT protocol is at the discretion of the clinician. Because MTs are a rare event in children, the notion of using clinical judgement in an ad hoc fashion can lead to inconsistent utilization and ineffective transfusion or blood product mismanagement. In an effort to improve the quality of MT implementation, some pediatric centers have simply designated every high acuity trauma activation as the single criteria for the release of the first packet of blood products.14 Other centers have taken a more selective approach by requiring the patient to “fail” initial crystalloid challenge as a partial or non-responder prior to activating the MTP.28 Given the paucity of data regarding which children will need massive transfusion, simple and straightforward criteria are the most likely to ensure timely access to blood products. Further investigation to establish novel pediatric-specific prediction algorithms or validate the existing adult criteria for children is needed.
Complications
As in the adult population, the transfusion of blood products into pediatric patients carries a significant risk of complications ranging from mild to lethal. These risks include electrolyte abnormalities, coagulation dysregulation, immunologic reactions to the blood, volume overload, or a combination of effects.
The most common complication of transfusion, regardless of age, is metabolic disturbances. Among these, post-transfusion hypocalcemia predominates because of the chelation of calcium by the citrate preservatives in PRBCs. While important for all children, this issue is extremely pertinent to the neonatal population, as these patients have a decreased ability to metabolize citrate. Hypocalcemia countermeasures for this group include decreasing the rate of transfusion or providing supplemental IV calcium.29 The second most common electrolyte disturbance is hyperkalemia, which arises from extracellular potassium which increases with time of blood stored. Post transfusion hyperkalemia is repeatedly implicated in fatal cardiac arrhythmias during pediatric MT. Measures to reduce this transfusion-related hyperkalemia include: early replacement of anticipated blood loss, the use of larger than 23 gauge peripheral intravenous access over central access to prevent hemolysis as the blood travels through long narrow plastic tubes, frequent electrolyte evaluation, and the replacement and use of the freshest packed red blood cells (PRBCs) in the blood bank.30 In an effort to avoid hyperkalemia from older blood stores, many blood banks have adopted a “last in, first out” approach wherein the newest blood is utilized preferentially over older blood stores, especially in the case of large medical centers where blood banks are servicing both adults and children.
Transfusion-induced coagulopathy is another significant complication associated with blood product administration. Like electrolyte disturbances, it is largely iatrogenic. However, inherent patient pathophysiology from severe injury or hemorrhage can accentuate the degree of dysregulation. For example, patients who have undergone severe trauma have activation of the protein C pathway. Protein C inactivates factor V and factor VIII, leading to impaired clot formation. What’s more, activated protein C depletes plasminogen activator inhibitor (PAI-1) leading to unregulated tissue plasminogen activator (tPA) which in turn leads to fibrinolysis and depletion of fibrinogen reserves.31 An abnormal PT, partial thromboplastin time (PTT), and platelet count are strongly associated with mortality. In one particular study, PT prolongation remained the most common abnormality that positively correlated to mortality even after multivariate analysis adjusted for injury severity score.32 These coagulation disturbances can be exacerbated by the delivery of blood products in an “unbalanced” fashion where certain products, like PRBCs, are given in ratios that far exceed the normal constituents of blood. For this reason, there has been considerable interest in transfusion medicine in determining the optimal ratio of individual blood products to improve mortality while limiting any transfusion-related morbidity.
In addition to electrolyte derangement and coagulopathy, immunologic reactions can arise resulting in ABO incompatibility, transfusion-related acute lung injury (TRALI), transfusion-associated circulatory overload (TACO), and alloimmunization which can all worsen the clinical condition.33 Graft versus host disease can be seen in family members who wish to donate to their children due to donor lymphocytes not being recognized as foreign and proliferating and mounting a response against the host.29 The safeguards enacted by modern United States Blood Banks and the FDA aim to ensure the proper donation, processing, testing and storage of blood products to prevent these reactions.
Ratios of Blood Products
The highest-quality evidence for optimal blood product ratios arises from the adult trauma literature and several of the prospective observational trials arising from the US military transfusion experience in Iraq and Afghanistan. These findings informed much of the pediatric care in those combat zones and have influenced the civilian population by extension.
In 2007, The Journal of Trauma published a retrospective study of combat massive transfusion data in adults that compared a FFP to PRBC ratio of 1:8, 1:2.5 and 1:1.4 and found that the high ratio, 1:1.4, was independently associated with improved survival.34 This description was quickly followed by reports in a civilian cohort of patients receiving 1:1 FFP:PRBCs. While there was no discernable survival benefit, the authors did note a decrease in mortality related to coagulopathy. These initial studies paved the way for prospective trials to help define optimal ratios of blood products.
The two major prospective clinical trials which formed the basis of the contemporary approach to massive transfusion were the Prospective Observational Multicenter Major Trauma Transfusion (PROMMTT) and the Pragmatic Randomized Optimal Platelet and FFP Ratios (PROPPR) trials. The PROMMTT Study Group included 10 level I trauma centers in the United States.35 A resuscitation intensity of four or more had a greater than two times increase in mortality at 6 h. They concluded that a higher ratio of FFP and platelet administration decreased mortality in patients who received at least three units of blood in the first 24 h. In the first 6 h, patients with ratios less than 1:2 FFP:PRBCs had increased mortality. However, after the first 24 h ratios did not correlate with mortality risk.36 The PROPPR trial evaluated FFP, platelets and PRBC ratio of 1:1:1 compared to 1:1:2 in patients needing massive transfusion at twelve level I trauma centers in North America in a 16 month period. They concluded no difference in mortality but decreased death by exsanguination and increased achievement of hemostasis in the 1:1:1 group. Complication rates were high in both groups, 87.9% in the 1:1:1 vs 90.6% in the 1:1:2 group and were widely varied to include systemic inflammatory response syndrome (SIRS), deep venous thrombi (DVTs) and infection to name a few.37 These prospective trials were monumental in supporting 1:1:1 balanced resuscitation in adults.
While the PROMMTT Study Group found that high early resuscitation intensity may be an indicator of mortality in adults, a review of the pediatric resuscitation practices using military data showed a shift towards a hemostatic resuscitation and that mortality actually decreased over time in pediatric patients who received MT.38 These military evaluations laid the framework for balanced resuscitation in pediatric MT.
These landmark trials provided the clinical rationale to investigate the benefits of a balanced transfusion at pediatric trauma patients. Cannon et al, studied pediatric trauma patients from the Department of Defense (DOD) Trauma Registry from 2001 to 2013. Defining massive transfusion as greater than or equal to 40 mL/kg total blood products in 24 h they concluded that a high FFP:PRBC ratio did not confer survival.21 More recently, Cunningham et al, published a retrospective review of the Pediatric TQIP data looking at low (less than 1:2), medium (greater than or equal to 1:2, less than 1:1) and high (greater than or equal to 1:1) FFP and platelet to PRBC ratios. They found a survival benefit in those in the high ratio group with regards to FFP, low vs medium vs high; at 4 h: 14% vs 14% vs 2%, p = <0.01 and at 24 h: 23% vs 24% vs 12%, p = 0.02. They found no difference with platelet groups.17 Noland et al also found survival benefit with a 1:1 ratio of PRBC:FFP in pediatric patients receiving massive transfusion.39 In the absence of high quality prospective observational trials, these retrospective analyses are the current best evidence to support use of 1:1 ratios of PRBCs and FFP in pediatric massive transfusion protocols.
Current Variations in Protocol
Obtaining admission labs and calculating injury severity scores is challenging in the midst of a complex trauma resuscitation and can impede protocol activation. Survivor bias, defined as excluding those patients who die before reaching the hospital or meeting the criteria for massive transfusion, presents another opportunity for protocol deviation. While a majority of protocols are in place with the knowledge that survivor bias occurs, others aim to reach more patients and lower the threshold of MT.9 As such, the concept of a “critical administration threshold” (CAT) can be helpful. CAT is defined as three units of blood in 1 h and provides insight into how aggressive the bleeding and the corresponding resuscitation actually is.
In the first 5 years of development of pediatric massive transfusion protocols, only two thirds of trauma centers had adopted a protocol, and the majority did not include FFP or platelets in their first set of products administered.8 As increased awareness of coagulopathy grew, this led to earlier transfusion of FFP and platelets than the initial protocols described. What’s more, testing for coagulopathy has become more sophisticated with increased use of thromboelastography and rotational thromboelastometry to guide resuscitation.40 These advancements have helped to standardize adult massive transfusion.
In pediatric massive transfusion, Horst et al have studied the majority of the conclusions we have about variation in protocol for pediatric massive transfusion protocols. First, without accurate pediatric MT prediction scoring systems, physician discretion is the current predominant factor for protocol activation. This introduces inherent variability. Second, the majority but not all sites use a greater than or equal to 1:2 FFP: PRBC ratio. Third, most sites have type-O PRBCs immediately available but less than half of the sites have thawed FFP immediately available. Finally, pediatric MTP initiation is very rare, with a median 6 activations per center per year.20 Overactivation leads to wasted blood products. Given these factors, a great deal of variability exists among pediatric massive transfusion protocols and highlights the need for further research to establish clear activation criteria.
Barriers to MTP Adherence and Implementation
Pediatric massive transfusion protocol activation is a fairly rare event and heralds a high probability of morbidity and mortality. The rarity poses a challenge in creating, implementing, standardizing and evaluating these pediatric massive transfusion protocols. However, the process of quality assurance (QA) requires accurate definition of terms. Pediatric massive transfusion protocol activation is a binary event, it is either activated or not.
There are many challenges that face institutions in their evaluation of adherence and implementation of pediatric massive transfusion protocols. These can be further subdivided into systems issues and in factors unique to that particular trauma resuscitation. Common systems issues include lack of properly formatted nurse recording sheets for MT or simply a failure to document the MTP activation at all, a lack of a principal investigator at the institution and inattentiveness to tracking blood-related complications. The Broxton MTP Evaluation Tool was created to standardize and collect information regarding MTP activations to improve the QA process.41 The creators examined their own institution’s processes and the barriers they encountered were mainly due to deficiencies in the electronic medical record to streamline data entry during MTP activation. There was no set EMR workspace for documentation of a massive transfusion, any attempts at retrospective evaluation of massive transfusion and its efficacy were particularly challenging. The immediate clinical barriers that can actually impact the patient at the point of care include unavailable supplies, delays in the arrival of blood products, lack of adherence to the appropriate resuscitative ratios and absence of an “after action” reporting system that would identify opportunities for improvement in the system or team performance. Horst et al analyzed 46 pediatric massive transfusion protocols.20 The analysis highlights many common challenges. Fifty-three percent of centers with a pediatric massive transfusion protocol had a ratio of 1:1 FFP:PRBCs established for their first round of resuscitation. Thus, little more than half of the centers are even attempting the initial recommended transfusion ratios. Additionally, 89% of responding pediatric trauma centers had emergency release type-O blood immediately available. Furthermore, less than half (48%) of centers had thawed FFP immediately available. Nationwide MT experience is still growing and robust data collection and accumulation will be required before any meaningful data-driven recommendations on best practice will be possible. At present, the paltry data can lead to erroneous assumptions about the ultimate morbidity and mortality benefit of MTP. For example, a recent literature review of outcomes before and after implementation of a pediatric massive transfusion protocol found no difference in mortality post protocol implementation despite the fact that the post-protocol patients did receive a higher ratio of platelets to RBCs and the transfusions tended to be more balanced. Yet, it is difficult to make any meaningful conclusion on data that encapsulates a total of 43 patients over 9 years.42 Clearly, more studies are needed to evaluate adherence of pediatric massive transfusion protocols and barriers to them.
Whole Blood
The increasing adoption of whole blood transfusion during the last 2 decades by the United States military during combat operations has resulted in a robust experience with whole blood in children cared for in military treatment facilities. As such, there is renewed interest in the use of whole blood in the pediatric trauma population whereas whole blood was largely relegated to adults. The often cited benefit of whole blood is that it already possesses the ideal ratio of red blood cells, FFP and platelets. In fact, separating whole blood into its constituent components results in the delivery of three times the volume of anticoagulant and additives compared to whole blood (Table 2).43 This is the reason that advocates for whole blood assert that it minimizes dilutional coagulopathy and essentially restores what was lost through hemorrhage (Table 3).44
 |
Table 2 Anticoagulants and Additives: Whole Blood versus Component Therapy |
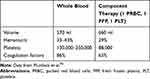 |
Table 3 Comparing Whole Blood to Component Therapy |
The major concern with widespread whole blood utilization is the theoretical risk of allogeneic transfusion reactions and massive hemolysis arising from a type-O donor’s anti-A or anti-B antibodies attacking the recipient's RBCs. To mitigate this risk, many adult trauma centers have implemented low-titer group O whole blood by using a platelet-sparing filter. Use of whole blood processed in this fashion resulted in no increased hemolysis, even days after transfusion. Despite growing preference amongst trauma practitioners for whole blood, the logistical challenges of whole blood availability and a sufficient pool of low-titer O negative donors have prevented more mainstream adoption in clinical practice. While in the adult population, the preference is for O-positive blood. In the pediatric population, the preference is for O-negative blood.45
Initial reports of pediatric whole blood transfusion during trauma bay resuscitation indicate that up to 20mL/kg of warm whole blood administered as the initial transfusion is safe and effective. This experience with eighteen patients greater than 15 kg from the Children’s Hospital of Pittsburgh allowed efficient delivery of a whole blood within 15 min of arrival without any transfusion-related complications. Additionally, concerns of platelet dysfunction arising from cold storage of the whole blood seem to be unfounded in early reports.46,47 Whole blood is safe, effective and less coagulopathic. Whole blood use should be considered in pediatric massive transfusion protocols.
Adjuncts to MTP
A majority of literature surrounding massive transfusion protocols in children and adults has focused on whole blood, packed red blood cells, FFP and platelet administration. While the importance of the balanced resuscitation concept has been repeatedly emphasized, more studies are needed. There is also a paucity of data to guide the use of adjuncts such as tranexamic acid and factor VII in children. Moving forward, the focus should broaden to include both a balanced transfusion strategy combined with adjuncts to combat the hyperfibrinolysis that accompanies the acute coagulopathy following massive hemorrhage.
Tranexamic acid (TXA) is a lysine analogue that prevents fibrinolysis by inhibiting plasminogen activation. This leads to inhibition of plasmin and the subsequent breakdown of fibrin leading to clot disruption. Multiple studies in both civilian and military trauma populations have provided only modest results. However, the United States and British military experiences and widespread usage of TXA in Operation Enduring Freedom prompted the evaluation of its use in severely injured children. The PED-TRAX study evaluated pediatric trauma patients with predominantly blast or penetrating injury mechanisms who received tranexamic acid as part of their massive transfusion in the same fashion as the adult counterparts treated at the same military hospital.48 Approximately 10% of their pediatric trauma patients received TXA and they found that it was independently associated with a decrease in mortality. No adverse effects were identified with its use. A recent meta-analysis of six randomized control trials showed that tranexamic acid administration had a statistically significant reduction in mortality and expanding hematoma with no statistically significant worse outcomes when compared to placebo.49 An additional recent study evaluated intraosseous administration of tranexamic acid in the setting were intravenous access was a challenge and demonstrated that intraosseous and intravenous tranexamic acid had similar efficacy.50 Based on these results, use of tranexamic acid should be considered in the severely bleeding child and administered by IV or IO access.
In addition to tranexamic acid, there are studies on the use of recombinant factor VIIa (rFVIIa) in the reversal of profound coagulopathy in patients needing massive transfusion. Compared to FFP, rFVIIa may provide similar efficacy with a much lower volume. Its use may be important in patients for whom fluid overload is a risk. This therapy serves as an adjunct to massive transfusion, but not as a means to replace endogenous factor VII (FVII) levels. There has been some controversy over its use due to concern that it may contribute to an increased risk of venous thromboembolism (VTE) formation. Studies have shown that rFVIIa can decrease total product needed during resuscitation, there is no increased VTE formation, and could be implemented into massive transfusion protocols to utilize adjuncts and decrease total product needed.11
While tranexamic acid and rFVIIa are useful adjuncts that can be administered to the patient, the notion of point of care coagulation testing to guide administration of blood products seems to hold the most promise. Thromboelastography (TEG) and rotational thromboelastometry (ROTEM) are two blood testing platforms that are now widely used in the adult trauma population.6 Often patients who require massive transfusion hemorrhage at a rate that exceeds the efficacy of typical laboratory studies to guide product administration. TEG and ROTEM provide real-time feedback to guide product administration and can identify the development of hyperfibrinolysis, prompting antifibrinolytic countermeasures such as tranexamic acid. While TEG and ROTEM parameters have been set for pediatric patients, their use is not mainstream in pediatric massive transfusion. Careful consideration should be given to the implementation of TEG and ROTEM in pediatric massive transfusion protocols and further research is needed to determine its true efficacy for children.
Future Directions
Opportunities abound for research of massive transfusion protocols, their efficacy, their complications and their adherence. Moving forward, it is important to consider common biases and pitfalls of outcomes research in order to mitigate these and produce the most critical conclusions. These biases are described by del Junco et al and include indication bias, survival bias, confounding bias and assumption bias.51 While guidelines and recommendations are coming into focus for adults, they are still needed for children. Kamyszek et al write of the need for a national focus to improve the implementation and standardization of massive transfusion protocols in children.52
The pediatric critical care community continues to search for new advances in resuscitation.53,54 The military is researching hemoglobin-based oxygen carriers (HBOCs). The current goal would be to be able to use HBOCs when PRBCs are not available. The challenge is to manufacture HBOCs with storage and production streamlined to keep costs to a minimum. HBOC has been evaluated in clinical trials and does not increase mortality when compared to up to 3 units of PRBCs. Massive transfusion for both adults and children is an expanding and evolving field with its own challenges and exciting new opportunities for research and protocol development.
Moving forward, judicious resource utilization will be a priority for health systems. At present, no one protocol exists for pediatric massive transfusion. Targeted transfusion protocols were found to have reduced overall product requirements than massive transfusion protocols but did not change overall mortality.55 These protocols were developed to guide the team through the resuscitation of patients with polytrauma with or without brain injury and penetrating trauma with brain injury. These protocols emphasize frequent re-evaluation of the patient, precision and optimization of RBC administration. Prospective multi-institutional data collection is occurring. Trappey et al have developed a consensus statement amongst four major pediatric centers and developed an algorithm for pediatric massive transfusion with the goal to study tranexamic acid in children.56 These resource conscious advancements and collaborative efforts by institutions will be vital to generating high-quality data to shape the future of pediatric massive transfusion.
Disclosure
The authors report no conflicts of interest in this work.
References
1. Dzik WH, Blajchman MA, Fergusson D, et al. Clinical review: Canadian National advisory committee on blood and blood products–massive transfusion consensus conference 2011: report of the panel. Crit Care. 2011;15(6):242. doi:10.1186/cc10498
2. Stewart RM, Nathens AB, Michael C, Chang MD, FACS, Chair TQIP Committee. 2016:128. NTDB Reports and Publications. American College of Surgeons. Available from: https://www.facs.org/quality-programs/trauma/tqp/center-programs/ntdb/docpub. Accessed May 2, 2020.
3. Edwards MJ, Lustik M, Eichelberger MR, Elster E, Azarow K, Coppola C. Blast injury in children: an analysis from Afghanistan and Iraq, 2002–2010. J Trauma Acute Care Surg. 2012;73(5):1278–1283. doi:10.1097/TA.0b013e318270d3ee
4. Tasker RC, Turgeon AF, Spinella PC. Pediatric Critical Care Transfusion and Anemia Expertise Initiative (TAXI), Pediatric Critical Care Blood Research Network (BloodNet), and the Pediatric Acute Lung Injury and Sepsis Investigators (PALISI) Network. Recommendations on RBC Transfusion in Critically Ill Children With Acute Brain Injury From the Pediatric Critical Care Transfusion and Anemia Expertise Initiative. Pediatr Crit Care Med. 2018;19(9SSuppl 1):S133–S136. doi:10.1097/PCC.0000000000001589
5. Paterson NA. Validation of a theoretically derived model for the management of massive blood loss in pediatric patients - a case report. Paediatr Anaesth. 2009;19(5):535–540. doi:10.1111/j.1460-9592.2009.02982.x
6. Diab YA, Wong ECC, Luban NLC. Massive transfusion in children and neonates. Br J Haematol. 2013;161(1):15–26. doi:10.1111/bjh.12247
7. Shroyer MC, Griffin RL, Mortellaro VE, Russell RT. Massive transfusion in pediatric trauma: analysis of the National Trauma Databank. J Surg Res. 2017;208:166–172. doi:10.1016/j.jss.2016.09.039
8. Schuster KM, Davis KA, Lui FY, Maerz LL, Kaplan LJ. The status of massive transfusion protocols in United States trauma centers: massive transfusion or massive confusion? Transfusion (Paris). 2010;50(7):1545–1551. doi:10.1111/j.1537-2995.2010.02587.x
9. Savage SA, Zarzaur BL, Croce MA, Fabian TC. Redefining massive transfusion when every second counts. J Trauma Acute Care Surg. 2013;74(2):
10. Stanworth SJ, Morris TP, Gaarder C, et al. Reappraising the concept of massive transfusion in trauma. Crit Care. 2010;14(6):R239. doi:10.1186/cc9394
11. Sihler KC, Napolitano LM. Massive transfusion: new insights. Chest. 2009;136(6):1654–1667. doi:10.1378/chest.09-0251
12. Sommer N, Schnüriger B, Candinas D, Haltmeier T. Massive transfusion protocols in nontrauma patients: a systematic review and meta-analysis. J Trauma Acute Care Surg. 2019;86(3):493–504. doi:10.1097/TA.0000000000002101
13. Pickett PM, Tripi PA. Massive transfusion protocol in pediatric trauma. Int Anesthesiol Clin. 2011;49(2):62–67. doi:10.1097/AIA.0b013e3181f955d8
14. Hendrickson JE, Shaz BH, Pereira G, et al. Implementation of a pediatric trauma massive transfusion protocol: one institution’s experience. Transfusion (Paris). 2012;52(6):1228–1236. doi:10.1111/j.1537-2995.2011.03458.x
15. Chidester SJ, Williams N, Wang W, Groner JI. A pediatric massive transfusion protocol. J Trauma Acute Care Surg. 2012;73(5):1273–1277. doi:10.1097/TA.0b013e318265d267
16. Neff LP, Cannon JW, Morrison JJ, Edwards MJ, Spinella PC, Borgman MA. Clearly defining pediatric massive transfusion: cutting through the fog and friction with combat data. J Trauma Acute Care Surg. 2015;78(1):
17. Cunningham ME, Rosenfeld EH, Zhu H, Naik-Mathuria BJ, Russell RT, Vogel AM. A high ratio of plasma: RBC improves survival in massively transfused injured children. J Surg Res. 2019;233:213–220. doi:10.1016/j.jss.2018.08.007
18. Cap AP, Spinella PC, Borgman MA, Blackbourne LH, Perkins JG. Timing and location of blood product transfusion and outcomes in massively transfused combat casualties. J Trauma Acute Care Surg. 2012;73(2 Suppl 1):S89–S94. doi:10.1097/TA.0b013e318260625a
19. Nosanov L, Inaba K, Okoye O, et al. The impact of blood product ratios in massively transfused pediatric trauma patients. Am J Surg. 2013;206(5):655–660. doi:10.1016/j.amjsurg.2013.07.009
20. Horst J, Leonard JC, Vogel A, Jacobs R, Spinella PC. A survey of US and Canadian hospitals’ paediatric massive transfusion protocol policies. Transfus Med. 2016;26(1):49–56. doi:10.1111/tme.12277
21. Cannon JW, Johnson MA, Caskey RC, Borgman MA, Neff LP. High ratio plasma resuscitation does not improve survival in pediatric trauma patients. J Trauma Acute Care Surg. 2017;83(2):211–217. doi:10.1097/TA.0000000000001549
22. Coté CJ, Grabowski EF, Stowell CP. Strategies for blood product management, reducing transfusions, and massive blood transfusion. In: A Practice of Anesthesia for Infants and Children.
23. Talving P, Lustenberger T, Lam L, et al. Coagulopathy after isolated severe traumatic brain injury in children. J Trauma. 2011;71(5):1205–1210. doi:10.1097/TA.0b013e31820d151d
24. Leeper CM, Neal MD, McKenna C, Billiar T, Gaines BA. Principal component analysis of coagulation assays in severely injured children. Surgery. 2018;163(4):827–831. doi:10.1016/j.surg.2017.09.031
25. Leeper CM, Neal MD, Billiar TR, Sperry JL, Gaines BA. Overresuscitation with plasma is associated with sustained fibrinolysis shutdown and death in pediatric traumatic brain injury. J Trauma Acute Care Surg. 2018;85(1):12–17. doi:10.1097/TA.0000000000001836
26. Shih AW, Al Khan S, Wang AY-H, et al. Systematic reviews of scores and predictors to trigger activation of massive transfusion protocols. J Trauma Acute Care Surg. 2019;87(3):717–729. doi:10.1097/TA.0000000000002372
27. Borgman MA, Maegele M, Wade CE, Blackbourne LH, Spinella PC. Pediatric trauma BIG score: predicting mortality in children after military and civilian trauma. Pediatrics. 2011;127(4):e892–e897. doi:10.1542/peds.2010-2439
28. Dehmer JJ, Adamson WT. Massive transfusion and blood product use in the pediatric trauma patient. Semin Pediatr Surg. 2010;19(4):286–291. doi:10.1053/j.sempedsurg.2010.07.002
29. Barcelona SL, Thompson AA, Coté CJ. Intraoperative pediatric blood transfusion therapy: a review of common issues. Part I: hematologic and physiologic differences from adults; metabolic and infectious risks. Paediatr Anaesth. 2005;15(9):716–726. doi:10.1111/j.1460-9592.2005.01548.x
30. Lee AC, Reduque LL, Luban NLC, Ness PM, Anton B, Heitmiller ES. Transfusion-associated hyperkalemic cardiac arrest in pediatric patients receiving massive transfusion. Transfusion (Paris). 2014;54(1):244–254. doi:10.1111/trf.12192
31. Callum JL, Rizoli S. Assessment and management of massive bleeding: coagulation assessment, pharmacologic strategies, and transfusion management. Hematol Am Soc Hematol Educ Program. 2012;2012:522–528. doi:10.1182/asheducation-2012.1.522
32. Hendrickson JE, Shaz BH, Pereira G, et al. Coagulopathy is prevalent and associated with adverse outcomes in transfused pediatric trauma patients. J Pediatr. 2012;160(2):204–209.e3. doi:10.1016/j.jpeds.2011.08.019
33. Sihler KC, Napolitano LM. Complications of massive transfusion. Chest. 2010;137(1):209–220. doi:10.1378/chest.09-0252
34. Borgman MA, Spinella PC, Perkins JG, et al. The ratio of blood products transfused affects mortality in patients receiving massive transfusions at a combat support hospital. J Trauma. 2007;63(4):805–813. doi:10.1097/TA.0b013e3181271ba3
35. Rahbar E, Fox EE, Del Junco DJ, et al. Early resuscitation intensity as a surrogate for bleeding severity and early mortality in the PROMMTT study. J Trauma Acute Care Surg. 2013;75(1 Suppl 1):S16–S23. doi:10.1097/TA.0b013e31828fa535
36. Holcomb JB, Del Junco DJ, Fox EE, et al. The prospective, observational, multicenter, major trauma transfusion (PROMMTT) study: comparative effectiveness of a time-varying treatment with competing risks. JAMA Surg. 2013;148(2):127–136. doi:10.1001/2013.jamasurg.387
37. Holcomb JB, Tilley BC, Baraniuk S, et al. Transfusion of plasma, platelets, and red blood cells in a 1:1:1 vs a 1:1:2 ratio and mortality in patients with severe trauma: the PROPPR randomized clinical trial. JAMA. 2015;313(5):471–482. doi:10.1001/jama.2015.12
38. Cannon JW, Neff LP, Pidcoke HF, et al. The evolution of pediatric transfusion practice during combat operations 2001–2013. J Trauma Acute Care Surg. 2018;84(6SSuppl 1):S69–S76. doi:10.1097/TA.0000000000001869
39. Noland DK, Apelt N, Greenwell C, et al. Massive transfusion in pediatric trauma: an ATOMAC perspective. J Pediatr Surg. 2019;54(2):345–349. doi:10.1016/j.jpedsurg.2018.10.040
40. Levi M, Fries D, Gombotz H, et al. Prevention and treatment of coagulopathy in patients receiving massive transfusions. Vox Sang. 2011;101(2):154–174. doi:10.1111/j.1423-0410.2011.01472.x
41. Broxton S, Medeiros R, Schumacher A. Evaluation tool for assessing a newly implemented massive transfusion protocol. J Trauma Nurs. 2017;24(3):164–169. doi:10.1097/JTN.0000000000000285
42. Hwu RS, Spinella PC, Keller MS, Baker D, Wallendorf M, Leonard JC. The effect of massive transfusion protocol implementation on pediatric trauma care. Transfusion (Paris). 2016;56(11):2712–2719. doi:10.1111/trf.13781
43. Spinella PC, Perkins JG, Grathwohl KW, Beekley AC, Holcomb JB. Warm fresh whole blood is independently associated with improved survival for patients with combat-related traumatic injuries. J Trauma. 2009;66(4 Suppl):S69–S76. doi:10.1097/TA.0b013e31819d85fb
44. Murdock AD, Berséus O, Hervig T, Strandenes G, Lunde TH. Whole blood: the future of traumatic hemorrhagic shock resuscitation. Shock. 2014;41(Suppl 1):62–69. doi:10.1097/SHK.0000000000000134
45. Young PP, Borge PD. Making whole blood for trauma available (again): the AMERICAN Red Cross experience. Transfusion (Paris). 2019;59(S2):1439–1445. doi:10.1111/trf.15166
46. Leeper CM, Yazer MH, Cladis FP, Saladino R, Triulzi DJ, Gaines BA. Use of uncross matched cold-stored whole blood in injured children with hemorrhagic shock. JAMA Pediatr. 2018;172(5):491–492. doi:10.1001/jamapediatrics.2017.5238
47. Leeper CM, Yazer MH, Cladis FP, Saladino R, Triulzi DJ, Gaines BA. Cold-stored whole blood platelet function is preserved in injured children with hemorrhagic shock. J Trauma Acute Care Surg. 2019;87(1):49–53. doi:10.1097/TA.0000000000002340
48. Eckert MJ, Wertin TM, Tyner SD, Nelson DW, Izenberg S, Martin MJ. Tranexamic acid administration to pediatric trauma patients in a combat setting: the pediatric trauma and tranexamic acid study (PED-TRAX). J Trauma Acute Care Surg. 2014;77(6):
49. The efficacy of tranexamic acid for brain injury: a meta-analysis of randomized controlled trials. - PubMed - NCBI. Available from: https://www-ncbi-nlm-nih-gov.go.libproxy.wakehealth.edu/pubmed/31706661.
50. Lallemand MS, Moe DM, McClellan JM, et al. No intravenous access, no problem: intraosseous administration of tranexamic acid is as effective as intravenous in a porcine hemorrhage model. J Trauma Acute Care Surg. 2018;84(2):379–385. doi:10.1097/TA.0000000000001741
51. Del Junco DJ, Fox EE, Camp EA, Rahbar MH, Holcomb JB; PROMMTT Study Group. Seven deadly sins in trauma outcomes research: an epidemiologic post mortem for major causes of bias. J Trauma Acute Care Surg. 2013;75(1 Suppl 1):S97–S103. doi:10.1097/TA.0b013e318298b0a4
52. Kamyszek RW, Leraas HJ, Reed C, et al. Massive transfusion in the pediatric population: a systematic review and summary of best-evidence practice strategies. J Trauma Acute Care Surg. 2019;86(4):744–754. doi:10.1097/TA.0000000000002188
53. Dubick MA. What’s new in shock. Shock. 2019;52(1S):1. doi:10.1097/SHK.0000000000001418
54. Pusateri AE, Glassberg E, Weiskopf RB. Reassessment of the need for an oxygen carrier for the treatment of traumatic hemorrhage when blood is not an option. Shock. 2019;52(1S):55. doi:10.1097/SHK.0000000000001417
55. Paydar S, Khalili H, Sabetian G, et al. Comparison of the impact of applications of targeted transfusion protocol and massive transfusion protocol in trauma patients. Korean J Anesthesiol. 2017;70(6):626–632. doi:10.4097/kjae.2017.70.6.626
56. Trappey AF, Thompson KM, Kuppermann N, et al. Development of transfusion guidelines for injured children using a modified delphi consensus process. J Trauma Acute Care Surg. 2019;87(4):935–943. doi:10.1097/TA.0000000000002432
 © 2020 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2020 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
