Back to Journals » Drug Design, Development and Therapy » Volume 12
Macrocyclic lactones inhibit nasopharyngeal carcinoma cells proliferation through PAK1 inhibition and reduce in vivo tumor growth
Authors Gallardo F, Mariamé B , Gence R, Tilkin-Mariamé AF
Received 28 April 2018
Accepted for publication 19 June 2018
Published 7 September 2018 Volume 2018:12 Pages 2805—2814
DOI https://doi.org/10.2147/DDDT.S172538
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 3
Editor who approved publication: Professor Anastasios Lymperopoulos
Franck Gallardo,1 Bernard Mariamé,2 Remi Gence,3 Anne-Francoise Tilkin-Mariamé4
1NeoVirTech, SAS, Institut for Advanced Technology in Life Science (ITAV), Toulouse, France; 2ITAV USR3505 CNRS/LBME, UMR 5099 CNRS, Toulouse, France; 3INSERM UMR 1037, CRCT, University of Toulouse, Toulouse, France; 4INSERM 1220, IRSD, University of Toulouse, INSERM, INRA, ENVT, UPS, Toulouse, France
Purpose: The Epstein–Barr virus (EBV)-associated cancer nasopharyngeal carcinoma (NPC) is rare in Europe and North America but is a real public health problem in some regions of the world, such as southern Asia, North Africa, and for Inuit populations. Due to the anatomy and location of the nasopharynx, surgery is rarely used to treat primary NPC cancers. Treatment by radiotherapy, combined or not with chemotherapy, are efficient for primary tumors but often do not protect against fatal relapses or metastases.
Methods: Search for new therapeutic molecules through high content screening lead to the identification of Ivermectin (IVM) as a promising drug. IVM is a US Food and Drug Administration-approved macrocyclic lactone widely used as anthelmintic and insecticidal agent that has also shown protective effects against cancers.
Results: We show here that IVM has cytotoxic activity in vitro against NPC cells, in which it reduces MAPKs pathway activation through the inhibition PAK-1 activity. Moreover, all macrocyclic lactones tested and a PAK1 inhibitor are cytotoxic in vitro for EBV-positive and EBV-negative NPC tumor cells. We have also shown that IVM intraperitoneal repeated injections, at US Food and Drug Administration-approved doses, have no significant toxicity and decrease NPC subcutaneous tumors development in nude mice.
Conclusion: Macrocyclic lactones appear as promising molecules against NPC targeting PAK-1 with no detectable adverse effect.
Keywords: nasopharyngeal carcinoma, ivermectin, macrocyclic lactones, P21-activated kinase 1, cytotoxicity
Plain language summary
We have shown that Ivermectin (IVM), a widely used antihelminthic and anti-insecticidal macrocyclic lactone also has a promising antitumor activity against nasopharyngeal carcinoma (NPC). This cancer develops in the nasopharyngeal cavity, is tightly linked with Epstein–Barr Virus (EBV) and presents a remarkable geographic distribution with regions of markedly elevated incidence, while it is rare in the rest of the world, including Europe and North America. We found that macrocyclic lactones, including IVM, kill EBV-positive and EBV-negative NPC cells in vitro through inhibition of a kinase (PAK1) involved in a wide range of biological activities. We also found that IVM has in vivo anti-NPC activity as intraperitoneal repeated injections of IVM decrease NPC subcutaneous tumors development in immunodepressed mice. Therefore, IVM appears as a promising molecule against NPC, which is particularly interesting because of the scarcity of available efficient treatments against advanced NPC tumors.
Introduction
Nasopharyngeal carcinoma (NPC), also known as cavum cancer, develops in the upper part of the pharynx. Among head and neck tumors, NPC is a distinct entity due to its particular epidemiology and nearly absolute association with Epstein–Barr virus (EBV).1 It occurs in adults and also in young people, and when advanced, presents a high lethality. It is a heterogeneous tumor composed of large epithelial malignant cells and massive lymphocyte infiltration. Environmental, biological, and genetic factors play important roles in NPC pathogenesis. Several proliferative pathways including Wnt, PI3-K, MAPKs, and EGFR are aberrantly activated and are thought to contribute to tumorigenesis.2
The association between NPC and EBV is very tight, and the virus could play a causal role.3,4 Indeed, elevated EBV antibody titers precede the development of detectable NPC,5 and the virus is clonal in the tumor.3,4 EBV is an ubiquitous herpes virus infecting in a latent manner more than 95% of the adult human world population.6 Despite EBV ubiquity, NPC presents a remarkable geographic distribution with regions of markedly elevated incidence. The highest incidence occurs in South China and Southeastern Asia. A moderate incidence is found in some African and Mediterranean populations, in Inuits from Greenland and Alaska, and in Malays from Singapore and Malaysia. In the rest of the world, including Europe and North America, this pathology is rare, classified as orphan cancer.
From a clinical point of view, the NPC tumor localization often precludes surgery, and for a long time, radiotherapy has been the treatment of choice. However, in a great proportion of patients, even if this treatment is efficient on primary tumor, it does not protect against relapses or metastases, most often resulting in fatality. Indeed, after radiotherapy, about 50% of patients develop recurrence or metastasis for which no effective treatment exists nowadays. Metastases often develop in cervical lymph node, but also in lungs, bones, liver, or distal lymph nodes. To date, different protocols of chemotherapy, combined or not with radiotherapy, are under evaluation, and cisplatin seems promising.7 Innovative immunotherapies are also currently evaluated targeting EBV through EBV-specific cytotoxic-T lymphocytes and therapeutic vaccines.8,9 However the numerous therapeutic failures and the scarcity of available efficient treatments largely justify the search for new therapeutic molecules.
We have previously found that macrocyclic lactones, including Ivermectin (IVM, [22, 23-dihydroavermectin B1a]), kill melanoma cells in vitro and decrease lung metastatic implantation of murine and human melanoma cells in immunodepressed mice.10,11 IVM and some other macrocyclic lactones are well-tolerated agents that are used to treat millions of people for river blindness and other parasite infections, including onchocerciasis, strongyloidiasis, pediculosis, and scabies.12 They induce hyperpolarization of invertebrate nerve and muscle cells mostly by activating the invertebrate-specific glutamate-gated chloride channels.13 Importantly, macrocyclic lactones including IVM inhibit distinct pathways in invertebrates and in mammal tumors cells. It has been shown that IVM displays antitumor,14,15 antimicrobial, antibacterial, and anti-inflammatory activities,16 and recent dermatological clinical studies of IVM show that its human tolerability is excellent.17
We have demonstrated that PAK1 is the macrocyclic lactones’ target involved in their cytotoxicity against melanoma cells.10,11 PAK-1 (also known as serine/threonine-protein kinase PAK1 or P21 protein [Cdc42/Rac]-activated kinase 1) contributes to MAPK pathway activation. In this pathway, PAK1 phosphorylates CRAF at Ser 338 and MEK1 at Ser 298. PAK1 is a critical effector that links the Rho GTPases, especially Rac and Cdc42, to cytoskeleton reorganization and nuclear signaling. PAK1 has been implicated in a wide range of biological activities, including roles in cell transformation, inflammation, cell motility and morphology, and tumor growth and tumorigenesis.18–20 We have previously patented21 and we show here that macrocyclic lactones and a PAK1 inhibitor appear as promising therapeutic molecules for NPC because they are cytotoxic in vitro against EBV-positive and EBV-negative NPC tumor cells and because IVM intraperitoneal (IP) injections decrease NPC subcutaneous tumor growth in nude mice.
Materials and methods
Mice
Six- to eight-week-old immunodepressed female NMRI-nu/nu mice (JANVIER Labs, Le Genest-Saint-Isle, France) were used for in vivo experiments to limit immune response against NPC cells. All the experiments involving mice were done using appropriate conditions of husbandry experimentation and care, under the control of the Regional Comity of Midi-Pyrénées (France). For the guidelines on animal welfare, we followed the European directive 2010/63/ EU. For administration of substances and removal of blood, including routes and volumes, we followed the good practice guide published in 2001 by Diehl et al.22 The ethics committee, which approved the research, is CEEA-122, chaired by Dr Nicolas Cenac.
NPC cell lines and thymocytes in vitro culture
The 3 human NPC cell lines HONE-1 (EBV negative), CNE-1 (EBV negative), and C666-1 (EBV positive) were provided by Dr P Busson (UMR 8126, Gustave Roussy, Villejuif, France). These cell lines were maintained in culture by serial passages in Roswell Park Memorial Institute 1640 (RPMI 1640) medium supplemented with 10% fetal bovine serum, 1 mM glutamine, and 1% penicillin–streptomycin–amphotericin B. They were monthly tested to be mycoplasm-free.
Thymuses were harvested from 4-week-old female C57BL/6 mice, minced, and single-cell suspensions of thymocytes prepared and cultivated at 5×106 cells/well in 48 wells culture plates in the same medium.
In vitro evaluation of macrocyclic lactones and PAK1 inhibitor cytotoxicity on human NPC cells and on normal murine thymocytes
HONE-1, CNE-1, and C666-1 NPC cells or normal thymocytes were treated in vitro for 72 hours with 4 macrocyclic lactones, IVM, Selamectin, Moxidectin, or Abamectin (purchased from Sigma-Aldrich Co., St Louis, MO, USA), and 1 PAK1 inhibitor, FRAX-597 (purchased from CliniScience, Nanterre, France), at doses ranging from 0 to 10 μM. NPC cells were plated in triplicate in 12-wells culture plates in 2 mL of RPMI 1640 medium supplemented with 10% fetal bovine serum, 1 mM glutamine, and 1% penicillin–streptomycin–amphotericin B. Different initial cell concentrations were used for the 3 NPC cell lines. After 24 hours of culture to allow NPC cell adhesion, the supernatants were eliminated and replaced by vehicle medium (RPMI/DMSO 0.25%) alone or with increasing concentrations, from 1 to 10 μM, of macrocyclic lactones or PAK1 inhibitor. Surviving cells were counted after 72 hours of culture with a BD Accuri C6 FACS analyzer (BD Biosciences, San Jose, CA, USA) or with a Cellomics Arrayscan microscope (Thermo Fisher Scientific, Waltham, MA, USA).
In vivo evaluation of IVM on NPC growth using murine xenograft models
To evaluate the impact of repeated IP IVM injections on local subcutaneous NPC tumor growth, NMRI-nu/nu mice were injected subcutaneously (SC) into the flank with 1×106 HONE-1 or 2×106 CNE-1 tumor cells. Two days later and every following day, mice were IP injected with 0.1 mL of vehicle (PBS/DMSO 0.25%) or 0.1 mL of vehicle containing 3.25 mg/kg of IVM, a dose approximately corresponding to what is given as anthelmintic agent. Tumor growth was monitored in mice every 2–4 days by palpation and tumor surfaces were measured using digital caliper. Tumor-bearing mice were sacrificed at day 18 after tumor cells implantation. At this time, all mice were alive, behaved normally, and did not lose weight, while the tumors did not display ulcerations. Two groups of mice injected IP with vehicle vs IVM were compared. After SC injection of 1×106 HONE-1 cells, palpable tumors appeared around day 6 and grew progressively while after SC injection of 2×106 CNE-1 cells, substantial tumors were already present at day 5 and their subsequent growth was slow.
Western blot analyses
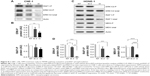
HONE-1 and CNE-1 cells were lysed in lysis buffer (20 mM Tris pH 7.6, 150 mM NaCl, 2 mM EDTA, 0.1% SDS, 1% Triton, proteases, and phosphatases inhibitor cocktail), and protein extracts were prepared by the standard procedure and then separated by sodium dodecyl sulfate–polyacrylamide (SDS-PAGE) gel electrophoresis (30 μg protein/lane). Proteins were blotted onto polyvinylidene difluoride (Hybond-P) membranes (GE Healthcare, Chicago, IL, USA). The filters were incubated at 4°C overnight with the different primary antibodies against p-ERK (Cell Signaling, Danvers, MA, USA, rabbit monoclonal antibody diluted at 1/3,000), ERK1/2 (Santa Cruz Biotechnology, Dallas, TX, USA, rabbit monoclonal antibody, diluted at 1/1,000), p-RAF1 (Merck Millipore, Billerica, MA, USA, mouse monoclonal antibody, diluted at 1/1,000), RAF1(R&D Systems, Minneapolis, MN, USA, mouse monoclonal antibody, diluted at 1/1,000), MEK 1/2 (rabbit monoclonal antibody from Cell Signaling, diluted at 1/1,000), and p-MEK (MEK 1/2 S217/221, rabbit monoclonal antibody from Cell Signaling, diluted at 1/1,000). Actin was used as a loading control (Chemicon; Merck Millipore). The Hybond-P membranes were then incubated with HRP-labeled secondary antibodies (R&D System and Cell Signaling) for 1 hour at room temperature and then detected with a chemiluminescence detection ECL kit (Thermo Scientific Pierce; Thermo Fisher Scientific). Immunoblots were revealed and quantified by chemiluminescence using a ChemiDoc MP system (Bio-Rad Laboratories Inc., Hercules, CA, USA).
Statistics
Significance was analyzed with Graph Pad (GraphPad InStat, GraphPad Software Inc., La Jolla, CA, USA) using impaired Student’s t-tests. A P-value <0.05 was considered significant. *P<0.05; **P<0.01, ***P<0.001, ****P<0.0001.
Results
Macrocyclic lactones and PAK1 inhibitor are cytotoxic for EBV-positive and EBV-negative NPC cells.
The capacity of the 4 macrocyclic lactones IVM, Selamectin, Moxidectin, and Abamectin and the PAK1 inhibitor FRAX-597 to kill NPC cells was tested using 3 human NPC cell lines, HONE-1, CNE-1, and C666-1. The cells were cultivated for 72 hours with several concentrations of macrocyclic lactones, PAK1 inhibitor, or with vehicle alone (RPMI/DMSO 0.25%). Surviving cells were counted using a BD Accuri C6 FACS analyzer (Figure 1) or a Cellomics Arrayscan microscope (Figure 2). Death of CNE-1 and HONE-1 cells, induced in vitro by IVM, is also illustrated in Figure 3. The 3 cell lines are similarly sensitive to the macrocyclic lactones and to PAK1 inhibitor (Figures 1 and 2) independently of their EBV status. The data allowed us to evaluate between 2 and 7 μM the IC50 of all compounds for the 3 NPC cell lines. All these data show that macrocyclic lactones and PAK1 inhibitor are cytotoxic in vitro to NPC cells independently of EBV presence.
Macrocyclic lactones and PAK1 inhibitor present no toxicity for normal thymocytes in vitro
Freshly extracted mouse thymocytes were used as normal control cells because T lymphocytes are very sensitive to anticancer therapy and white blood cells often collapse in patients receiving chemotherapy. As for NPC cells, thymocytes were cultivated for 72 hours with increasing concentrations of macrocyclic lactones, PAK1 inhibitor, or with vehicle alone (RPMI/DMSO 0.25%). Surviving thymocytes were counted using a BD Accuri C6 FACS analyzer (Figure 4). The data show that macrocyclic lactones and PAK1 inhibitor are not toxic for these control cells up to the dose of 10 μM.
IVM IP injections are not toxic in vivo in nude mice
To determine whether IVM could be used in preclinical phases for the treatment of NPC cells, we investigated the toxicity of this molecule in mice. The molecule was administered daily by IP injections in nude mice.
Thirteen nude mice were weighed before and after the repeated daily injections of vehicle (PBS/DMSO 0.25%) or IVM at 3.25 mg/kg for 18 days. Before injections, the average weight of mice was 25.8 g. After vehicle injections, the average weight was 27.7 g, and after IVM injections the average weight was 26.7 g. These results show that weight loss after IVM repeated IP injections compared to vehicle injections represents <4% and is not significant (Figure 5). Furthermore, mice behavior was not modified by these IP injections, and the number of circulating white blood cells in peripheral blood of mice after these injections showed no decrease (data not shown). Altogether, these results show that IVM repeated injections at the US Food and Drug Administration (FDA)-approved concentration, which we used in our experiments, are not toxic for mice in vivo.
IVM IP injections decrease NPC SC tumor growth in nude mice
In order to test the efficacy of IVM against NPC development in vivo, the 2 EBV-negative NPC cell lines were used. C666-1 cell line was discarded to avoid introducing the EBV in an unadapted animal facility. First, HONE-1 cells were injected SC in the flank of immunodepressed nude mice at a dose of 1×106 cells/mouse to produce local SC tumors. After tumor cell implantation, mice were left untreated for 48 hours, in order to allow HONE-1 cells to implant. Then, 6 mice were daily injected IP with 0.1 mL of vehicle and 5 mice with 3.25 mg/kg of IVM for 15 days. Tumors growth was measured every 2–3 days, showing that 4 out of 6 mice injected with vehicle alone (67%) but only 1 out of 5 mice injected with IVM (20%) developed growing tumors. Some tumor-bearing mice subsequently rejected their tumor, but only 1 (out of 5) in the control group (20%) vs 2 (out of 3) in the IVM-injected group (67%) (Figure 6A and B). Tumors sizes were reduced in IVM-injected group as compared to control group, but the difference was not significant due to the small number of mice in each group and the heterogeneity of tumor sizes (P=0.2 for day 14 and P=0.3 for days 16 and 18) (Figure 6C).
To confirm that IVM IP injections could reduce NPC tumor development in nude mice, CNE-1 cells were SC injected in 11 nude mice at a dose of 2×106 cells/mouse to produce local SC tumors. As in the previous experiment, mice were left untreated for 48 hours, in order to allow CNE-1 cells to implant and then divided in 2 groups: 4 mice were subsequently daily injected with vehicle (PBS/DMSO 0.25%) and 7 mice with IVM at 3.25 mg/kg. In the group of mice injected with vehicle alone, the tumors initially grew and then decreased slowly from day 8, suggesting that a spontaneous NK-induced antitumor immune response progressively reduced tumor size (Figure 7A). On the contrary, 6 out of the 7 mice injected with IVM significantly reduced their tumor and only 1 mouse of this group developed a tumor (Figure 7B). In both groups, 1 mouse had rejected its tumor at day 14 (Figure 7A and B). Tumors sizes were reduced in IVM-injected group as compared to control group, and the difference was significant at least until day 8 (P=0.004 for days 6 and 7 and P=0.01 for day 8 [Figure 7C]).
Altogether, in vivo results obtained with SC xenografts of 2 human NPC cell lines in nude mice show that IP IVM injections inhibit local development of NPC tumors. Data obtained with CNE-1 cells suggest that nude mice spontaneously develop a NK cell-induced anti-NPC response.
The kinase PAK1 as a target of IVM involved in NPC cells killing
It was previously shown that IVM inhibits the kinase PAK1 in tumor cells, leading to reduced tumor growth of ovarian, NF2 human cancer cells,19 colorectal cancers15 and melanoma.10 PAK1 phosphorylates Raf1 on Ser338 and MEK1 on Ser 298, which contribute to MAPK pathway activation and tumor growth. To test whether these molecules are involved in the IVM-induced NPC cells death, we tested the effect of IVM on MEK1/2, ERK1/2 and RAF1 phosphorylation in these tumor cells. HONE-1 and CNE-1 cells treated for 72 hours with 0.3 and 3 μM IVM were lysed and their extracts analyzed by Western blot experiments. As shown in Figure 8, significant inhibitions of MAPK pathway and RAF1 activation were detected. These inhibitions were limited at 0.3 μM of IVM but drastic at 3 μM (Figure 8A–D). These results strongly suggest that IVM-induced inactivation of PAK1 results in marked RAF1, ERK1/2, and MEK decreased phosphorylation and is involved in NPC cells death. Moreover the capacity of another highly selective PAK1 inhibitor, FRAX-597, to similarly kill these NPC cells (Figure 1) confirmed the impact of PAK1 inhibition in IVM-induced NPC cells death.
Discussion
IVM is a well-tolerated and clinically approved compound (European Medicine Agency- and FDA-approved) used to treat parasite infections. IVM’s and other macrocyclic lactones’ action against parasites have been ascribed to their inhibition of parasite-specific glutamate-gated chloride channels. Importantly, macrocyclic lactones including IVM inhibit pathways that are different in invertebrates and in mammal tumor cells. Here, we show that IVM and 3 other macrocyclic lactones, used at low doses (3.25 mg/kg) corresponding to the doses used in humans and domestic mammals to treat helminthic infections, are efficient to kill NPC cells in vitro, regardless of their EBV status. This IVM-induced cytotoxic activity against NPC cells being independent of the presence of EBV in tumor cells, this drug could probably be associated with other anti-NPC therapies or immunotherapies targeting EBV antigens as nearly all these tumors contain the viral genome. The use of this combinatorial treatment simultaneously targeting different pathways or antigens, associated to NPC, could drastically reduce the appearance of resistant cancer cells, because it is unlikely that cells should acquire at the same time resistance to both treatments.
As previously shown for melanomas and colorectal cancers,10,11,16 inhibition of PAK1 activity is involved in the macrocyclic lactones-induced anti-NPC cells cytotoxicity. This inhibition reduces the MAPKs protumor pathway, which is involved and important for the development of these cancers.16,20 A natural occurring plant product, Nobiletin, has also been tested for potential anti-NPC effect. It is a citrus polymethoxyflavonoid inhibiting NPC cell line migration/invasion through inhibition of MMP-2 expression. Unlike IVM, Nobiletin does not inhibit in vitro proliferation of NPC cells. But, interestingly the anti-NPC effects of Nobiletin like IVM involve inhibition of ERK1/2 phosphorylation, and both molecules suppressed NPC tumor development in xenograft models in vivo.23 These similarities between Nobiletin and IVM highlight the importance of the inhibition of ERK1/2 in the antitumor activity of macrocyclic lactones.
Combinatorial therapies are not restricted to the combination of signaling pathways inhibitors. It is likely that the combination between IVM and anti-EBV therapies gives additive or synergic effect. Also, promising combinatorial therapies for NPC could include oncolytic viruses, such as oncolytic viruses derived from Herpes virus or Adenovirus.24,25 Studying the combination of IVM with oncolytic virus can be a tremendous asset in the control of NPC growth and metastasis in vivo.
Conclusion
In vivo repeated IP injections of IVM at a FDA-approved dose are effective to reduce subcutaneous NPC tumors development with no discernable side effects on the injected nude mice. In the HONE-1 model, 20% of IVM-injected mice vs 67% of the control mice developed growing tumors. Results obtained with xenografted CNE1 cells show that IVM injections reduce tumor sizes in 87% of mice, while tumor slowing down was observed in only 25% of control mice. In the CNE-1 model, IVM antitumor efficiency is maximum at the beginning of the tumor growth likely because the mice started to control or reduce their CNE-1 tumors 8 days after tumor implantation, even in the absence of IVM. These results are most probably due to an NK-induced immune response, which is highly efficient to reject human tumor cells. Even if our results strongly suggest that IVM, like other macrocyclic lactones and PAK1 inhibitors, could be an interesting anti-NPC drug, preclinical experiments have to be confirmed in a recently described more relevant orthotopic model of metastatic NPC.26 This model is based on the injection of human NPC cells in the nasopharyngeal cavity of deeply immunodeficient Scid/Beige mice, which do not develop any immune antitumor response. Interestingly, this murine model remarkably mimics the human disease with locally invasive tumor and occurrence of spontaneous metastases. By using this model, one could more reliably test the antimetastatic effect of IVM on NPC. We expect that IVM could have an antimetastatic effect because we previously described its antimetastatic effect in murine and human melanoma models.10,11 Moreover, with this preclinical model it will be possible to describe IVM anti-NPC activities directly at the primary site of tumor growth and confirm that IVM can reach the cavum. Indeed, IVM suffers from poor bioavailability due to its low aqueous solubility. It is poorly absorbed or metabolized because 80% of the administered IVM is eliminated as the initial compound. For antiparasite treatments, IVM is typically given as solid tablets in 1 or 2 oral doses. We intend to test different formulations solubilizing the IVM, to allow orthotopic injections in mice and, subsequently, nebulization into the human nasopharyngeal cavity. Altogether, our results pave the way for new therapies of NPC and are particularly interesting because of the scarcity of available efficient treatments against advanced NPC.
Acknowledgments
The authors thank Dr P Busson for the human nasopharyngeal carcinoma cell lines. This work was supported by Institut National de la Santé et de la Recherche Médicale (INSERM, France) and by La Ligue Régionale contre le Cancer de Toulouse. The authors also thank the Center Régional d’Exploration Fonctionnelle et Ressources Expérimentales (CREFRE-US006, Toulouse), in particular Rachel Balouzat and Romain Escalard, and the microscopy and chemistry Platform at ITAV for expert technical assistance.
Disclosure
The authors report no conflicts of interest in this work.
References
Chua MLK, Wee JTS, Hui EP, Chan ATC. Nasopharyngeal carcinoma. Lancet. 2016;387(10022):1012–1024. | ||
Chou J, Lin YC, Kim J, et al. Nasopharyngeal carcinoma – review of the molecular mechanisms of tumorigenesis. Head Neck. 2008;30(7):946–963. | ||
Wu L, Li C, Pan L. Nasopharyngeal carcinoma: a review of current updates. Exp Ther Med. 2018;15(4):3687–3692. | ||
Gu AD, Zeng MS, Qian CN. The criteria to confirm the role of Epstein–Barr virus in nasopharyngeal carcinoma initiation. Int J Mol Sci. 2012;13(10):13737–13747. | ||
Ji MF, Wang DK, Yu YL, et al. Sustained elevation of Epstein–Barr virus antibody levels preceding clinical onset of nasopharyngeal carcinoma. Br J Cancer. 2007;96(4):623–630. | ||
Stanfield BA, Luftig MA. Recent advances in understanding Epstein–Barr virus. F1000Res. 2017;6:386. | ||
Lv JW, Qi ZY, Zhou GQ, et al. Optimal cumulative cisplatin dose in nasopharyngeal carcinoma patients receiving additional induction chemotherapy. Cancer Sci. 2018;109(3):751–763. | ||
Teow SY, Yap HY, Peh SC. Epstein–Barr virus as a promising immunotherapeutic target for nasopharyngeal carcinoma treatment. J Pathog. 2017;2017:7349268. | ||
Huang J, Fogg M, Wirth LJ, et al. Epstein–Barr virus-specific adoptive immunotherapy for recurrent, metastatic nasopharyngeal carcinoma. Cancer. 2017;123(14):2642–2650. | ||
Gallardo F, Teiti I, Rochaix P, et al. Macrocyclic lactones block melanoma growth, metastases development and potentiate activity of anti-BRAF V600 inhibitors. Clin Skin Cancer. 2016;1(1):4–14. | ||
Patent Methods and pharmaceutical composition for the treatment of melanoma. EPO Form 1001E-TILKIN-M14404AN. Patent PCT EP15305339.2. 2015 Mar 3. | ||
Laining R, Gillan V, Devaney E. Ivermectin – old drug, new tricks? Trends Parasitol. 2017;33(6):463–472. | ||
Lynagh T, Lynch JW. Molecular mechanisms of Cys-loop ion channel receptor modulation by ivermectin. Front Mol Neurosci. 2012;5:60. | ||
Hashimoto H, Messerli SM, Sudo T, Maruta H. Ivermectin inactivates the kinase PAK1 and blocks the PAK1-dependent growth of human ovarian cancer and NF2 tumor cell lines. Drug Discov Ther. 2009;3(6):243–246. | ||
Melotti A, Mas C, Kuciak M, et al. The river blindness drug ivermectin and related macrocyclic lactones inhibit WNT-TCF pathway responses in human cancer. EMBO Mol Med. 2014;6(10):1263–1278. | ||
González P, González FA, Ueno K. Ivermectin in human medicine, an overview of the current status of its clinical applications. Curr Pharm Biotechnol. 2012;13(6):1103–1109. | ||
Abokwidir M, Fleischer AB. An emerging treatment: topical ivermectin for papulopustular rosacea. J Dermatolog Treat. 2015;26(4):379–380. | ||
Ong CC, Jubb AM, Jakubiak D, et al. P21-activated kinase 1 (PAK1) as a therapeutic target in BRAF wild-type melanoma. J Natl Cancer Inst. 2013;105(9):606–607. | ||
Ong CC, Jubb AM, Haverty PM, et al. Targeting p21-activated kinase 1 (PAK1) to induce apoptosis of tumor cells. Proc Natl Acad Sci U S A. 2011;108(17):7177–7182. | ||
Khare V, Dammann K, Asboth M, Krnjic A, Jambrich M, Gasche C. Overexpression of PAK1 promotes cell survival in inflammatory bowel diseases and colitis-associated cancer. Inflamm Bowel Dis. 2015;21(2):287–296. | ||
Tilkin-Mariame AF, Mariame B, Gallardo F, inventor; INSERM (Institut National de la Santé et de la Recherche Médicale), Université Paul Sabatier Toulouse Iii, Centre National De La Recherche Scientifique (Cnrs), Neovirtech, assignee. Methods and pharmaceutical compositions for the treatment of nasopharyngeal carcinoma. European patent EP 16305207: TILKIN15449AN. 2016, extension PCT/EP2017/054084. 2017. | ||
Diehl KH, Hull R, Morton D, et al. A good practice guide to the administration of substances and removal of blood, including routes and volumes. J Appl Toxicol. 2001;21(1):15–23. | ||
Chien SY, Hsieh MJ, Chen CJ, Yang SF, Chen MK. Nobiletin inhibits invasion and migration of human nasopharyngeal carcinoma cell lines by involving ERK1/2 and transcriptional inhibition of MMP-2. Expert Opin Ther Targets. 2015;19(3):307–320. | ||
Liu RY, Zhou L, Zhang YL, et al. An oncolytic adenovirus enhances antiangiogenic and antitumoral effects of a replication-deficient adenovirus encoding endostatin by rescuing its selective replication in nasopharyngeal carcinoma cells. Biochem Biophys Res Commun. 2013;442(3–4):171–176. | ||
Wang JN, Hu P, Zeng MS, Liu RB. Anti-tumor effect of oncolytic herpes simplex virus G47delta on human nasopharyngeal carcinoma. Chin J Cancer. 2011;30(12):831–841. | ||
Smith PA, Merritt D, Barr L, Thorley-Lawson DA. An orthotopic model of metastatic nasopharyngeal carcinoma and its application in elucidating a therapeutic target that inhibits metastasis. Genes Cancer. 2011;2(11):1023–1033. |
 © 2018 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2018 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.