Back to Archived Journals » Open Access Animal Physiology » Volume 7
Long-term morphine addiction reduces neurogenesis and memory performance and alters emotional reactivity and anxiety levels in male rats
Authors Famitafreshi H, Karimian M , Marefati N
Received 30 April 2015
Accepted for publication 9 June 2015
Published 17 July 2015 Volume 2015:7 Pages 129—136
DOI https://doi.org/10.2147/OAAP.S87674
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 2
Editor who approved publication: Dr Peter Koulen
Hamidreza Famitafreshi, Morteza Karimian, Narges Marefati
Department of Physiology, Tehran University of Medical Sciences-International Campus, Tehran, Iran
Introduction: Substance abuse is a behavioral disorder associated with a wide variety of devastating effects. Neurogenesis in dentate gyrus of hippocampus is essential for brain functions like memory formation. Therefore, this may play an important role in achieving successful withdrawal.
Methods and materials: Twenty Sprague-Dawley rats were randomly divided into two experimental groups: control and addiction. To induce morphine dependence, animals received morphine (0.75 mg/rat) for 21 days. The performance of animals in Morris water maze and elevated plus maze tests was evaluated after day 20. At the end of the study, the rats were decapitated, and their brains were sectioned to study neurogenesis by counting BrdU-positive cells.
Results: Hippocampal neurogenesis was significantly reduced in rats in the addicted group. Also, reference and working memory performance were impaired in animals in the addicted group. A decrease in emotional reactivity and anxiety was observed in animals in the addicted group when compared with that in the control group.
Conclusion: Addiction adversely affects brain functions and neurogenesis; thus treatment to increase neurogenesis is the better option for the persons with substance abuse.
Keywords: hippocampal neurogenesis, morphine addiction, striatum, morphine-induced, hippocampus, substance abuse
Introduction
Neurogenesis is a process that occurs in some parts of brain like dentate gyrus of hippocampus and subventricular zone of lateral ventricles.1 Adequate neurogenesis is essential for brain functions, but it may be influenced by many pathological conditions like Alzheimer, depression, and addiction.2–4 Drug addiction, a compulsive urge to take drugs like cocaine, heroin, and alcohol, can adversely affect mesolimbic system.5 Mesolimbic system is the most known region of brain that is responsible for side effects of drugs.6 It is important to study adult hippocampal neurogenesis because cognitive functions are an important part of brain function and healthy life.7–10 Also, hippocampus, as part of the limbic system, may be necessary for tolerance of drugs and reduction of relapse. However, mechanisms involving addiction-induced side effects are not elucidated to date. Previous studies have shown that cocaine has adverse effect on neurogenesis; however, the effect of long-term use of other substances such as morphine has not been studied. The aim of this research is to study the effect of long-term morphine addiction on neurogenesis and brain functions like memory, emotional reactivity, and anxiety levels to delineate the behaviors that help addiction exerts and improves its various adverse effects. We further studied to find whether improved neurogenesis can prevent drug relapse.
Methods and materials
Animals
Twenty male Sprague-Dawley rats (weighing 200–250 g) were used in this study. Animals were divided into two groups (n=10 each): control and addiction. Housing took place under standard 12 hour day/night cycle at room temperature 22°C, and animals had free access to water and chow ad libitum. All the experiments were performed in accordance with the guidelines for the care and use of laboratory animals published by the US National Institutes of Health (NIH Publication No 85-23, revised 1996). The experimental protocol was approved by the institutional care and use committee of Tehran University of Medical Sciences (Tehran, Iran).
Experimental procedure
At the start of experiment, rats were injected with BrdU (50 mg/kg) and morphine (0.75 mg) intraperitoneally, and these injections lasted for 21 days. On day 20, the memory of rats was first tested by Morris water maze for two days, and then anxiety was tested by elevated plus maze. Then rats were anesthetized with ketamine (100 mg/kg) and xylazine (10 mg/kg) and then sacrificed. The brain was perfused with paraformaldehyde 4% for fixation of brain, and then it was removed for preparing sections.
Addiction
To induce drug dependence and addiction, animals received 0.75 mg morphine Sulfate intraperitoneally for 3 weeks.
Morris water maze
This apparatus was used to assess two types of memory: working and reference memory. During this task, animals had to find extra-maze cues to locate the platform. This setup consisted of a large circular black tank (diameter 183 cm) placed in the center of the room. After performing the first trial for visible test with clear water, the tank was filled with water (27°C) that was made opaque with nontoxic black tempera paint and divided into four quarters (North, East, West, and South). An escape platform (10 cm in diameter) was submersed 0.5 cm below the surface of water in the southeast quadrant. Furthermore, non motile visual cues were placed around the water tank. Since the animals were kept in opaque water and the platform was not visible, they had to rely on external maze cues to find their route of escape. During the probe trial, the platform was retracted from the bottom of the maze to assess if animals can swim to the quadrant where the platform was located previously. Large red geometrical shapes with white backgrounds were placed around the maze to provide external cues. During the entire experiment, the animals’ performance was videotaped using a video camera mounted above the maze and interfaced with a computerized tracking system. In this 3 days experiment, two trials were performed each day. However, on the third day, the probe trial was performed. The first trial was considered for the visible test.11 First trial of each session was considered as the test for reference memory and the later one for working memory. In addition, swimming speed was also recorded to evaluate the emotional state of the animal.12
Visible test
This test was used to assess nonspatial learning and to rule out the possibility that the spatial learning deficit detected might actually be a product of deficient escape motivation or impairment of vision and/or motor skills. In this test, the location of platform was highlighted by attaching it to a high-contrast striped flag rising above the water. This gave spatial cues to animals for escaping from water. This simple associative nonspatial task is believed to be independent of hippocampal function.
Spatial acquisition test
In these trials, the animals had to locate the hidden platform using extra-maze cues and find the platform that is now located under water in the southeast of the quadrant of the tank. A transparent Lucite platform (10×10 cm) was submerged beneath the surface of the water (0.5 cm) in the southeast quadrant of the tank. Each rat participated in 16 spatial trials with invariable start positions. For each trial, a 60-second time period was provided to reach the platform. If the animal had failed to reach the platform within 60 seconds, the experimenter placed the animal on the platform. A 20-second rest period was observed between each trial. Swimming time (seconds), swim distance (cm), and swim speed (s/cm) were recorded. For swimming time and distance, lower values were indicative of better performance, whereas higher values indicated better swimming speed.
Probe Trial for Spatial Memory
This trial was performed to evaluate how well the animal had learnt the location of platform. The platform was removed and made unavailable for 60 seconds. For time spent in the fourth quadrant and swim distance in the fourth quadrant, higher numbers were indicative of better performance in contrast to the swimming speed.
- Fourth quadrant is the quadrant in which the platform had been placed and was removed in the probe trial.
Elevated plus maze
During this task, the level of anxiety of animals was assessed. It consisted of a plus-shaped apparatus with two open and two closed arms, each with an open roof. The arms were elevated 70 cm from the floor. Each animal was placed in the center of the apparatus and then allowed to move freely in the four arms for 5 minutes. The number of entries to the open arms and time spent in the open arms were recorded. Reduced anxiety levels were indicated by more time spent and increased number of entries into open arms.13
Immunohistochemistry
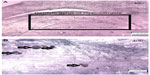
For 21 days, all animals received 50 mg/kg BrdU intraperitoneally. On day 22, rats were anesthetized using ketamine (100 mg/kg) and xylazine (10 mg/kg) and then sacrificed. After thoracotomy, all animals were first perfused with normal saline and then with paraformaldehyde 4% via intracranial infusion. After fixation, the brains were removed from skull. For the first 2 days, the brains were kept in PBS + paraformaldehyde 4% and then on day 3, in sucrose 10% + paraformaldehyde 4% + PBS. Throughout day 4, the brains were kept in sucrose 20% + paraformaldehyde 4% + PBS, and for the remaining days, they were kept in sucrose 30% + paraformaldehyde 4% + PBS. The cryosections (30 μm) were prepared from dentate gyrus of the hippocampal region. Immunohistochemistry was performed for ten sections from each brain, five of which were stained for BrdU-positive neurons with anti-BrdU antibody kit (5-Bromo-2′-dU Labeling and Detection Kit ll; Roche, Germany; Cat No 11299964001-en-17). BrdU-positive cells in dentate gyrus were counted using light microscope (400×).14
Statistics
Data were analyzed using SPSS version 22 and Graph pad prism 5. Independent two-tailed sample t-test was performed for all experiments. Data were represented as mean ± SEM, and P<0.05 was considered significant.
Results
Morris water maze
Working memory
In animals in the addicted group, working memory performance was markedly reduced when compared with that of the control group (34.45 vs 28.54, P<0.0344, Figure 1).
  | Figure 1 Working memory performance in control and addicted rats. |
Spatial task acquisition
Swimming speed, as an indicator of emotional reactivity, was lower in animals in the addicted groups than the animals in the control group (30.28 cm/s vs 27.04 cm/s, P<0.0246, Figure 2).
  | Figure 2 Speed of swimming in spatial task acquisition (n=10). |
Probe trials
Animals in the control group spent more time in the fourth quadrant than the animals in the addicted group (25.93 seconds vs 20.30 seconds, P=0.0388, Figure 3A). Speed of swimming was found to be significantly higher in rats in the addicted group than that of rats in the control group (33.77 cm/s vs 26.73 cm/s, P=0.0139, Figure 3B). These results indicate better reference memory performance in the control group.15
Elevated plus maze
Anxiety levels
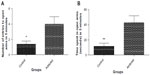
Rats in the addicted group showed higher number of entries (3.667 vs 1.4, P=0.0268, Figure 4A) and also spent more time in the open arms (42.78 seconds vs 11.7 seconds, P=0.0049, Figure 4B) in comparison with the animals in the control group. These measures are suggestive of lower anxiety levels in rats in the addicted groups.
Neurogenesis
The number of BrdU-positive cells in dentate gyrus of hippocampus was considerably higher in animals in the control group compared with that of the addicted group (251.4 vs 158.2, P=0.0386, Figures 5 and 6).
  | Figure 5 Neurogenesis in dentate gyrus of hippocampus (n=6). |
Discussion
In this study, we examined the effect of long-term morphine abuse on neurogenesis and brain functions. We found that morphine addiction reduces neurogenesis and memory performance in rats in the addicted group. Increased anxiety and emotional state was observed in rats in the control group.
Adequate neurogenesis is important for brain functioning. Therefore, morphine-induced reduction in neurogenesis can adversely affect memory performance, learning, emotional reactivity, and anxiety levels. Furthermore, intact learning abilities are required for successful drug abstinence. It is possible that along with reduction in learning abilities, adaptation in rewarding system occurs, and morphine abusers cannot overcome this dysfunction for avoiding morphine abuse.16 As we know, along with abuse of morphine, brain activates rewarding system to discard morphine abuse. Two types of rewarding systems are involved: 1) one for quitting drug abuse that activates behaviors that are necessary for life such as feeding, and 2) the other is the rewarding system that causes relapse due to adverse effect on mesolimbic dopaminergic system.17 Exercise results in enhancement of neurogenesis, angiogenesis, and neuroplasticity, which in turn reduces side effects caused by drug abuse.18 Another study shows overall enhancement in neurogenesis, angiogenesis, and neuroplasticity, and this shows that one possible reason such as angiogenic factor can result in enhancement of all of them.19 But in drug addiction, this pathway decreases and can result in loss of normal pleasurable activities. In drug addiction, pleasure center of the brain is hijacked by drugs, so it is the drug that gives brain the sense of joy.17 So if improvement in neurogenesis helps this lost function of pleasure center, tolerance of drugs can be easier. This can be somehow because of loss of neuroplasticity in brain regions and improvement in neurogenesis directly or indirectly compensate this defect.20 By destroying cells in dentate gyrus of hippocampus, drug abuse was increased. This suggests that rats’ desire for drugs has increased.21 Noonan et al showed that self-administration of cocaine decreases neurogenesis, whereas cocaine withdrawal normalizes proliferation and survival of adult-generated neurons and enhances maturity of adult-generated neurons in subgranular zone of rats’ brain.21 This indicates that cognitive functions are vital for drug abstinence, and restoration of neurogenesis is essential to reduce the risk of drug relapse.
In Morris water maze, different aspects of memory, such as reference memory and working memory, were assessed in a two-phase test: spatial task acquisition trial and probe trial. Our results showed that morphine abuse impairs reference memory and working memory; this is suggestive of morphine-induced disruption in brain signaling required for intact memory.15 Previously, it has been documented that successful drug withdrawal requires intact memory and proper prefrontal cortex function.22,23 This implies that adequate neurogenesis can improve brain functions, which may result in drug abstinence. During spatial task acquisition trial, we observed high emotional reactivity in rats in the control group.12 This result suggests that decreased emotional state in rats in the addicted group may cause more depression and augment adverse effect of morphine. Striatum is a part of brain implicated in regulation of emotions and memory.24,25 In this study, along with the reduction in neurogenesis, memory and emotions were disturbed. So hippocampal neurogenesis is required for proper memory function and emotional regulations. Galanin is a neurotransmitter proposed to regulate cognitive functions and craving for drugs.26–28
Broadly speaking, fear and anxiety are two different terms. Evidence of this difference is found in studies of humans and animals.29 In animals, fear causes them to move away from danger, whereas anxiety causes the animal to move toward danger. This suggests that the defensive direction is an important factor.30 Conversely, it has been implicated that the demarcation between fear and anxiety is independent of the conditioned or unconditioned nature of the stimuli.30 We used elevated plus maze to access anxiety levels in rats. We observed that animals in the addicted group entered more frequently and spent more time in open arms, which indicates low anxiety levels and fear in these animals. These results imply that morphine abuse affects activity in amygdala, striatum, and cingulate cortex31 and further, addiction-induced reduction in neurogenesis may aggravate the outcome.32 Sufficient anxiety levels are required to combat the harmful effects of addiction. With reduction of anxiety level, the defending action of animals reduces because anxiety may recruit body compensatory mechanisms to prevent bad situations. Thus, our findings elaborate that reduced neurogenesis in animals in addicted group can affect memory, anxiety levels, and emotional reactivity.33 Liu et al demonstrated the role of interleukin-17 in hippocampus in altering anxiety levels.34
Comorbidity of mood and anxiety disorders is well documented in literature. Mood disturbances are attributed to alterations in circadian rhythms, and effectiveness of antidepressants is partially mediated by their ability to regularize abnormal circadian rhythms.35 However, a number of experiments have been performed to study if hippocampal neurogenesis can independently cause anxiety. Some of these studies have observed that reduction of anxiety is associated with reduced neurogenesis.36 In addition, Revest et al divided hippocampus into two parts: rostral for cognition and ventral for anxiety level regulation.36 Taken together, neurogenesis can prevent devastating effects of drug abuse.
Treatments for improving neurogenesis
- Deep brain stimulation: Electroconvulsive therapy (ECT) has been used in medicine for more than a century. The mechanism, which adjusts its effectiveness in treatment of neurological diseases, has been associated with neurogenesis.37,38 It has been shown that dose-dependent electroconvulsive brain stimulation can increase number and age of neurons.37,38 Evidence of effectiveness of increasing neurogenesis in treatment of depression comes from disruption of fear memory. ECT increases proliferation of quiescent progenitor cells, followed by that of amplifying progenitor cells.39,40 Also, applying chronic ECT causes synaptic rearrangements in dentate gyrus, which is associated with increase in long-term potentiation.41 Improved long-term potentiation further increases the number of new born neurons in the dentate gyrus,42 which in turn alters hippocampal circuits. The mechanism behind ECT-induced neurogenesis is not fully understood. However, studies have suggested the participation of some factors like brain-derived neurotrophic factor (BDNF), fibroblast growth factor-2, and vascular endothelial growth factor.43–45
- Long-term use of some antidepressants also induces neurogenesis. Among them, selective serotonin reuptake inhibitors are the most commonly used drugs. These drugs increase progenitor cells in dentate gyrus of hippocampus.3 A study using nestin-cyan fluorescent protein has shown that fluoxetine increases number of amplifying neuronal progenitor cells but does not exert any effect on stem cells.46 Furthermore, Wang et al47 have also reported fluoxetine-induced hippocampal neurogenesis.48 Some factors such as BDNF and vascular endothelial growth factor are considered to regulate the microenvironment for adult neurogenesis.49–54 N-acetylecysteine has also shown useful properties for treatment of addiction.55
- Exercise therapy: Exercise induces neurogenesis,56–59 which improves memory performance and learning abilities in rats56,59 and humans.60 Human brain continues to undergo neurogenesis; running helps to increase neurogenesis to exert its effect. A study on mice has shown that running increases BrdU-positive cells, learning, and long-term potentiation.59 In addition, exercise can also activate quiescent cells in the hippocampus.57 These neurons eventually become mature, and hence improve hippocampal function.
Conclusion
These findings suggest that drug abuse has devastating effects on the brain, and it impairs proper functioning of the brain. Thus, neurogenesis may be essential with successful drug abstinence.
Disclosure
The authors declare they have no conflicts of interest in this work.
References
Ming GL, Song H. Adult neurogenesis in the mammalian brain: significant answers and significant questions. Neuron. 2011;70(4):687–702. | |
Maruszak A, Pilarski A, Murphy T, Branch N, Thuret S. Hippocampal neurogenesis in Alzheimer’s disease: is there a role for dietary modulation? J Alzheimers Dis. 2014;38(1):11–38. | |
Sahay A, Hen R. Adult hippocampal neurogenesis in depression. Nat Neurosci. 2007;10(9):1110–1115. | |
Sudai E, Croitoru O, Shaldubina A, et al. High cocaine dosage decreases neurogenesis in the hippocampus and impairs working memory. Addict Biol. 2010;16(2):251–260. | |
Mandyam CD, Koob GF. The addicted brain craves new neurons: putative role for adult-born progenitors in promoting recovery. Trends Neurosci. 2012;35(4):250–260. | |
Pierce RC, Kumaresan V. The mesolimbic dopamine system: the final common pathway for the reinforcing effect of drugs of abuse? Neurosci Biobehav Rev. 2006;30(2):215–238. | |
Mandyam CD, Wee S, Crawford EF, Eisch AJ, Richardson HN, Koob GF. Varied access to intravenous methamphetamine self-administration differentially alters adult hippocampal neurogenesis. Biol Psychiatry. 2008;64(11):958–965. | |
Noonan MA, Bulin SE, Fuller DC, Eisch AJ. Reduction of adult hippocampal neurogenesis confers vulnerability in an animal model of cocaine addiction. J Neurosci. 2010;30(1):304–315. | |
Abrous DN, Adriani W, Montaron MF, et al. Nicotine self-administration impairs hippocampal plasticity. J Neurosci. 2002;22(9):3656–3662. | |
Eisch AJ, Barrot M, Schad CA, Self DW, Nestler EJ. Opiates inhibit neurogenesis in the adult rat hippocampus. Proc Natl Acad Sci U S A. 2000;97(13):7579–7584. | |
Anisman H, McIntyre DC. Conceptual, spatial, and cue learning in the Morris water maze in fast or slow kindling rats: attention deficit comorbidity. J Neurosci. 2002;22(17):7809–7817. | |
van der Staay FJ, Schuurman T, van Reenen CG, Korte SM. Emotional reactivity and cognitive performance in aversively motivated tasks: a comparison between four rat strains. Behav Brain Funct. 2009;5:50. | |
Vila-Luna S, Cabrera-Isidoro S, Vila-Luna L, et al. Chronic caffeine consumption prevents cognitive decline from young to middle age in rats, and is associated with increased length, branching, and spine density of basal dendrites in CA1 hippocampal neurons. Neuroscience. 2012;202:384–395. | |
Spritzer MD, Ibler E, Inglis W, Curtis MG. Testosterone and social isolation influence adult neurogenesis in the dentate gyrus of male rats. Neuroscience. 2011;195:180–190. | |
Tanda K, Nishi A, Matsuo N, et al. Abnormal social behavior, hyperactivity, impaired remote spatial memory, and increased D1-mediated dopaminergic signaling in neuronal nitric oxide synthase knockout mice. Mol Brain. 2009;2:19. | |
Bocklisch C, Pascoli V, Wong JC, et al. Cocaine disinhibits dopamine neurons by potentiation of GABA transmission in the ventral tegmental area. Science. 2013;341(6153):1521–1525. | |
Kennett J, Matthews S, Snoek A. Pleasure and addiction. Front Psychiatry. 2013;4:117. | |
Scheewe TW, Backx FJ, Takken T, et al. Exercise therapy improves mental and physical health in schizophrenia: a randomised controlled trial. Acta Psychiatr Scand. 2013;127(6):464–473. | |
Font MA, Arboix A, Krupinski J. Angiogenesis, neurogenesis and neuroplasticity in ischemic stroke. Curr Cardiol Rev. 2010;6(3):238–244. | |
Niu L, Cao B, Zhu H, et al. Impaired in vivo synaptic plasticity in dentate gyrus and spatial memory in juvenile rats induced by prenatal morphine exposure. Hippocampus. 2009;19(7):649–657. | |
Noonan MA, Choi KH, Self DW, Eisch AJ. Withdrawal from cocaine self-administration normalizes deficits in proliferation and enhances maturity of adult-generated hippocampal neurons. J Neurosci. 2008; 28(10):2516–2526. | |
Perry JL, Joseph JE, Jiang Y, et al. Prefrontal cortex and drug abuse vulnerability: translation to prevention and treatment interventions. Brain Res Rev. 2011;65(2):124–149. | |
Goldstein RZ, Volkow ND. Dysfunction of the prefrontal cortex in addiction: neuroimaging findings and clinical implications. Nat Rev. 2011;12(11):652–669. | |
Rolls ET. Neurophysiology and cognitive functions of the striatum. Rev Neurol (Paris). 1994;150(8–9):648–660. | |
Gasbarri A, Tomaz C. Memory and motivational/emotional processes. Front Behav Neurosci. 2012;6:71. | |
Lori A, Tang Y, O’Malley S, et al. The galanin receptor 1 gene associates with tobacco craving in smokers seeking cessation treatment. Neuropsychopharmacology. 2011;36(7):1412–1420. | |
Heberlein A, Muschler M, Frieling H, et al. Decreased galanin serum levels are associated with alcohol-craving during withdrawal. Prog Neuropsychopharmacol Biol Psychiatry. 2011;35(2):568–572. | |
Mechenthaler I. Galanin and the neuroendocrine axes. Cell Mol Life Sci. 2008;65(12):1826–1835. | |
Perkins AM, Ettinger U, Davis R, Foster R, Williams SC, Corr PJ. Effects of lorazepam and citalopram on human defensive reactions: ethopharmacological differentiation of fear and anxiety. J Neurosci. 2009;29(40):12617–12624. | |
McNaughton N, Corr PJ. A two-dimensional neuropsychology of defense: fear/anxiety and defensive distance. Neurosci Biobehav Rev. 2004;28(3):285–305. | |
Duval ER, Javanbakht A, Liberzon I. Neural circuits in anxiety and stress disorders: a focused review. Ther Clin Risk Manag. 2015;11:115–126. | |
Schoenfeld TJ, Gould E. Stress, stress hormones, and adult neurogenesis. Exp Neurol. 2012;233(1):12–21. | |
Imayoshi I, Sakamoto M, Ohtsuka T, et al. Roles of continuous neurogenesis in the structural and functional integrity of the adult forebrain. Nat Neurosci. 2008;11(10):1153–1161. | |
Liu Y, Ho RC, Mak A. The role of interleukin (IL)-17 in anxiety and depression of patients with rheumatoid arthritis. Int J Rheum Dis. 2012; 15(2):183–187. | |
Rosenwasser AM. Alcohol, antidepressants, and circadian rhythms. Human and animal models. Alcohol Res Health. 2001;25(2):126–135. | |
Revest JM, Dupret D, Koehl M, et al. Adult hippocampal neurogenesis is involved in anxiety-related behaviors. Mol Psychiatry. 2009;14(10):959–967. | |
Madsen TM, Treschow A, Bengzon J, Bolwig TG, Lindvall O, Tingstrom A. Increased neurogenesis in a model of electroconvulsive therapy. Biol Psychiatry. 2000;47(12):1043–1049. | |
Malberg JE, Eisch AJ, Nestler EJ, Duman RS. Chronic antidepressant treatment increases neurogenesis in adult rat hippocampus. J Neurosci. 2000;20(24):9104–9110. | |
Segi-Nishida E, Warner-Schmidt JL, Duman RS. Electroconvulsive seizure and VEGF increase the proliferation of neural stem-like cells in rat hippocampus. Proc Natl Acad Sci U S A. 2008;105(32):11352–11357. | |
Scott BW, Wojtowicz JM, Burnham WM. Neurogenesis in the dentate gyrus of the rat following electroconvulsive shock seizures. Exp Neurol. 2000;165(2):231–236. | |
Stewart C, Reid I. Electroconvulsive stimulation and synaptic plasticity in the rat. Brain Res. 1993;620(1):139–141. | |
Bruel-Jungerman E, Davis S, Rampon C, Laroche S. Long-term potentiation enhances neurogenesis in the adult dentate gyrus. J Neurosci. 2006;26(22):5888–5893. | |
Newton SS, Collier EF, Hunsberger J, et al. Gene profile of electroconvulsive seizures: induction of neurotrophic and angiogenic factors. J Neurosci. 2003;23(34):10841–10851. | |
Nibuya M, Morinobu S, Duman RS. Regulation of BDNF and trkB mRNA in rat brain by chronic electroconvulsive seizure and antidepressant drug treatments. J Neurosci. 1995;15(11):7539–7547. | |
Gwinn RP, Kondratyev A, Gale K. Time-dependent increase in basic fibroblast growth factor protein in limbic regions following electroshock seizures. Neuroscience. 2002;114(2):403–409. | |
Encinas JM, Vaahtokari A, Enikolopov G. Fluoxetine targets early progenitor cells in the adult brain. Proc Natl Acad Sci U S A. 2006; 103(21):8233–8238. | |
Wang JW, David DJ, Monckton JE, Battaglia F, Hen R. Chronic fluoxetine stimulates maturation and synaptic plasticity of adult-born hippocampal granule cells. J Neurosci. 2008;28(6):1374–1384. | |
Czeh B, Welt T, Fischer AK, et al. Chronic psychosocial stress and concomitant repetitive transcranial magnetic stimulation: effects on stress hormone levels and adult hippocampal neurogenesis. Biol Psychiatry. 2002;52(11):1057–1065. | |
Sairanen M, Lucas G, Ernfors P, Castren M, Castren E. Brain-derived neurotrophic factor and antidepressant drugs have different but coordinated effects on neuronal turnover, proliferation, and survival in the adult dentate gyrus. J Neurosci. 2005;25(5):1089–1094. | |
Neto FL, Borges G, Torres-Sanchez S, Mico JA, Berrocoso E. Neurotrophins role in depression neurobiology: a review of basic and clinical evidence. Curr Neuropharmacol. 2011;9(4):530–552. | |
Saarelainen T, Hendolin P, Lucas G, et al. Activation of the TrkB neurotrophin receptor is induced by antidepressant drugs and is required for antidepressant-induced behavioral effects. J Neurosci. 2003;23(1):349–357. | |
Li Y, Luikart BW, Birnbaum S, et al. TrkB regulates hippocampal neurogenesis and governs sensitivity to antidepressive treatment. Neuron. 2008;59(3):399–412. | |
Warner-Schmidt JL, Duman RS. VEGF as a potential target for therapeutic intervention in depression. Curr Opin Pharmacol. 2008; 8(1):14–19. | |
Palmer TD, Markakis EA, Willhoite AR, Safar F, Gage FH. Fibroblast growth factor-2 activates a latent neurogenic program in neural stem cells from diverse regions of the adult CNS. J Neurosci. 1999; 19(19):8487–8497. | |
Madayag A, Lobner D, Kau KS, et al. Repeated N-acetylcysteine administration alters plasticity-dependent effects of cocaine. J Neurosci. 2007;27(51):13968–13976. | |
van Praag H, Shubert T, Zhao C, Gage FH. Exercise enhances learning and hippocampal neurogenesis in aged mice. J Neurosci. 2005; 25(38):8680–8685. | |
Lugert S, Basak O, Knuckles P, et al. Quiescent and active hippocampal neural stem cells with distinct morphologies respond selectively to physiological and pathological stimuli and aging. Cell Stem Cell. 2010; 6(5):445–456. | |
Creer DJ, Romberg C, Saksida LM, van Praag H, Bussey TJ. Running enhances spatial pattern separation in mice. Proc Natl Acad Sci U S A. 2010;107(5):2367–2372. | |
van Praag H, Christie BR, Sejnowski TJ, Gage FH. Running enhances neurogenesis, learning, and long-term potentiation in mice. Proc Natl Acad Sci U S A. 1999;96(23):13427–13431. | |
Erickson KI, Voss MW, Prakash RS, et al. Exercise training increases size of hippocampus and improves memory. Proc Natl Acad Sci U S A. 2011;108(7):3017–3022. |
 © 2015 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2015 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.