Back to Journals » Clinical Interventions in Aging » Volume 14
Long-term icariin treatment ameliorates cognitive deficits via CD4+ T cell-mediated immuno-inflammatory responses in APP/PS1 mice
Authors Zhu T, Zhang F, Li H, He Y, Zhang G , Huang N, Guo M, Li X
Received 8 March 2019
Accepted for publication 15 April 2019
Published 7 May 2019 Volume 2019:14 Pages 817—826
DOI https://doi.org/10.2147/CIA.S208068
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 2
Editor who approved publication: Dr Zhi-Ying Wu
Tianrui Zhu,1 Feng Zhang,1 Heng Li,1 Yi He,2 Guitao Zhang,1 Nana Huang,1 Mingming Guo,3 Xiaohong Li1
1Department of Neurology, Jinan Central Hospital Affiliated to Shandong University, Jinan, Shandong 250013, People’s Republic of China; 2Department of Neurology, The Second Affiliated Hospital of Xi’an Medical University, Xi’an, Shaanxi 710021, People’s Republic of China; 3Department of Breast Surgery, The Second Hospital of Shandong University, Jinan, Shandong 250033, People’s Republic of China
Background: Alzheimer’s disease (AD) is the most common neurodegenerative disorder that also involves neuroinflammation in addition to many other features. Icariin (ICA) as one of the active ingredients of Chinese herbal medicine has the immunomodulating function. This study aimed to investigate the immunotherapeutic potential of ICA on AD.
Methods: APP/PS1 mice and wild type C57BL/6 mice were subjected to orally ICA administration (60 mg/kg/d) for 8 months. Then, the ethological and biochemical experiments, such as Morris water maze assay, Aβ ELISA, blood T cell flow cytometry, and plasma and brain cytokines array, were conducted to evaluate the effects of ICA administration.
Results: ICA significantly improved spatial learning and memory retention in APP/PS1 mice. Long-term application of ICA could also reduce hippocampus Aβ deposition, modulate the differentiation of CD4+ T cells, and modulate the release of inflammatory cytokines in plasma and brain tissue.
Conclusion: ICA shows the neuroprotective effects via modulating the CD4+ T lymphocyte-related immuno-inflammatory responses in APP/PS1 mice and may be a promising drug against AD progression.
Keywords: Alzheimer’s disease, icariin, T lymphocyte, neuroinflammation, cytokines
Introduction
Alzheimer’s disease (AD) is the most common neurodegenerative disorder and the main cause of dementia, which is characterized by progressive memory loss and cognitive deterioration.1 It is estimated that there is a new case every 3 s in the world, and this community is likely to rise to about 152 million people by 2050.2 The neuropathological hallmarks of AD include the extracellular aggregation of amyloid-β (Aβ) peptide in plaques and the formation of intraneuronal neurofibrillary tangles (NFTs) by hyperphosphorylated tau protein.3,4 Although the mechanisms causing the neuropathological lesions are largely unknown, increasing documents initially underlined that neuroinflammation may be a major driver in the pathogenesis of AD.5,6
Growing evidence indicates that deposition of Aβ induces an inflammatory response by activating microglia in the brain.7,8 T cells have been identified in neuritic plaques and microglia in the post-mortem AD brain.9 T cells from peripheral immune system may play a critical role in driving the neuroinflammatory changes and the associated neuronal degeneration.10 Approximately, over 80% of the T lymphocytes in the cerebrospinal fluid are CD4+ cells.11 Accumulating evidence suggests increased intracerebral infiltration and enhanced peripheral CD4+ T cell responses to Aβ in patients suffering from AD.10,12,13 Regulatory T cells (Tregs) have been proven to play a beneficial role in the pathophysiology of AD, by modulating microglial response to Aβ.14 Regulation of CD4+ T cells differentiation may be a feasible way to affect the pathophysiological changes of AD. Our previous study found that human cord blood-derived stem cells could alter Tregs and alleviate neuroinflammation in AD transgenetic mice.15 Despite current advances in the underlying mechanisms of AD, there remains no effective treatment to cure the intractable disease in clinical practice.
Icariin (ICA), a natural flavonoid glucoside from Epimedium, has been proved to have various pharmacological activities, such as anti-inflammatory,16,17 and neuroprotective effects.18 It presents an activity to prevent neuronal degeneration in AD animal models, by inhibiting neuronal apoptosis and Aβ deposition.19,20 Moreover, ICA negatively regulates the activation of T lymphocytes and pro-inflammatory cytokine profiles in CD4+ T cells.21,22 Meanwhile, ICA plays a regulatory effect on T-helper (Th) 17 and Tregs function.23 These findings indicate that ICA has a potent immune regulating function to prevent chronic neuroinflammatory and might be a promising therapeutic agent for AD.
Transgenic animal models of AD can simulate the behavioral and pathological changes in AD patients, thus are considered as an optimal alternative for testing novel therapeutics.24 The APP/PS1 mouse model, first developed from a cross between the mutant amyloid precursor protein (APP) transgenic line Tg2576 and mutant presenilin 1 (PS1) transgenic mice,25 presents substantial, age-related neuron loss in the pyramidal layer of the hippocampus, which has been widely used to explore underlying AD pathophysiology. Thus, in the present study, we attempted to investigate the specific effect of ICA on Aβ deposition and CD4+ T lymphocyte-related immuno-inflammatory responses in peripheral immune system and brain parenchyma in double transgenic APP/PS1 mice.
Materials and methods
Reagent
ICA (I8760, purity ≥98% via HPLC) was purchased from Beijing Solarbio Science & Technology Co., Ltd. (Beijing, China).
Mice
C57BL/6 mice (n=16, male, 4 months old) and mutant C57BL/6J-TgN (APP/PS1) double transgenic mice (n=16, male, 4 months old) were purchased from Beijing HFK Bio-Technology, Institute of Laboratory Animal Science (Beijing, China). All of the mice were housed in a thermostatic 12-hr light/dark cycle condition with free access to water and food. Animal protocols and procedures have been approved and in accordance with the guidelines of the Ethical Committee for Animal Experiments of Shandong University.
Administration
All mice were randomly divided into four groups (each n=8), which were wild type (WT), WT+ICA, APP/PS1 mice (Tg), and Tg+ICA. ICA was dissolved in distilled water. After 2 weeks of adaptive feeding, animals were orally administered by gavage with ICA (60 mg/kg/d) or distilled water for 8 months. Excluding death and severe weak laboratory animals, 6 experimental animals in each group were randomly selected for the subsequent experiment.
Morris water maze test
The Morris water maze test was performed to assess spatial learning and memory deficits. The pool (120 cm in diameter and 50 cm in height) was filled with water (opaqued with powdered milk) and maintained at 23±1°C. The test was performed 1 week after the last gavage. During the next 5 days, the navigation test was conducted 4 trials per day at 4 different start points, to find the hidden platform located under the water 1.0 cm. Each trial lasted until the animal found the platform or 60 s. Animals failing to find the platform within 60 s were guided to the hidden platform, and stayed for 15 s. Escape latency and the number of platform crossing times were recorded during the platform trials. The escape latency was recorded as 60 s. On the sixth day, the spatial probe test was performed, and the time in the target quadrant was measured. An automatic photographic recording and analysis system (Smart3.0, Panlab) was used to track and analyze behavioral parameters.
Hippocampus Aβ measurement by ELISA
After the behavioral test, mice were anesthetized using chloral hydrate (4 mL/kg). After harvesting peripheral blood, the brains were removed on ice immediately, and hippocampus was separated. Then, the hippocampus tissue was transferred to liquid nitrogen for storage. The levels of soluble and insoluble Aβ40 and Aβ42 in hippocampus were quantified according to previous procedure26 and were determined by ELISA kit (BioLegend) in accordance with the manufacturer’s instructions. Each experimental sample was run in duplicate. The data were recorded at 620 nm using a microplate reader (Biorad, USA).
Flow cytometry
Peripheral blood was harvested in tubes with heparin sodium through angular veins. After centrifuging at 1000 rpm for 30 mins at 4°C, the supernatant plasma was collected for cytokine array. For flow cytometry, the resuspended cells with PBS were isolated by mouse lymphocyte separation medium (Dakewe, China) according to the instruction. Then, cells were incubated with antibodies against CD4+ T, CD8+ T, Th1, Th2, Th17, and Tregs. The antibodies (BioLegend Inc) used in the experiment included anti-mouse FITC-conjugated CD4 (identifying CD4+ T cells), anti-mouse APC/CY7-conjugated CD8 (identifying CD8+ T cells), anti-mouse PE/CY7-conjugated IFN-γ, anti-mouse PE-conjugated IL-4, anti-mouse APC-conjugated IL-17, anti-mouse AF647-conjugated CD25, anti-mouse PE-conjugated Foxp3. Th1 cells were defined as CD4+ T cells expressing IFN-γ, Th2 cells were defined as CD4+ T cells expressing IL-4, Th17 cells were defined as CD4+ T cells expressing IL-17, and Treg cells were defined as CD4+ T cells expressing the molecules CD25 and FOXP3. Cells were resuspended with PBS and were analyzed by a flow cytometer (Beckman CytoFLEX) using CytExpert software.
Cytokine array analysis
In the present study, multiple cytokine levels in the brain and serum were measured using LEGENDplex assay (BioLegend, Beijing Ltd.) according to the instructions of the manufacturer.
Statistical analysis
The data were presented as mean±SEM. Graphs were obtained with GraphPad Prism 6, and comparisons between groups were evaluated by two-way ANOVA followed by Fisher’s LDS multiple comparsions. P-value less than 0.05 was considered to be statistically significant.
Results
ICA treatment alleviates cognitive deficits in APP/PS1 mice
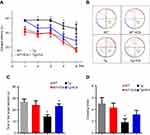
The Morris water maze test was used to examine the effect of ICA on the amelioration of spatial learning and cognitive deficits in APP/PS1 mice. In the place navigation test, APP/PS1 mice presented an increased escape latency to find the hidden platform compared with WT mice, but ICA treatment could significantly decrease the escape latency (Figure 1A, P<0.05). APP/PS1 mice with ICA treatment required less time to locate the original platform position than those with saline lavage in the fourth and fifth training days (Figure 1B). Time spent in the target zone by APP/PS1 mice was significantly shorter than that by WT mice, while ICA treatment could significantly increase the time spent in the target section relative to APP/PS1 mice (Figure 1C, P<0.05). APP/PS1 mice showed obviously decreased number of platform crossing times relative to WT mice (Figure 1D, P<0.05). APP/PS1 mice under ICA treatment moderately increased the crossing times compared with untreated mice, but no significance was observed (Figure 1D). These findings demonstrated that ICA treatment may alleviate cognitive and memory deficits in APP/PS1 mice.
ICA treatment reduces hippocampus Aβ deposition in APP/PS1 mice
Given that Aβ deposition is a major component in AD brain and hippocampus is the key region for memory and cognitive function, we performed ELISA assay to detect both soluble and insoluble Aβ40 and Aβ42 levels in the hippocampus. The levels of insoluble Aβ40 and Aβ42 in the hippocampus of APP/PS1 mice were higher than that in the WT group (Figure 2A and B, P<0.05), and ICA treatment remarkably lowered the level of insoluble Aβ40 and Aβ42 in Tg mice (Figure 2 A and B, P<0.05). Soluble Aβ40 and Aβ42 in the Tg group were significantly increased relative to that in the WT group, while treatment with ICA for 8 months remarkably decreased soluble Aβ40 deposition only (Figure 2C and D, P<0.05). ICA treatment moderately decreased the level of soluble Aβ42 in Tg mice, but no significance was observed (Figure 2D), that might because Aβ42 aggregates more easily to form an insoluble state. Our results indicated that the deposition of Aβ in APP/PS1 mice could be ameliorated under ICA treatment.
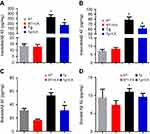
ICA treatment modulates the differentiation of CD4+ T cells in peripheral blood of APP/PS1 mice
To observe the potential of ICA in the regulation of T cell subtypes, we performed a flow cytometry to test the proportion of CD4+and CD8+ T cells. The results showed that the rate of CD4+ T cells in the Tg group was significantly increased compared with that in the WT group (Figure 3A and F, P<0.05), but ICA treatment did not change the proportion of total CD4+ cells in both Tg and WT mice (Figure 3A and F). There was no statistical significance in the proportion of CD8+ T cells among four groups (Figure 3A and G).
Th1, Th2, and Th17 cells were determined by measuring the intracellular cytokines IFN-γ, IL-4 and IL-17, respectively, in CD4+ T cells. The data indicated that Th1 cell proportion was markedly increased in the Tg group compared with that in the WT group, and ICA treatment could decrease the polarization of Th1 in Tg mice (Figure 3B and H, P<0.05). There was no significant difference for Th2 cell proportion among four groups (Figure 3C and I). Tg mice presented significantly increased Th17 cell proportion compared with WT mice, and ICA treatment could decrease the proportion of Th17 cells in Tg mice, but not in WT mice (Figure 3D and J).
Tregs (CD4+CD25+Foxp3+) in APP/PS1 mice were tested according to our previous study.15 As shown in Figure 3E and K, we observed that there was no statistical difference of the percentage of Tregs between the Tg and the WT groups. Whereas, ICA treatment could increase the percentage of Tregs both in the Tg and the WT groups (Figure 3E and K).
ICA treatment alleviates neuroinflammation and regulates the release of inflammatory cytokines in the plasma and brain
Cytokine induction is a critical pathologic event in inflammatory and anti-inflammatory responses in AD. To further study the effect of ICA on immune-inflammatory response, the secretion of several pro-inflammatory cytokines (IL-1α, IL-1β, IL-6, IL-10, IL-12p70, IL-17A, IL-23, IL-27, MCP-1, IFN-β, IFN-γ, TNF-α, and GM-CSF) was detected in both plasma and brain tissues using the multiple detection kit.
The plasma results showed the levels of IL-1β, IFN-γ, IL-6, MCP-1, IL-10, and IL-17A in Tg mice were significantly higher than that in WT mice (Figure 4). ICA treatment could remarkably decrease the levels of IL-1β, TNF-α, IFN-γ, MCP-1, IL-17A, and GM-CSF in Tg mice (Figure 4). There was no statistical difference in the level of TNF-α, GM-CSF, IL-1α, IFN-β, IL-23, IL-12-p70, and IL-27 between the Tg and the WT groups (Figure 4). ICA treatment had no significant effect on cytokines IL-6, IL-10, IL-1α, IFN-β, IL-23, IL-12-p70, and IL-27 (Figure 4).
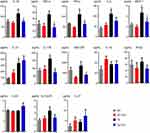 | Figure 4 Alterations of cytokine levels in the plasma. *P<0.05 compared to WT, #P<0.05 compared to Tg. |
In brain tissues, the data showed that the level of IL-1β, TNF-α, IL-6, IL-17A, and GM-CSF were higher in Tg mice than that in WT mice (Figure 5). ICA treatment could significantly lower the levels of IL-1β, IL-17A, IL-12p70, and MCP-1 (Figure 5). Interestingly, ICA treatment could elevate the IL-10 level in the brain tissues of Tg mice (Figure 5). There was no statistical significance in the levels of IL-23, IL-12p70, MCP-1, IL-10, IL-1α, IFN-γ, IFN-β, and IL-27 between WT and Tg mice (Figure 5). ICA treatment had no regulation role for TNF-α, IL-1α, IFN-γ, IFN-β, GM-CSF, and IL-27 in Tg mice (Figure 5).
 | Figure 5 Alterations of cytokine levels in the brain homogenate. *P<0.05 compared to WT, #P<0.05 compared to Tg. |
Discussion
Immune-related abnormalities in the peripheral system of patients are associated with the risk of AD. An effective intervention in immune responses might be a promising strategy to tackle this tough disorder. ICA, as an effective component of traditional Chinese medicine, has been proved to have anti-inflammation and neuroprotective effects.27,28 In the present study, we administered ICA for Tg mice to ameliorate AD pathology. Our results showed that ICA could improve spatial learning and memory of Tg mice, and contribute to the reduced hippocampus Aβ deposition and modulated release of pro-inflammatory cytokines in Tg mice. These effects might be closely related to the function of regulating CD4+ T cell subgroup.
Previous evidence has demonstrated that the proportion of activated CD4+ and CD8+ T cells is elevated in the cerebrospinal fluid of AD patients and the brain of AD mice, compared with that in the controls.29 Our results also suggested an increased proportion of CD4+ cells in the peripheral blood of Tg mice relative to WT mice. Whereas, there was no significant difference of CD8+ cell proportion between Tg and WT mice. The result indicated that the pathological change of AD is accompanied by the change of activated CD4+ T cells in Tg mice.
CD4+ T cells play an important role in the immune system by activating and directing other immune cells. There are at least four distinct Th cell subsets (Th1, Th2, Th17, and Tregs) that have been demonstrated to control immune response. Although Th2 and Tregs have a protective role in the brain, the entry of activated Th1 or Th17 cells is likely to escalate the Aβ-induced glia activation and inflammatory cascade.10 In our study, we observed increased proportion of Th1 and Th17 cells in Tg mice indicating that the imbalance of Th cells might result in more Th1 and Th17 cells infiltrating into brain tissues. The changes in the ratio of pro-inflammatory and anti-inflammatory T cells might be a characteristic of the aggravation of AD, while this inference needs further experimental confirmation.
Several herbal drugs have been reported to have anti-inflammatory action, such as turmeric,30 ginger,31 and resveratrol.32 In this work, we focused on the anti-inflammatory of ICA in APP/PS1 mice. The potential role of ICA in inflammatory responses has been excavated preliminarily. Shen R et al33 found that ICA could suppress the frequencies of Th1 and Th17 cells in the splenocytes and lymph node and inhibit Th17 cells in CNS mononuclear cells, mediating by modulation of dendritic cells. Tao et al21 found that ICA inhibited the Th1/Th17 responses via suppressing STAT1 and STAT3 activation. Our results suggested that ICA could inhibit the differentiation of Th1 and Th17 cells in Tg mice. It is supposed that ICA makes less infiltrate of Th1 and Th17 cells into the brain and lower levels of pro-inflammatory cytokines produced by Th1 and Th17 in peripheral plasma and brain tissues. Dansokho et al14 found that Tregs play a beneficial role in AD pathophysiology by slowing disease progression and modulating microglial response to Aβ deposition. Meanwhile, ICA has influence on the proliferation and differentiation of Tregs.23 In this study, we observed that ICA could improve the differentiation of Tregs in both Tg and WT mice, indicating that ICA might perform inflammation regulatory function by modulating Tregs differentiation.
ICA may have multiple mechanisms of action to bring about the beneficial effect in APP/PS1 mice model of AD. We have demonstrated in this paper that the immuno-inflammatory effect would be one possible mechanism. It is, however, possible that Icariin may have addition mechanism of action to affect autophagy,34 affecting adult neurogenesis or neurogenic stem cell niche,35–37 reducing oxidative stress,38 synaptic injury,38–40 or epigenetic modulation.41
Our study can be further explored by studying the CD4+ cells with the brain. In light of advanced multiplexing mass cytometry technology, this study may be expanded to gain in-depth insight at single cell or single synapse level and can offer insight into progression of disease.40,42 It is known that synaptic injury is cardinal and earliest features of neurogenerative diseases, including AD and Parkinson’s disease. Mass synaptometry offers to study 34 markers from single synapses and thus offers unprecedented bioassay tool to study synapse. Studying ICA or turmeric or any disease-modifying compounds administration early in disease progression may prevent the synaptic degeneration will be powerful novel assay for evaluation of drugs in neurodegeneration in general and AD in particular.40
Conclusion
CD4+T cell-mediated immuno-inflammatory response has been proven to participate in the pathological process of AD. ICA appears to be a promising drug to treat chronic inflammatory responses. Thus, there is practical value to explore the therapeutic potential of ICA in AD. Our research demonstrates the pathological changes of APP/PS1 mice along with the chronic inflammation and the change of CD4+ T subsets ratio. Long-term administration of ICA could decrease the cognitive deficits, reduce Aβ deposition, regulate the differentiation of Th1, Th17, and Tregs cells, and modulate inflammatory cytokines. Taken together, ICA could reduce Aβ deposition and modulate CD4+ T cell-mediated immuno-inflammatory responses in APP/PS1 mice. Our results suggest ICA as a promising drug in the protection against AD.
Acknowledgments
We also appreciate the central laboratory of the Jinan Central Hospital and the School of Medicine of Shandong University for providing experimental places and facilities.
This work was supported by Jinan Municipal Science and Technology Development Program (Grant No. 201212012), Primary Research & Development Plan of Shandong Province (Grant No. 2016GSF121044) and National Natural Science Foundation of China (Grant No. 81373635).
Disclosure
The authors report no conflicts of interest in this work.
References
1. Masters CL, Bateman R, Blennow K, Rowe CC, Sperling RA, Cummings JL. Alzheimer‘s disease. Nat Rev Dis Primers. 2015;1:15056. doi:10.1038/nrdp.2015.56
2.
3. Selkoe DJ, Hardy J. The amyloid hypothesis of Alzheimer‘s disease at 25 years. EMBO Mol Med. 2016;8:595–608. doi:10.15252/emmm.201606210
4. Scheltens P, Blennow K, Breteler MM, et al. Alzheimer‘s disease. Lancet. 2016;388:505–517. doi:10.1016/S0140-6736(15)01124-1
5. Calsolaro V, Edison P. Neuroinflammation in Alzheimer‘s disease: current evidence and future directions. Alzheimers Dement. 2016;12:719–732. doi:10.1016/j.jalz.2016.02.010
6. Kinney JW, Bemiller SM, Murtishaw AS, Leisgang AM, Salazar AM, Lamb BT. Inflammation as a central mechanism in Alzheimer‘s disease. Alzheimers Dement (NY). 2018;4:575–590. doi:10.1016/j.trci.2018.06.014
7. Vom Berg J, Prokop S, Miller KR, et al. Inhibition of IL-12/IL-23 signaling reduces Alzheimer‘s disease-like pathology and cognitive decline. Nat Med. 2012;18:1812–1819. doi:10.1038/nm.2965
8. Heneka MT, Kummer MP, Stutz A, et al. NLRP3 is activated in Alzheimer‘s disease and contributes to pathology in APP/PS1 mice. Nature. 2013;493:674–678. doi:10.1038/nature11729
9. Rogers J, Luber-Narod J, Styren SD, Civin WH. Expression of immune system-associated antigens by cells of the human central nervous system: relationship to the pathology of Alzheimer‘s disease. Neurobiol Aging. 1988;9:339–349.
10. McManus RM, Mills KH, Lynch MA. T cells-protective or pathogenic in Alzheimer‘s disease? J Neuroimmune Pharmacol. 2015;10:547–560. doi:10.1007/s11481-015-9612-2
11. Kivisakk P, Mahad DJ, Callahan MK, et al. Human cerebrospinal fluid central memory CD4+ T cells: evidence for trafficking through choroid plexus and meninges via P-selectin. Proc Natl Acad Sci U S A. 2003;100:8389–8394. doi:10.1073/pnas.1433000100
12. Monsonego A, Nemirovsky A, Harpaz I. CD4 T cells in immunity and immunotherapy of Alzheimer‘s disease. Immunology. 2013;139:438–446. doi:10.1111/imm.12103
13. Wolf SA, Steiner B, Akpinarli A, et al. CD4-positive T lymphocytes provide a neuroimmunological link in the control of adult hippocampal neurogenesis. J Immunol. 2009;182:3979–3984. doi:10.4049/jimmunol.0801218
14. Dansokho C, Ait Ahmed D, Aid S, et al. Regulatory T cells delay disease progression in Alzheimer-like pathology. Brain. 2016;139:1237–1251. doi:10.1093/brain/awv408
15. He Y, Li H, Zhang F, et al. Immunotherapeutic effects of lymphocytes co-cultured with human cord blood-derived multipotent stem cells transplantation on APP/PS1 mice. Behav Brain Res. 2016;315:94–102. doi:10.1016/j.bbr.2016.08.025
16. Xu CQ, Liu BJ, Wu JF, et al. Icariin attenuates LPS-induced acute inflammatory responses: involvement of PI3K/Akt and NF-kappaB signaling pathway. Eur J Pharmacol. 2010;642:146–153. doi:10.1016/j.ejphar.2010.05.012
17. Liu L, Zhao Z, Lu L, et al. Icariin and icaritin ameliorated hippocampus neuroinflammation via inhibiting HMGB1-related pro-inflammatory signals in lipopolysaccharide-induced inflammation model in C57BL/6J mice. Int Immunopharmacol. 2019;68:95–105. doi:10.1016/j.intimp.2018.12.055
18. He XL, Zhou WQ, Bi MG, Du G-H. Neuroprotective effects of icariin on memory impairment and neurochemical deficits in senescence-accelerated mouse prone 8 (SAMP8) mice. Brain Res. 2010;1334:73–83. doi:10.1016/j.brainres.2010.03.084
19. Jin F, Gong QH, Xu YS, et al. Icariin, a phosphodiesterase-5 inhibitor, improves learning and memory in APP/PS1 transgenic mice by stimulation of NO/cGMP signalling. Int J Neuropsychopharmacol. 2014;17:871–881. doi:10.1017/S1461145713001533
20. Li F, Dong HX, Gong QH, Wu Q, Jin F, Shi JS. Icariin decreases both APP and Abeta levels and increases neurogenesis in the brain of Tg2576 mice. Neuroscience. 2015;304:29–35. doi:10.1016/j.neuroscience.2015.06.010
21. Tao F, Qian C, Guo W, Luo Q, Xu Q, Sun Y. Inhibition of Th1/Th17 responses via suppression of STAT1 and STAT3 activation contributes to the amelioration of murine experimental colitis by a natural flavonoid glucoside icariin. Biochem Pharmacol. 2013;85:798–807. doi:10.1016/j.bcp.2012.12.002
22. Li X, Hu Y, He L, et al. Icaritin inhibits T cell activation and prolongs skin allograft survival in mice. Int Immunopharmacol. 2012;13:1–7. doi:10.1016/j.intimp.2012.02.011
23. Wei Y, Liu B, Sun J, et al. Regulation of Th17/Treg function contributes to the attenuation of chronic airway inflammation by icariin in ovalbumin-induced murine asthma model. Immunobiology. 2015;220:789–797. doi:10.1016/j.imbio.2014.12.015
24. Li X, Bao X, Wang R. Experimental models of Alzheimer‘s disease for deciphering the pathogenesis and therapeutic screening (Review). Int J Mol Med. 2016;37:271–283. doi:10.3892/ijmm.2015.2428
25. Holcomb L, Gordon MN, McGowan E, et al. Accelerated Alzheimer-type phenotype in transgenic mice carrying both mutant amyloid precursor protein and presenilin 1 transgenes. Nat Med. 1998;4:97–100.
26. Imbimbo BP, Giardino L, Sivilia S, et al. CHF5074, a novel gamma-secretase modulator, restores hippocampal neurogenesis potential and reverses contextual memory deficit in a transgenic mouse model of Alzheimer‘s disease. J Alzheimers Dis. 2010;20:159–173. doi:10.3233/JAD-2010-1366
27. Schluesener JK, Schluesener H. Plant polyphenols in the treatment of age-associated diseases: revealing the pleiotropic effects of icariin by network analysis. Mol Nutr Food Res. 2014;58:49–60. doi:10.1002/mnfr.201300409
28. Zhang Y, Liu M, Sun H, Yin K. Matrine improves cognitive impairment and modulates the balance of Th17/Treg cytokines in a rat model of Abeta1-42-induced Alzheimer‘s disease. Cent Eur J Immunol. 2015;40:411–419. doi:10.5114/ceji.2015.56961
29. Laurent C, Dorothee G, Hunot S, et al. Hippocampal T cell infiltration promotes neuroinflammation and cognitive decline in a mouse model of tauopathy. Brain. 2017;140:184–200. doi:10.1093/brain/aww270
30. de Souza Tavares W, Akhtar Y, Goncalves GL, et al. Turmeric powder and its derivatives from Curcuma longa rhizomes: insecticidal effects on cabbage looper and the role of synergists. Sci Rep. 2016;6:34093. doi:10.1038/srep34093
31. Gunathilake K, Rupasinghe H. Recent perspectives on the medicinal potential of ginger. Botanics. 2015;5:55–63.
32. Moussa C, Hebron M, Huang X, et al. Resveratrol regulates neuro-inflammation and induces adaptive immunity in Alzheimer‘s disease. J Neuroinflammation. 2017;14:1. doi:10.1186/s12974-016-0779-0
33. Shen R, Deng W, Li C, Zeng G. A natural flavonoid glucoside icariin inhibits Th1 and Th17 cell differentiation and ameliorates experimental autoimmune encephalomyelitis. Int Immunopharmacol. 2015;24:224–231. doi:10.1016/j.intimp.2014.12.015
34. Uddin MS, Stachowiak A, Mamun AA, et al. Autophagy and Alzheimer‘s disease: from molecular mechanisms to therapeutic implications. Front Aging Neurosci. 2018;10:04. doi:10.3389/fnagi.2018.00004
35. Choi SH, Bylykbashi E, Chatila ZK, et al. Combined adult neurogenesis and BDNF mimic exercise effects on cognition in an Alzheimer‘s mouse model. Science. 2018;361:eaan8821. doi:10.1126/science.aan8821
36. Gajera CR, Emich H, Lioubinski O, et al. LRP2 in ependymal cells regulates BMP signaling in the adult neurogenic niche. J Cell Sci. 2010;123:1922–1930. doi:10.1242/jcs.065912
37. Kur E, Christa A, Veth KN, et al. Loss of Lrp2 in zebrafish disrupts pronephric tubular clearance but not forebrain development. Dev Dyn. 2011;240:1567–1577. doi:10.1002/dvdy.22624
38. Tonnies E, Trushina E. Oxidative stress, synaptic dysfunction, and Alzheimer‘s disease. J Alzheimers Dis. 2017;57:1105–1121. doi:10.3233/JAD-161088
39. Serrano-Pozo A, Frosch MP, Masliah E, Hyman BT. Neuropathological alterations in Alzheimer disease. Cold Spring Harb Perspect Med. 2011;1:a006189. doi:10.1101/cshperspect.a006189
40. Gajera CR, Fernandez R, Postupna N, et al. Mass synaptometry: high-dimensional multi parametric assay for single synapses. J Neurosci Methods. 2019;312:73–83. doi:10.1016/j.jneumeth.2018.11.008
41. Grinan-Ferre C, Corpas R, Puigoriol-Illamola D, et al. Understanding epigenetics in the neurodegeneration of Alzheimer‘s disease: SAMP8 mouse model. J Alzheimers Dis. 2018;62:943–963. doi:10.3233/JAD-170664
42. Spitzer MH, Nolan GP. Mass cytometry: single cells, many features. Cell. 2016;165:780–791. doi:10.1016/j.cell.2016.04.019
 © 2019 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2019 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.