Back to Journals » Drug Design, Development and Therapy » Volume 14
LncRNA PVT1 Suppresses the Progression of Renal Fibrosis via Inactivation of TGF-β Signaling Pathway
Authors Cao L, Qin P, Zhang J, Qiao H, Shi P, Huo H
Received 8 January 2020
Accepted for publication 31 July 2020
Published 26 August 2020 Volume 2020:14 Pages 3547—3557
DOI https://doi.org/10.2147/DDDT.S245244
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 2
Editor who approved publication: Professor Anastasios Lymperopoulos
Lu Cao,1 Peng Qin,2 Jianjiang Zhang,1 Huiju Qiao,1 Peipei Shi,1 Huali Huo1
1Department of Pediatrics, The First Affiliated Hospital of Zhengzhou University, Zhengzhou, Henan 450000, People’s Republic of China; 2Department of Cancer Immunotherapy, The Affiliated Cancer Hospital of Zhengzhou University, Zhengzhou, Henan 450000, People’s Republic of China
Correspondence: Lu Cao Email [email protected]
Background: Renal fibrosis is a frequent pathway leading to end-stage kidney dysfunction. In addition, renal fibrosis is the ultimate manifestation of chronic kidney diseases (CKD). Long noncoding RNAs (lncRNAs) are known to be involved in occurrence of renal fibrosis, and lncRNA plasmacytoma variant translocation 1 (PVT1) has been reported to act as a key biomarker in renal diseases. However, the role of PVT1 in renal fibrosis remains unclear.
Materials and Methods: HK-2 cells were treated with TGF-β 1 to mimic renal fibrosis in vitro. Gene and protein expressions in HK-2 cells were measured by qRT-PCR and Western-blot, respectively. ELISA was used to test the level of creatinine (CR) and blood urea nitrogen (BUN) in serum of mice. Additionally, unilateral ureteral obstruction (UUO)-induced renal fibrosis mice model was established to investigate the effect of PVT1 on renal fibrosis in vivo.
Results: PVT1 was upregulated in TGF-β 1-treated HK-2 cells. In addition, TGF-β 1-induced upregulation of α-SMA and fibronectin in HK-2 cells was significantly reversed by PVT1 knockdown. Meanwhile, PVT1 bound to miR-181a-5p in HK-2 cells. Moreover, miR-181a-5p directly targeted TGF-βR1. Furthermore, miR-181a-5p antagonist could significantly reverse the anti-fibrotic effect of PVT1 knockdown. Besides, knockdown of PVT1 notably attenuated the symptom of renal fibrosis in vivo.
Conclusion: Knockdown of PVT1 significantly inhibited the progression of renal fibrosis in vitro and in vivo. Thus, PVT1 may serve as a potential target for the treatment of renal fibrosis.
Keywords: PVT1, renal fibrosis, miR-181a-5p, TGF-βR1
Introduction
Chronic kidney disease (CKD) is a serious health problem all over the world. It has been reported that hypertension and diabetes mellitus are histopathologically characterized by fibrosis.1 In contrast to the adult population, very little is known about the epidemiology of CKD in children, especially in the US.2 A previous report indicated that the prevalence of CKD in children was only 4% lower than that in adults.3 Moreover, the occurrence of CKD in children may due to family inheritance or obesity,4 which causes great injuries to the happiness of children and their parents. As we know, renal fibrosis is a terminal manifestation of CKD.5 Additionally, most of patients with CKD eventually develop to renal fibrosis, following with end-stage renal disease (ESRD).6 In that situation, transplantation is the only effective therapeutic strategy.7 Meanwhile, it has been reported that patients with renal fibrosis accounts for 10% of the world’s population.8 Therefore, novel effective strategies that suppress progression of renal fibrosis are of great importance.
LncRNAs are a class of non-coding RNA transcripts with the length of about 200 nucleotides.9 LncRNAs are key mediators that are notably participated in the progression of multiple diseases.10,11 Previous studies have shown a close correlation between lncRNAs and fibrosis.12,13 For instance, knockdown of lncRNA-ATB could inhibit the progression of renal fibrosis in vitro.14 Meanwhile, lncRNA PVT1 has been found to be upregulated in CKD.15 However, the role of PVT1 in renal fibrosis remains unclear.
TGF-β1 was found to promote fibronectin and collagen production by transcriptional activation of the relevant genes.16 Recent studies have indicated that TGF‑β1 acts as a key biomarker during the progression of renal fibrosis.17–19 Meanwhile, Epithelial-Mesenchymal Transition (EMT) has been noted to play a critical role in embryonic development and tumor metastasis; however, a recent study has reported EMT in chronic lesions leading to renal fibrosis, and there is controversy over its role in renal fibrosis.20 Furthermore, it has been previously reported that activation of TGF‑βsignaling pathway could promote EMT process during the renal fibrosis.21 Based on these backgrounds, TGF-β/EMT plays an important role during renal fibrosis.
In the current study, we sought to investigate the effect of PVT1 on renal fibrosis. We hope our finding may supply a new strategy for the treatment of renal fibrosis.
Materials and Methods
Cell Culture
Human renal tubular epithelial cell lines (HK-2) and HEK-293T cell lines were purchased from ATCC (Manassas, VA, USA) and maintained in RPMI-1640 medium (Thermo Fisher Scientific, Waltham, MA, USA), supplemented with 10% fetal bovine serum (FBS), 1% penicillin (Thermo Fisher Scientific) and 1% streptomycin (Thermo Fisher Scientific) in a humidified incubator with 5% CO2 at 37°C. To establish in vitro model of renal fibrosis, HK-2 cells were treated with TGF-β1 (Pepro Tech, Rocky Hill, NJ, USA) for 48 h.
Reverse Transcription-Quantitative Polymerase Chain Reaction (qRT-PCR)
Total RNA was extracted from HK-2 cell lines using TRIzol reagent (TaKaRa, Tokyo, Japan) according to the manufacturer’s protocol. cDNA was synthesized using the reverse transcription kit (TaKaRa, Ver.3.0) according to the manufacturer’s protocol. qRT-PCRs were performed in triplicate under the following protocol: 2 minutes at 94°C, followed by 35 cycles (30 s at 94°C and 45 s at 55°C). The specific primers used were PVT1 F: 5’-GGCGAGGACTTTAATCTTGGTG-3’, R: 5’- AGACACCACTTTGCCATCCACT-3’; miR-181a-5p F: 5’-GGGGAACATTCAACGCTGT-3’, R: 5’-CTCAACTGGTGTCGTGGAGTC-3’; TGF-βR1, F: 5’-TCGAACTTTGACAGCGACAAG-3’, R: 5’-TTCAGGGCGAGGACCATAG-3’; U6, F: 5’-CTCGCTTCGGCAGCACAT-3’, R: 5’-AACGCTTCACGAATTTGCGT-3’; GAPDH, F: 5’- CATCATCCCTGCCTCTACTGG-3’, R: 5’-GTGGGTGTCGCTGTTGAAGTC-3’. The relative fold changes were calculated using the 2-ΔΔCt method by the formula: 2-(sample ΔCt – control ΔCt), where ΔCt is the difference between the amplification fluorescent thresholds of the gene of interest and the internal reference gene (U6 or GAPDH) used for normalization.
Cell Transfection and Transduction
HEK-293T cells (5×106/well) were transfected with pLVX-IRES-Puro (GenePharma, Shanghai, China) expressing PVT1 short-hairpin RNA (PVT1 shRNA1 or shRNA2; 1 μg/μL, GenePharma, Shanghai, China) or empty vector (pLVX-IRES-Puro). The helper packaging vectors (pLP/VSVG, pLP1 and pLP2, 1 μg/μL) were obtained from Invitrogen (Thermo Fisher Scientific). After transfection, cells were incubated at 37°C for 48 h. Subsequently, the HEK-293T lentiviral supernatant, containing the retroviral particles, was harvested using centrifugation (956×g, 15 min). For transduction, supernatant was passed through a 45 μm filter (Costar, Cambridge, MA, USA) to obtain viral particles, which were then added to HK-2 cells (5×106/well). After transduction for 48 h, cells were selected with puromycin (Sigma, St. Louis, MA, USA). The efficiency of knockdown was verified by qRT-PCR.
For miR-181a-5p transfection, HK-2 cells were transfected with miR-181a-5p agonist, miR-181a-5p antagonist or NC by Lipofectamine 2000 according to the previous reference.22 MiR-181a-5p agonist, miR-181a-5p antagonist and negative control RNAs were purchased from GenePharma (Shanghai, China).
Dual Luciferase Reporter Assay
The partial sequences of PVT1 and 3’-UTR of TGF-βR1 containing the putative binding sites of miR-181a-5p were synthetized and obtained from Sangon Biotech (Shanghai, China), which were then cloned into the pmirGLO Dual-Luciferase miRNA Target Expression Vectors (Promega, Madison, WI, USA) to construct wild-type or mutate type reporter vectors PVT1 (WT/MT) and TGF-βR1 (WT/MT), respectively. The PVT1 (WT/MT) or TGF-βR1 (WT/MT) were transfected into HK-2 cells together with control, vector-control (NC) or miR-181a-5p agonist using Lipofectamine 2000 (Thermo Fisher Scientific) according to the manufacturer’s instructions. The relative luciferase activity was analyzed by the Dual-Glo Luciferase Assay System (Promega). The sequences were as follows: PVT1: 5’- … UGAUCUGUCUUGAUGUGAAUGUG … −3’ (WT); 5’- … UGAUCUGUCUUGAUGCCAGCCUG … −3’ (MT). TGF-βR1: 5’- … CCUUGAUUCACACUUUGAAUGUA … −3’ (WT); 5’- … CCUUGAUUCACACUUCACCGUUA … −3’ (MT).
Western-Blot Detection
Total protein was isolated from cell lysates or tissues by using RIPA buffer. The concentration of protein was detected with a BCA protein kit (Thermo Fisher Scientific). Then, proteins (40 μg per lane) were separated with 10% SDS-PAGE gel and then transferred into polyvinylidene fluoride (PVDF, Thermo Fisher Scientific) membranes. After that, the membranes were blocked with 5% skim milk in TBST for 1 h at room temperature, and incubated with the primary antibodies at 4°C overnight. Then, the membranes were incubated with HRP-conjugated secondary antibody (Abcam; ab6721, 1:5000) for 1 h at room temperature. Finally, the membranes were detected by Enhanced Chemiluminescence (ECL) kit (Thermo Fisher Scientific). The primary antibodies used in this study as follows: anti-TGF-βR1 (Abcam, Cambridge, MA, USA; ab31013, 1:1000), anti-Fibronectin (Abcam; ab2413, 1:1000), anti-α-SMA (Abcam; ab240678, 1:1000), anti-E-cadherin (Abcam; ab233611, 1:1000), anti-Smad3 (Abcam; ab40854, 1:1000), and anti-β-actin (Abcam; ab8226, 1:1000). β-actin was used as an internal control.
RNA Pulldown
RNA pull-down assay was conducted using the Biotin RNA Labeling Mix (Roche, Basel, Switzerland). The biotinylated PVT1 and negative control (bio-NC) were generated via GenePharma and coated to streptavidin-conjugated magnetic beads. HK-2 cells were lysed and then incubated with the magnetic beads for 6 h. The RNA on the beads was isolated and the enrichment level of miR-181a-5p was detected by PCR.
In vivo Study
The experimental protocol was approved by the Ethics Committee for Animal Experimentation of Zhengzhou University and was performed according to the Guidelines for Animal Experimentation of Zhengzhou University. The ethical approval reference number of the study is 20,191,015. Healthy wild type male mice on C57BL/6 (body weight 18 ± 2g) were purchased from the Laboratory Animal Center of Zhengzhou University with SPF-class breeding. Mice were housed under standard conditions with a 12 h light/12 h dark cycle at 22–24°C and allowed free access to water and food. After one week of adaptive feeding, mice were randomly divided into five groups: 1) control (n=12, treated with distilled water); 2) UUO (n=12, treated with distilled water); 3) UUO plus PVT1-NC (n=12, mice were injected with PVT1-NC through tail vein); 4) UUO plus PVT1 shRNA1 (n=12, mice were injected with PVT1 shRNA1 through tail vein); and 5) Control plus PVT1 shRNA1 (n=12, mice were injected with PVT1 shRNA1 through tail vein). For establishment of UUO model, the left ureter of mice was ligated at two points and the latter part was ligated to the uretero-pelvic junction, under anesthesia with intraperitoneal injection of sodium pentobarbital except for control and control plus PVT1 shRNA1 group as previously described.23 Twenty-four hours after UUO surgery, mice were injected through tail vein with either 2×107 Pfu Lv-PVT1 (200 μL) or PVT1-NC (200 μL) twice weekly. At the end of study, the kidney of each mouse was dissected and then the paraffin-embedded renal tissue sections (2 μm) were stained by hematoxylin-eosin (HE) and Masson’s trichrome to evaluate the symptom of renal fibrosis. The severity of fibrosis was demonstrated by selecting 10 non-interfering fields of each section to calculate the ratio of blue-stained scarred areas to the total area.
Measurement of Creatinine (CR) and Blood Urea Nitrogen (BUN)
Serum CR was measured by the sarcosine oxidase method with a Creatinine Assay kit (Jiancheng Bioengineering Institute, Nanjing, China). Meanwhile, BUN was measured by urease method with Urea Assay Kit (Jiancheng Bioengineering Institute, Nanjing, China). The measurement of CR and BUN was carried out on an enzyme-labeled instrument (Thermo Fisher Scientific) as previously described.24
Statistical Analysis
Each group were performed at least three independent experiments and all data were expressed as the mean ± standard deviation (SD). Differences were analyzed using one-way analysis of variance (ANOVA) followed by Tukey’s test (more than 2 groups, Graphpad Prism7). P < 0.05 was considered to indicate a statistically significant difference.
Results
PVT1 shRNA1 Significantly Suppressed the Progression of Renal Fibrosis in vitro
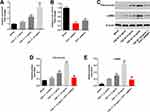
To mimic renal fibrosis in vitro, HK-2 cells were treated with different concentrations of TGF-β (2, 5 or 10 ng/mL). As indicated in Figure 1A, 5 or 10 ng/mL TGF-β1 notably increased the expression of PVT1 in HK-2 cells, while 2 ng/mL TGF-β1 had very limited effect on PVT1 expression. In addition, the level of PVT1 in HK-2 cells was significantly decreased by PVT1 shRNAs (Figure 1B). Since HK-2 cells were more sensitive to PVT1 shRNA1 than PVT1 shRNA2, PVT1 shRNA1 were selected of use in the following experiments. Furthermore, TGF-β1 increased the expression of fibrotic proteins (Fibronectin and α-SMA) in HK-2 cells in a dose-dependent manner (Figure 1C–E). Thus, 10 ng/mL TGF-β1 was selected of our use in the following experiments. Meanwhile, TGF-β1-induced upregulation of Fibronectin and α-SMA was significantly reversed by PVT1 shRNA1 (Figure 1C–E). All these results suggested that silencing of PVT1 significantly inhibited the progression of renal fibrosis in vitro.
PVT1 Sponged miR-181a-5p in HK-2 Cells
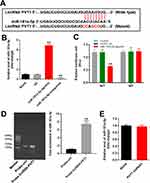
In order to explore the mechanism by which PVT1 mediated the progression of renal fibrosis, miRDB (http://www.mirdb.org/) and starbase (http://starbase.sysu.edu.cn/) were used. As demonstrated in Figure 2A, PVT1 had a putative targeting site of miR-181a-5p. Moreover, co-transfection of the wild-type PVT1 vector (WT-PVT1) with miR-181a-5p agonist significantly reduced luciferase activities compared with mutant PVT1 vector (MT-PVT1) (Figure 2B). Meanwhile, the expression of miR-181a-5p in HK-2 cells was upregulated by miR-181a-5p agonist but inhibited in the presence of miR-181a-5p antagonist (Figure 2C). In addition, the result of RNA pull down further confirmed that PVT1 could bind to miR-181a-5p (Figure 2D). However, PVT1 shRNA1 had very limited effect on miR-181a-5p expression (Figure 2E). Altogether, PVT1 sponged miR-181a-5p in HK-2 cells.
MiR-181a-5p Directly Targeted TGF-βR1 in HK-2 Cells
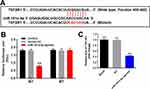
To explore the target of miR-181a-5p, targetscan (http://www.targetscan.org/vert_71/) and miRDB (http://www.mirdb.org/) were performed. As indicated in Figure 3A, TGF-βR1 was found to be the direct target of miR-181a-5p. In addition, co-transfection of the wild-type TGF-βR1 vector (WT-TGF-βR1) with miR-181a-5p agonist significantly reduced luciferase activities compared with mutant TGF-βR1 vector (MT-TGF-βR1) (Figure 3B). Moreover, the expression of TGF-βR1 in HK-2 cells was significantly downregulated by miR-181a-5p agonist (Figure 3C). Taken together, TGF-βR1 was the direct target of miR-181a-5p in HK-2 cells.
MiR-181a-5p Antagonist Significantly Reversed the Anti-Fibrotic Effect of PVT1 shRNA1 in vitro
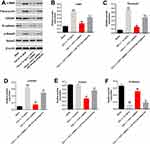
For the purpose of verifying the mechanism by which PVT1 mediated the progression of renal fibrosis, Western blot was performed. As shown in Figure 4A–D, the expressions of α-SMA, Fibronectin and p-Smad3 in HK-2 cells were significantly upregulated by TGF-β1, which was significantly reversed by PVT1 shRNA1. Moreover, PVT1 shRNA1 significantly decreased the expression of TGF-βR1 in TGF-β1-treated HK-2 cells (Figure 4A and E). In contrast, TGF-β1-induced decrease of E-cadherin expression was significantly reversed by PVT1 knockdown (Figure 4A and F). However, the effect of PVT1 shRNA1 on these proteins was significantly inhibited by miR-181a-5p (Figure 4A–F). In summary, miR-181a-5p antagonist significantly reversed the anti-fibrotic effect of PVT1 shRNA1 in vitro.
Silencing of PVT1 Obviously Attenuated the Symptom of Renal Fibrosis in vivo
Finally, the UUO model was established to investigate the effect of PVT1 on renal fibrosis in vivo. As demonstrated in Figure 5A, PVT1 shRNA1 significantly inhibited UUO-induced renal fibrosis in mice. Consistently, as measured by masson staining, the symptom of renal fibrosis was significantly increased in UUO mice, which was notably reduced by knockdown of PVT1 (Figure 5B and C). However, PVT1 shRNA1 alone had very limited effect on control mice (Figure 5A–C). Furthermore, the expression of PVT1 in tissues of mice was notably decreased by UUO, which was partially reversed in the presence of PVT1 shRNA (Figure 5D). Moreover, UUO-induced increase of CR and BUN level in mice was greatly inhibited by PVT1 knockdown (Figure 5E and F). Besides, the protein expression of Fibronectin and p-Smad3 in tissues of mice were significantly upregulated by UUO, and this phenomenon was obviously reversed in the presence of PVT1 shRNA (Figure 6A–C). Meanwhile, the expression of TGF-βR1 in UUO-treated mice was notably inhibited by PVT1 knockdown (Figure 6A and D). Altogether, these data confirmed that the silencing of PVT1 obviously attenuated the symptom of renal fibrosis in vivo via inactivation of TGF-β signaling.
Discussion
Recent studies have indicated that lncRNAs played an important role in the progression of renal fibrosis.12,25 In this research, we confirmed that the expression of PVT1 was significantly upregulated in renal fibrosis. Huang et al found that PVT1 was upregulated in LPS-induced kidney injury via regulation of JNK/NF-κB.26 Our study was similar to this previous data. JNK/NF-κB are known to be a key mediator in kidney injury.27 Meanwhile, our study revealed that PVT1 shRNA inhibited the progression of renal fibrosis via regulation of TGF-β signaling. Thus, this similarity may result from similar function between JNK/NF-κB and TGF-β pathway. Additionally, lncRNA PVT1 could promote the progression of multiple diseases.28–30 Our current research further confirmed these results, suggesting that lncRNA PVT1 could act as a pro-inflammatory agent. In addition, our study firstly found that PVT1 played a key role in the occurrence of renal fibrosis, which supplemented the biological function of PVT1.
Knockdown of lncRNA PVT1 inhibited the renal fibrosis via downregulating the expression of α-SMA and upregulating the expression of E-cadherin. Wang et al found that α-SMA played a regulatory role in progression of fibrosis.31 On the other hand, Notch3 exhibited anti-fibrotic effect through inhibiting the expression of α-SMA.32 Similar to these results, silencing of PVT1 could suppress renal fibrosis via inactivation of α-SMA in TGF-β1-treated HK-2 cells. In addition, E-cadherin and α-SMA played crucial roles in EMT process.33–35 Our results suggested that PVT1 inactivation could inhibit the expression of E-cadherin and α-SMA. Our data were similar to the previous study, indicating that knockdown of PVT1 exhibited the anti-fibrotic effect via downregulation of the EMT process.
We further investigated the mechanism by which PVT1 silencing suppressed the progression of renal fibrosis in vitro and in vivo. The result of Dual luciferase reporter assay indicated that miR-181a-5p was the downstream target gene of PVT1. MiRNAs are highly conserved ncRNAs that exert versatile biological functions.36–38 Wu et al indicated that PVT1 silencing could prevent the development of uveal melanoma through impairing miR-17-3p-dependent MDM2 upregulation.39 Furthermore, PVT1 could modulate the proliferation and apoptosis of lens epithelial cells in diabetic cataract through regulation miR-214-3p/MMP2 axis.30 Besides, it has been previously reported that miR-181a-5p could mediate the progression of renal fibrosis.40 Our study was similar to these previous findings, further indicating that PVT1 knockdown inhibited the progression of renal fibrosis via sponging miR-181a-5p.
TGF-β signaling plays a crucial role in fibrosis.41,42 It was persistently activated in many types of fibrosis.41,43 It has been reported that TGF-β1 can activate Smad3, and the latter induce transcription of pro-fibrotic agents, factors that can interference this signaling pathway may cause fibrosis.44 In the current study, we found that silencing of PVT1 downregulated the expression of p-smad3 in TGF-β1-induced HK-2 cells. Based on these data, the mechanism underlying the anti-fibrosis effects of PVT1 knockdown in vitro and in vivo was associated with the suppression of TGF-β signaling pathways. According to Liu et al, silibinin attenuated the symptoms of fibrotic responses through inhibition of TGF-β/Smad 2/3 signaling.45 Moreover, PVT1 regulated atrial fibrosis via miR-128-3p-SP1-TGF-β-Smad axis in atrial fibrillation.15 Our present study was consistent with these previous data. Besides, we also found that the expression of E-cadherin and α-SMA were notably upregulated in TGF-β1-treated HK-2 cells. Wang et al has found that activation of TGF-βsignaling could promote the EMT process of renal fibrosis.44 Similar to these findings, our data suggested that TGF-βsignaling could promote EMT process during the fibrosis. Taken together, PVT1 was closely associated with TGF-β/Smad3 signaling. Meanwhile, Zhang et al found that PVT1 was also upregulated in pancreatic cancer and it could promote the tumorigenesis of pancreatic cancer via mediation of TGF-β/Smad signaling.46 TGF-β is involved in tumorigenesis.47 Thus, the similarity may due to various functions of TGF-β.
Frankly speaking, this research had some limitations. For instance, this study focused only on the effect of PVT1 on TGF-β signaling pathway. In addition, we found only one miRNA sponged by PVT1. Since bFGF/PI3K/ESRP1 signaling pathways are involved in the fibrotic process,48 further studies are needed to explore the role of lncRNA PVT1 on bFGF/PI3K/ESRP1 pathway. Besides, we will explore more miRNAs sponged by PVT1 in renal fibrosis.
In conclusion, PVT1 shRNA could inhibit the progression of renal fibrosis via mediation of miR-181a-5p/TGF-βR1 axis. Therefore, PVT1 may serve as a potential target for the treatment of renal fibrosis.
Disclosure
The authors declared no competing interests in this research.
References
1. Li N, Cui Y, Yin M, Liu F. Screening potential prognostic biomarkers of long non-coding RNAs for predicting the risk of chronic kidney disease. Braz J Med Biol Res. 2019;52(11):e8333. doi:10.1590/1414-431x20198333
2. Ahn SY, Moxey-Mims M. CKD in children: the importance of a national epidemiologic study. Am J Kidney Dis. 2018;72(5):628–630. doi:10.1053/j.ajkd.2018.07.005
3. Aurelle M, Basmaison O, Ranchin B, et al. Intermittent cholecalciferol supplementation in children and teenagers followed in pediatric nephrology: data from a prospective single-center single-arm open trial. Eur J Pediatr. 2020;179:661–669.
4. Tuttle KR, Alicic RZ, Duru OK, et al. Clinical characteristics of and risk factors for chronic kidney disease among adults and children: an analysis of the CURE-CKD registry. JAMA Netw Open. 2019;2(12):e1918169. doi:10.1001/jamanetworkopen.2019.18169
5. Haffner D, Leifheit-Nestler M. Treatment of hyperphosphatemia: the dangers of aiming for normal PTH levels. Pediatr Nephrol. 2020;35:485–491.
6. Price AM, Hayer MK, Vijapurapu R, et al. Myocardial characterization in pre-dialysis chronic kidney disease: a study of prevalence, patterns and outcomes. BMC Cardiovasc Disord. 2019;19(1):295. doi:10.1186/s12872-019-1256-3
7. Uchida L, Tanaka T, Saito H, et al. Effects of a prolyl hydroxylase inhibitor on kidney and cardiovascular complications in a rat model of chronic kidney disease. Am J Physiol Renal Physiol. 2020;318:F388–F401.
8. Ai K, Zhu X, Kang Y, Li H, Zhang L. miR-130a-3p inhibition protects against renal fibrosis in vitro via the TGF-β1/Smad pathway by targeting SnoN. Exp Mol Pathol. 2019;112:104358. doi:10.1016/j.yexmp.2019.104358
9. Li J, Guo W, Xue W, et al. Long noncoding RNA AURKAPS1 potentiates malignant hepatocellular carcinoma progression by regulating miR-142, miR-155 and miR-182. Sci Rep. 2019;9(1):19645. doi:10.1038/s41598-019-56036-3
10. Zhang T, Cheng G, Sun L, et al. Transcriptome alteration spectrum in rat lung induced by radiotherapy. Sci Rep. 2019;9(1):19701. doi:10.1038/s41598-019-56027-4
11. Xun W, Cen W, Dahai Y, et al. LncRNA miR143HG suppresses miR-21 through methylation to inhibit cell invasion and migration. Laryngoscope. 2019. doi:10.1002/lary.28474
12. Puthanveetil P, Gutschner T, Lorenzen J. MALAT1: a therapeutic candidate for a broad spectrum of vascular and cardiorenal complications. Hypertens Res. 2019:1–8.
13. Wu K, Jiang Y, Zhou W, et al. Long noncoding RNA RC3H2 facilitates cell proliferation and invasion by targeting microRNA-101-3p/EZH2 axis in OSCC. Mol Ther Nucleic Acids. 2020;20:97–110. doi:10.1016/j.omtn.2020.02.006
14. Zhou J, Jiang H. Livin is involved in TGF-β1-induced renal tubular epithelial-mesenchymal transition through lncRNA-ATB. Ann Transl Med. 2019;7(18):463. doi:10.21037/atm.2019.08.29
15. Cao F, Li Z, Ding WM, Yan L, Zhao QY. LncRNA PVT1 regulates atrial fibrosis via miR-128-3p-SP1-TGF-β1-Smad axis in atrial fibrillation. Mol Med. 2019;25(1):7. doi:10.1186/s10020-019-0074-5
16. Li C, Sun X, Li A, Mo M, Zhao Z. S-Allylmercaptocysteine attenuates Bleomycin-induced pulmonary fibrosis in mice via suppressing TGF-beta1/Smad and oxidative stress pathways. Int Immunopharmacol. 2019;79:106110. doi:10.1016/j.intimp.2019.106110
17. Wang K, Wei H, Zhan J, et al. GSPE alleviates renal fibrosis by inhibiting the activation of C3/ HMGB1/ TGF-β1 pathway. Chem Biol Interact. 2020;316:108926
18. Kilari S, Yang B, Sharma A, McCall DL, Misra S. Increased transforming growth factor beta (TGF-β) and pSMAD3 signaling in a murine model for contrast induced kidney injury. Sci Rep. 2018;8(1):1–12. doi:10.1038/s41598-018-24340-z
19. Wang Z, Zhang B, Chen Z, et al. The long noncoding RNA myocardial infarction-associated transcript modulates the epithelial-mesenchymal transition in renal interstitial fibrosis. Life Sci. 2020;241:117187. doi:10.1016/j.lfs.2019.117187
20. Wang YJ, Chen YY, Hsiao CM, et al. Induction of autophagy by pterostilbene contributes to the prevention of renal fibrosis via attenuating NLRP3 inflammasome activation and epithelial-mesenchymal transition. Front Cell Dev Biol. 2020;8:436. doi:10.3389/fcell.2020.00436
21. Yang Z, Zhan YW, Huang YY, et al. Regulation of epithelial mesenchymal transition by the renin-angiotensin system: a role for klotho in renal tubular epithelial cells. J Biol Regul Homeost Agents. 2020;34(1):57–67. doi:10.23812/19-410-A-27
22. Lin P, Li Q, Lv X, et al. HMGA1 promotes the development of esophageal squamous cell carcinoma by mediating miR-671-5p/lncRNA DLEU1. Panminerva Med. 2020.
23. Liu P, Zhang B, Chen Z, et al. m6A-induced lncRNA MALAT1 aggravates renal fibrogenesis in obstructive nephropathy through the miR-145/FAK pathway. Aging. 2020;12(6):5280–5299. doi:10.18632/aging.102950
24. Wang J, Zhou T, Liu J, et al. Application of 1H-MRS in end-stage renal disease with depression. BMC Nephrol. 2020;21(1):225. doi:10.1186/s12882-020-01863-0
25. Zhou C, Wang P, Tu M, et al. Long non-coding RNA part1 promotes proliferation, migration and invasion of hepatocellular carcinoma cells via miR-149-5p/MAP2K1 axis. Cancer Manag Res. 2020;12:3771–3782. doi:10.2147/CMAR.S246311
26. Huang W, Lan X, Li X, et al. Long non-coding RNA PVT1 promote LPS-induced septic acute kidney injury by regulating TNFα and JNK/NF-κB pathways in HK-2 cells. Int Immunopharmacol. 2017;47:134–140. doi:10.1016/j.intimp.2017.03.030
27. Wang L, Liang Q, Lin A, et al. Puerarin increases survival and protects against organ injury by suppressing NF-κB/JNK signaling in experimental sepsis. Front Pharmacol. 2020;11:560. doi:10.3389/fphar.2020.00560
28. Zhan J, Hu P, Wang Y. lncRNA PVT1 aggravates doxorubicin-induced cardiomyocyte apoptosis by targeting the miR-187-3p/AGO1 axis. Mol Cell Probes. 2020;49:101490.
29. Luo K, He J, Yu D, Acil Y. MiR-149-5p regulates cisplatin chemosensitivity, cell growth, and metastasis of oral squamous cell carcinoma cells by targeting TGFβ2. Int J Clin Exp Pathol. 2019;12(10):3728–3739.
30. Yang J, Zhao S, Tian F. SP1-mediated lncRNA PVT1 modulates the proliferation and apoptosis of lens epithelial cells in diabetic cataract via miR-214-3p/MMP2 axis. J Cell Mol Med. 2020;24(1):554–561. doi:10.1111/jcmm.14762
31. Wang Y, Jin BJ, Chen Q, Yan BJ, Liu ZL. MicroRNA-29b upregulation improves myocardial fibrosis and cardiac function in myocardial infarction rats through targeting SH2B3. Eur Rev Med Pharmacol Sci. 2019;23(22):10115–10122. doi:10.26355/eurrev_201911_19581
32. Zhao JL, Zhang T, Shao X, Zhu JJ, Guo MZ. Curcumin ameliorates peritoneal fibrosis via inhibition of transforming growth factor-activated kinase 1 (TAK1) pathway in a rat model of peritoneal dialysis. BMC Complement Altern Med. 2019;19(1):280. doi:10.1186/s12906-019-2702-6
33. Zhang H, Li Z. microRNA-16 via twist1 inhibits EMT induced by PM2.5 exposure in human hepatocellular carcinoma. Open Med. 2019;14:673–682. doi:10.1515/med-2019-0078
34. Yu Y, Zhu T, Li Y, et al. Repeated intravenous administration of silica nanoparticles induces pulmonary inflammation and collagen accumulation via JAK2/STAT3 and TGF-β/Smad3 pathways in vivo. Int J Nanomedicine. 2019;14:7237–7247. doi:10.2147/IJN.S209458
35. Guo LP, Chen LM, Chen F, Jiang NH, Sui L. Smad signaling coincides with epithelial-mesenchymal transition in a rat model of intrauterine adhesion. Am J Transl Res. 2019;11(8):4726–4737.
36. Parashar D, Geethadevi A, Aure MR, et al. miRNA551b-3p activates an oncostatin signaling module for the progression of triple-negative breast cancer. Cell Rep. 2019;29(13):4389–4406 e4310. doi:10.1016/j.celrep.2019.11.085
37. Wang W, Zhang K, Zhang H, et al. Underlying genes involved in atherosclerotic macrophages: insights from microarray data mining. Med Sci Monit. 2019;25:9949–9962. doi:10.12659/MSM.917068
38. Yang M, He X, Huang X, et al. LncRNA MIR4435-2HG-mediated upregulation of TGF-beta1 promotes migration and proliferation of nonsmall cell lung cancer cells. Environ Toxicol. 2019;35:582–590.
39. Wu S, Chen H, Han N, Zhang C, Yan H. Long noncoding RNA PVT1 silencing prevents the development of uveal melanoma by impairing microRNA-17-3p-dependent MDM2 upregulation. Invest Ophthalmol Vis Sci. 2019;60(14):4904–4914. doi:10.1167/iovs.19-27704
40. Xu P, Guan MP, Bi JG, et al. High glucose down-regulates microRNA-181a-5p to increase pro-fibrotic gene expression by targeting early growth response factor 1 in HK-2 cells. Cell Signal. 2017;31:96–104. doi:10.1016/j.cellsig.2017.01.012
41. Jones IC, Espindola MS, Narayanan R, et al. Targeting MAP3K19 prevents human lung myofibroblast activation both in vitro and in a humanized SCID model of idiopathic pulmonary fibrosis. Sci Rep. 2019;9(1):19796. doi:10.1038/s41598-019-56393-z
42. Liang M, Lv J, Jiang Z, et al. The leukotriene B4 -leukotriene B4 receptor 1 axis promotes myofibroblast differentiation and tissue fibrosis in systemic sclerosis. Arthritis Rheumatol. 2019.
43. Wang CJ, Li BB, Tan YJ, et al. MicroRNA-31/184 is involved in transforming growth factor-β-induced apoptosis in A549 human alveolar adenocarcinoma cells. Life Sci. 2019;242:117205. doi:10.1016/j.lfs.2019.117205
44. Wang K, Wei H, Zhan J, et al. GSPE alleviates renal fibrosis by inhibiting the activation of C3/ HMGB1/ TGF-β1 pathway. Chem Biol Interact. 2019;316:108926.
45. Liu K, Zhou S, Liu J, et al. Silibinin attenuates high-fat diet-induced renal fibrosis of diabetic nephropathy. Drug Des Devel Ther. 2019;13:3117–3126. doi:10.2147/DDDT.S209981
46. Zhang X, Feng W, Zhang J, et al. Long non‑coding RNA PVT1 promotes epithelial-mesenchymal transition via the TGF-β/Smad pathway in pancreatic cancer cells. Oncol Rep. 2018;40(2):1093–1102. doi:10.3892/or.2018.6462
47. Takagi K, Midorikawa Y, Takayama T, et al. Effects of pyrrole-imidazole polyamides targeting human TGF-β1 on the malignant phenotypes of liver cancer cells. Molecules. 2020;25(12):2883. doi:10.3390/molecules25122883
48. Weng CM, Li Q, Chen KJ, et al. Bleomycin induces epithelial-to-mesenchymal transition via bFGF/PI3K/ESRP1 signaling in pulmonary fibrosis. Biosci Rep. 2020;40.
 © 2020 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2020 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.