Back to Journals » Drug Design, Development and Therapy » Volume 13
Levonadifloxacin, a Novel Broad-Spectrum Anti-MRSA Benzoquinolizine Quinolone Agent: Review of Current Evidence
Authors Bhagwat SS , Nandanwar M, Kansagara A, Patel A, Takalkar S, Chavan R , Periasamy H, Yeole R , Deshpande PK, Bhavsar S, Bhatia A, Ahdal J , Jain R , Patel M
Received 4 September 2019
Accepted for publication 17 November 2019
Published 24 December 2019 Volume 2019:13 Pages 4351—4365
DOI https://doi.org/10.2147/DDDT.S229882
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 2
Editor who approved publication: Prof. Dr. Tin Wui Wong
Sachin S Bhagwat,1 Manohar Nandanwar,2 Atul Kansagara,2 Anusuya Patel,3 Swapna Takalkar,1 Rajesh Chavan,4 Hariharan Periasamy,1 Ravindra Yeole,5 Prasad K Deshpande,6 Satish Bhavsar,6 Ashima Bhatia,7 Jaishid Ahdal,8 Rishi Jain,8 Mahesh Patel9
1Department of Microbiology, Wockhardt Research Centre, Aurangabad, India; 2Department of Toxicology, Wockhardt Research Centre, Aurangabad, India; 3Department of Safety Pharmacology, Wockhardt Research Centre, Aurangabad, India; 4Department of Drug Metabolism and Pharmacokinetics, Wockhardt Research Centre, Aurangabad, India; 5Department of Analytical Chemistry, Wockhardt Research Centre, Aurangabad, India; 6Department of Medicinal Chemistry, Wockhardt Research Centre, Aurangabad, India; 7Global Clinical Operations, Wockhardt Ltd, Mumbai, India; 8Department of Medical Affairs, Wockhardt Ltd, Mumbai, India; 9Drug Discovery Research, Wockhardt Research Centre, Aurangabad, India
Correspondence: Sachin S Bhagwat
Wockhardt Research Centre, Aurangabad 431006, India
Tel +91240-669-4185
Email [email protected]
Abstract: Levonadifloxacin and its prodrug alalevonadifloxacin are novel broad-spectrum anti-MRSA agents belonging to the benzoquinolizine subclass of quinolone, formulated for intravenous and oral administration, respectively. Various in vitro and in vivo studies have established their antimicrobial spectrum against clinically significant Gram-positive, Gram-negative, atypical, and anaerobic pathogens. The potent activity of levonadifloxacin against MRSA, quinolone-resistant Staphylococcus aureus, and hetero-vancomycin-intermediate strains is an outcome of its well-differentiated mechanism of action involving preferential targeting to DNA gyrase. Potent anti-staphylococcal activity of levonadifloxacin was also observed in clinically relevant experimental conditions such as acidic pH, the intracellular environment, and biofilms, suggesting that the drug is bestowed with enabling features for the treatment of difficult-to-treat MRSA infections. Levonadifloxacin also retains clinically relevant activity against resistant respiratory pathogens such as macrolide- and penicillin-resistant Streptococcus pneumoniae, Streptococcus pyogenes, Haemophilus influenzae, and Moraxella catarrhalis and, in conjunction with clinically established best-in-class human epithelial lung fluid concentration, has promising potential in the management of recalcitrant respiratory infections. Attractive features, such as resistance to NorA efflux, divergent mechanism of action in S. aureus, cidality against high-inoculum cultures, and low mutant prevention concentration, are likely to confer favorable resistance-suppression features to both agents. In vivo studies have shown promising efficacy in models of acute bacterial skin and skin structure infection, respiratory infections, pyelonephritis, and peritonitis at human-equivalent mouse doses. Both formulations were well tolerated in multiple phase I studies and overall showed a dose-dependent exposure. In particular, oral alalevonadifloxacin showed excellent bioavailability (∼90%), almost mirroring the pharmacokinetic profile of intravenous levonadifloxacin, indicating the prodrug’s seamless absorption and efficient cleavage to release the active parent drug. Hepatic impairment studies showed that clinical doses of levonadifloxacin/alalevonadifloxacin are not required to be adjusted for various degrees of hepatic impairment. With the successful completion of phase II and phase III studies for both levonadifloxacin and alalevonadifloxacin, they represent clinically attractive therapeutic options for the treatment of infections caused by multi-drug-resistant Gram-positive organisms. Herein, we review the current evidence on therapeutically appealing attributes of levonadifloxacin and alalevonadifloxacin, which are based on a range of non-clinical in vitro and in vivo investigations and clinical studies.
Keywords: levonadifloxacin, WCK 771, alalevonadifloxacin, WCK 2349, broad-spectrum antibiotic, MRSA
Introduction
The emergence of resistance to antibiotics has resulted in an ongoing battle since the introduction of the first antibiotic, “Penicillin G”. Antibiotic resistance is now viewed as a risk to the world’s sustainable development.1 The Centers for Disease Control and Prevention (CDC) identified certain bacterial pathogens associated with urgent, serious, and concerning threat levels that necessitate attention from all the stakeholders involved in healthcare delivery.2 Among various resistant bacterial pathogens isolated around the world, methicillin-resistant Staphylococcus aureus (MRSA) is one of the major public health concerns.3 The World Health Organization (WHO) estimates a 64% higher likelihood of mortality due to MRSA compared to infection with non-resistant staphylococcal isolates.4 Furthermore, resistance or safety deficiency-linked limitations of current antibiotics and stalled anti-infective drug development pose a heightened risk of various resistance mechanisms spreading globally.5 Besides typical bacteria, incidences of atypical bacterial infections are also on the rise and have attracted global attention to the spread of resistance.6,7 Given the serious hazards of multi-drug-resistant (MDR) bacterial infections and the limitation of in-use antibiotics, there is a need for newer broad-spectrum antibacterials with superior efficacy and safety.8
A new benzoquinolizine subclass of quinolone derivatives, levonadifloxacin (WCK 771, intravenous), which is an arginine salt of the active S(–) isomer of nadifloxacin, has been shown to be more potent than the R(+) isomer and twice as active as the racemic form of nadifloxacin against Gram-positive and Gram-negative bacteria.9 Levonadifloxacin and its prodrug alalevonadifloxacin (WCK 2349, oral) have been evaluated in non-clinical and clinical studies for the treatment of acute bacterial skin and skin structure infections (ABSSSI), community-acquired bacterial pneumonia (CABP) and other types of infections.10 These molecules have undergone a multitude of preclinical in vitro, in vivo (efficacy, safety, and toxicity) and clinical phase I studies. Several phase I studies have been completed in the USA (ClinicalTrials.gov identifiers: NCT01875939, NCT02253342, NCT02244827, NCT02217930) and a phase II study has been conducted in India. Both oral and intravenous forms have been evaluated for the indication of ABSSSI and diabetic foot infections in comparison to oral and intravenous linezolid, in India (ClinicalTrials.gov identifier: NCT03405064). In this review, we discuss the current evidence supporting the therapeutic potential of levonadifloxacin and alalevonadifloxacin.
Drug Profile: Levonadifloxacin (WCK 771) and Alalevonadifloxacin (WCK 2349)
Both levonadifloxacin (WCK 771) and its oral prodrug alalevonadifloxacin (WCK 2349) are being developed by Wockhardt Limited.
Chemistry
Levonadifloxacin belongs to the novel benzoquinolizine subclass of quinolone antibiotics and is being developed as a parenteral formulation in the form of L-arginine salt (WCK 771). In addition, the L-alanine ester prodrug of levonadifloxacin (WCK 2349; alalevonadifloxacin) is being developed as a mesylate salt for oral administration. Chemically, WCK 771 is S-(–)-9-fluoro-6,7-dihydro-8-(4-hydroxypiperidin-1-yl)-5-methyl-1-oxo-1H,5H-benzo[i,j] quinolizine-2-carboxylic acid L-arginine salt tetrahydrate and WCK 2349 is (S)-(–)-9-fluoro-8-(4-L-alaninyl oxypiperidin-1-yl)-5-methyl-6,7-dihydro-1-oxo-1H,5H-benzo[i,j] quinolizine-2-carboxylic acid, methane sulfonic acid salt. The molecular formulae of WCK 771 and WCK 2349 are C25H35FN6O6 0.4H2O and C22H26FN3O5•CH3SO3H, respectively. The molecular weight of WCK 771 is 606.6 g/mol and that of WCK 2349 is 527.6 g/mol. Figure 1 depicts the chemical structures of both molecules. Structurally, levonadifloxacin differs from ciprofloxacin (quinolone core with basic methyl piperazine side chain), moxifloxacin (8-methoxy quinolone core with basic bicyclic side chain), and levofloxacin (benzoxazine core with basic methyl piperazine side chain) by having a benzoquinolizine core attached to a non-basic hydroxy piperidine side chain. Because of its non-basic side chain, levonadifloxacin remains in un-ionized form in acidic pH, which facilitates its entry into the bacterial cell. As a result, there is a significant increase in the potency of levonadifloxacin in acidic environments.9,11,12 This feature could be beneficial for intracellular activity and antibacterial action in infections with an acidic environment.
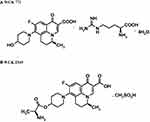 |
Figure 1 Chemical structures of (A) WCK 771 and (B) WCK 2349. Data from De Souza NJ, Gupte SV, Deshpande PK, et al. A chiral benzoquinolizine-2-carboxylic acid arginine salt active against vancomycin-resistant staphylococcus aureus. J Med Chem. 2005;48(16):5232–5242.9 |
Mechanism of Action of WCK 771
DNA gyrase and topoisomerase IV are two bacterial enzymes that are critical for cellular functions. They produce double-stranded breaks in the bacterial chromosome that play an important role in bacterial DNA replication. The quinolones exhibit cidal activity by increasing the concentration of enzyme–DNA cleavage complexes. The cleavage introduced by these complexes leads to permanent chromosomal breaks since bound quinolone physically blocks subsequent ligation reactions.13 Once a large number of DNA strands has broken, this overwhelms other DNA repair mechanisms, leading to bacterial cell death.
In Gram-positive bacteria, most quinolones approved to date are reported to have primary affinity for topoisomerase IV rather than DNA gyrase.14 Since exposures of fluoroquinolones at 1.5–2× minimum inhibitory concentration (MIC) tend to select strains with mutations in the high-affinity target (primary target),14 identification of the primary target of WCK 771 was undertaken through genomic characterization of WCK 771 (along with other comparator fluoroquinolones) in selected mutants of Staphylococcus aureus. These mutants were generated in the laboratory, employing two wild-type S. aureus strains (S. aureus ISP 794 and S. aureus ATCC 29213). Mutants were selected by spreading 109 cells on to Mueller–Hinton agar medium at 1.5× MIC of WCK 771 (2× MIC was mutant prevention concentration [MPC], a concentration at which no mutants were selected) and 2× MIC of moxifloxacin and trovafloxacin. Subsequently, “hot-spot” regions known to harbor mutations responsible for quinolone resistance, quinolone resistance-determining regions (QRDRs) of topoisomerases (DNA gyrase gyrA, gyrB; DNA topoisomerase IV grlA, grlB), of parents as well as mutants, were analyzed using polymerase chain reaction (PCR) and sequencing. WCK 771 mutants (N=8, four from each strain) harbored either S80F or G82D mutation selectively in DNA gyrase (GyrA), while amino acid substitutions in topoisomerase IV (GrlA) were observed in mutants selected on trovafloxacin and moxifloxacin. These results indicated that DNA gyrase is the primary target site for WCK 771 in S. aureus.14,15 This observation was further substantiated by the observed two-fold increase in MICs of WCK 771 in strains with GyrA mutations and no increase in MICs for grlA mutants. MICs of WCK 771 and clinafloxacin, another dual-acting quinolone having high affinity towards DNA gyrase, were superior to trovafloxacin, moxifloxacin, and garenoxacin for the strains harboring up to two and three mutations in QRDR, suggesting their potent activity in sequentially selected mutants.14 This demonstrates that preferential targeting of DNA gyrase by WCK 771 is beneficial in providing superior potency, especially against quinolone-resistant S. aureus harboring multiple mutations in QRDR.
Resistance-suppression studies have shown that in high-level quinolone-resistant (ciprofloxacin MIC ≥16 mg/L) S. aureus clinical isolates, the MPCs determined for WCK 771 were 2 mg/L and 4 mg/L for 68% and 28% of the strains, respectively.15 For moxifloxacin, the MPCs were 16 mg/L and, unlike WCK 771, such concentrations for moxifloxacin were therapeutically not attainable.15
WCK 771: Spectrum of Activity
Gram-Positive Pathogens
WCK 771 shows potent activity against a variety of Gram-positive aerobic bacteria, including S. aureus resistant to methicillin (i.e. MRSA), vancomycin, i.e. hetero-vancomycin-intermediate S. aureus (hVISA) and vancomycin-resistant S. aureus (VRSA), and daptomycin-intermediate/resistant strains. Coagulase-negative staphylococci were also susceptible to WCK 771. VRSA strains resistant to other quinolones were also found to be susceptible to WCK 771. Activity against quinolone-resistant Staphylococcus epidermidis has also been demonstrated. WCK 771 is active against Streptococcus pneumoniae, having variable sensitivity to penicillin and macrolides. Moreover, it is active against other streptococcal species as well.16–20
Gram-Negative Pathogens
WCK 771 demonstrates potent activity against Escherichia coli causing urinary tract infection.12 Activity against Acinetobacter baumannii, E. coli, Haemophilus influenzae, Klebsiella pneumoniae, and Pseudomonas aeruginosa is comparable to other clinically available quinolones.19,20
Atypical Pathogens
Activity against Mycoplasma pneumoniae, M. genitalium, M. hominis, Ureaplasma spp. (including macrolide-, tetracycline-, and levofloxacin-resistant strains), Chlamydophila pneumoniae, and Legionella pneumophila is established.21,22
Anaerobes
WCK 771 was found to be active against Gram-positive anaerobes such as Clostridium perfringens and C. difficile. Similarly, Gram-negative anaerobes Bacteroides fragilis, 'Prevotella and Porphyromonas strains, and β-lactamase-producing Fusobacteria were found to be susceptible to WCK 771.23
Activity Against Bioterror Pathogens
WCK 771 has demonstrated activity against a wide range of bioterror pathogens, including Bacillus anthracis, Burkholderia pseudomallei, Francisella tularensis, Burkholderia mallei, and Yersinia pestis. Potent activity against ciprofloxacin-resistant B. anthracis has also been reported by a leading team from the University of Florida.24
The above studies have established broad-spectrum activity as a feature of WCK 771, along with remarkable activity against methicillin- and/or quinolone-resistant staphylococci.
In Vitro Studies Characterizing Potency and Bactericidal Effect
Most studies are focused on Gram-positive bacteria isolated from a range of hospital- or community-acquired infections. A brief account of these studies is provided below.
In Vitro Activity Against Gram-Positive Organisms
In Vitro Activity Against S. aureus and S. epidermidis
In vitro activity of WCK 771 was determined against US hospital- and community-acquired S. aureus isolates with various resistotypes. In total, 101 methicillin-susceptible Staphylococcus aureus (MSSA) and 196 MRSA isolates were tested. Among the MRSA strains, 110 isolates were hospital acquired and 86 were community acquired. Two hVISA, 25 VISA, and seven VRSA isolates made up the subsets with raised vancomycin and daptomycin MICs. WCK 771 demonstrated MIC50 and MIC90 of 0.03 and 1 mg/L, respectively, against all isolates, whereas the MIC50 and MIC90 of moxifloxacin were 0.25 and 16 mg/L, respectively. For community-acquired and hospital-acquired MRSA strains, WCK 771 MIC50/90 were 0.03/0.5 mg/L and 0.5/1 mg/L, respectively. The numbers of MRSA strains that were inhibited by WCK 771, moxifloxacin, and vancomycin at 2 mg/L were 195, 108, and 178, respectively. Against hVISA, VISA, and VRSA strains, WCK 771 MIC50 and MIC90 were 0.25 and 0.5 mg/L, respectively.10
A study carried out at the Hershey Medical Center, USA, by Bozdogan et al demonstrated low MIC (0.5 mg/L) for WCK 771 against a VRSA strain, HMC3 (isolated from a heel wound), which was resistant to most available bactericidal drugs.17
In a study by Jacobs et al, the in vitro activity of WCK 771 was compared to that of ciprofloxacin, levofloxacin, gatifloxacin, moxifloxacin, clinafloxacin, vancomycin, and linezolid against both quinolone-susceptible and -resistant staphylococci. Against all 116 S. aureus isolates (43 QSSA, 53 QRSA, and 20 VISA), WCK 771 showed MIC50 and MIC90 of 0.5 mg/L and 1 mg/L, respectively. In addition, there were three VISA isolates for which WCK 771 MIC was 1 mg/L (Table 1).16
Strong activity of WCK 771 was also demonstrated against S. epidermidis (N=114), for which MIC50/90 were 0.5/0.5 mg/L. Further, WCK 771 activity was better than other quinolones and comparable to clinafloxacin, a quinolone antibacterial once considered a promising anti-MRSA drug candidate. For the quinolone-resistant S. epidermidis subset, MIC50 and MIC90 of 0.5 and 1 mg/L, respectively, were observed. The study also showed that the NorA efflux pump had no adverse impact on the activity of WCK 771, suggesting that, unlike ciprofloxacin and norfloxacin, WCK 771 is not a substrate of the staphylococcal efflux pump (MICs of 0.015–0.03 mg/L).16
Hackel et al evaluated WCK 771 for its activity against Gram-positive and Gram-negative isolates collected between 2010 and 2012 from various sites throughout the USA and Europe. Against 146 S. aureus isolates, WCK 771 showed MIC90 of 0.5 mg/L. The comparators levofloxacin and moxifloxacin exhibited MIC90 of >16 and 8 mg/L, respectively.20 As part of the SENTRY surveillance program, the in vitro activity of WCK 771 was assessed against a global collection of Gram-positive and Gram-negative isolates (N=12,474) by Flamm et al.19 These isolates were collected from 154 medical centers spread across four continents (USA, Europe, Latin America, and Asia-Pacific). Most of the isolates were collected in the year 2014. The sources of the isolates were patients hospitalized with pneumonia (17.8%), community-acquired pneumonia (18.1%), bloodstream infections (19.6%), urinary tract infections (5.5%), ABSSSIs (28.9%), intra-abdominal infections (2.4%), and other infection types (7.7%). WCK 771 exhibited potent activity against 4077 S. aureus strains with MIC90 of 1 mg/L and 96.8% of all isolates were inhibited at ≤2 mg/L. With regard to the MRSA subset, WCK 771 inhibited 91.5% of isolates at ≤2 mg/L. WCK 771 was also active against methicillin-resistant coagulase-negative staphylococci, with MIC90 of 2 mg/L.19
As described in the previous section, several in vitro MIC determination studies have shown that WCK 771 retains high potency against quinolone-resistant S. aureus.10 To evaluate its bactericidal action, time-kill studies were undertaken. employing an initial inoculum of 106 CFU/mL, wherein pronounced killing was observed (up to 3 logs in 24 h) at 2–8 times of WCK 771 MIC against MRSA (N=2). When initial inoculum was further raised to challenging levels of 108 CFU/mL, WCK 771 continued to demonstrate potent bactericidal activity (at 5 mg/L), whereas the comparator drug, moxifloxacin, failed to do so even at higher concentrations. Apart from demonstrating bactericidal activity at higher density inoculum, WCK 771 was also evaluated against fast- and slow-growing MRSA strains, where it demonstrated good bactericidal activity by 24 h under both conditions. Effective killing of 2 log10 was observed by WCK 771 against slow-growing MRSA strains. The comparator moxifloxacin again failed to show any cidal action in such strains.16 Finally, higher inoculum time-kill studies were also performed against NorA-expressing strains, where the presence of NorA did not impact the activity of WCK 771.16
Bactericidal activity of WCK 771 was monitored against MRSA/QRSA strains by employing an IVPM at human-simulated peak serum concentration (Cmax) and elimination half-life (t1/2) of WCK 771. Various concentrations corresponding to free Cmax of 2, 3, and 4 mg/L of WCK 771 were evaluated, with t1/2 of 5 h. WCK 771 at free Cmax of 4 mg/L with 12 h dosing (BID, twice a day) showed an area under the concentration–time curve (AUC) of 54 µg·h/mL, which was found to be sufficient for cidal action against QRSA/MRSA strains and also prevented the emergence of resistant mutants up to 36 h.25
Dubois and Dubois determined the intracellular activity of WCK 771. Within a clinically achievable concentration range, WCK 771 manifested intracellular bactericidal activity against MSSA and MRSA strains phagocytosed in a human THP-1 monocyte cell line. This observation suggests the potential for clinically relevant activity of WCK 771 in the management of intracellular staphylococcal infections.11
The above-described studies involving staphylococci establish a therapeutically interesting pharmacodynamic profile of WCK 771 against one of the most important Gram-positive genera.
In Vitro Activity Against S. pneumoniae and S. pyogenes
Pneumococcal resistance to penicillin and macrolides has increased worldwide. The in vitro susceptibility of WCK 771 and comparators was determined against S. pneumoniae isolates obtained from the USA and countries in Western Europe. In total, 300 S. pneumoniae strains (consisting of 108 penicillin-susceptible, 92 penicillin-intermediate, and 100 penicillin-resistant S. pneumoniae strains) were studied. MIC50/90 values of WCK 771 for these isolates were 0.5/0.5 mg/L, whereas clinafloxacin exhibited MIC50/90 of 0.12/0.12 mg/L. In quinolone-resistant pneumococcal isolates (N=25), WCK 771 showed raised MIC50/90 values of 4/8 mg/L. Comparator quinolones exhibited varying MIC50/90 values: clinafloxacin 0.5/1 mg/L, gatifloxacin and moxifloxacin 2/4 mg/L, levofloxacin 8/16 mg/L, and ciprofloxacin 16/>32 mg/L. Out of 25 quinolone-resistant pneumococcal isolates, 12 strains showed the presence of efflux by demonstrating a change in MIC for the other quinolone in the presence of reserpine, whereas the MICs of WCK 771 were unaffected. Thus, WCK 771 retained an edge over levofloxacin against quinolone-resistant pneumococcal isolates.26
Promising in vitro potency of WCK 771 against S. pneumoniae was also noted by Al-Lahham et al.27 WCK 771 MIC50/90 values were 0.25/0.5 mg/L against 119 quinolone-susceptible S. pneumoniae, which included 54 penicillin-susceptible, 53 penicillin-intermediate, and 12 penicillin-resistant strains. Consistent with previous results,26 Al-Lahham et al also found an increased WCK 771 MIC50/90 (4/16 mg/L) for quinolone-resistant S. pneumoniae (N=40). WCK 771 was also shown to be highly active (MIC50/90 of 0.25/0.25 mg/L) against S. pyogenes.27 Against S. pneumoniae (N=53), in unpublished data, WCK 771 was found to display lower MICs (MIC50/90 of 0.25/0.25 mg/L) compared to levofloxacin (0.5/1 mg/L). In the unpublished data, WCK 771 showed superior activity against viridans streptococci (N=29; MIC50/90 0.25/0.25 mg/L) and β-hemolytic streptococci (N=64; MIC50/90 0.25/0.25 mg/L). Better anti-pneumococcal activity of WCK 771 against quinolone-susceptible pneumococci (N=177) was also demonstrated by Pankuch et al.28 Furthermore, in a large global surveillance study conducted by Flamm et al, potent activity against S. pneumoniae (N=1196) was observed as 98.1% isolates were inhibited at a 0.5 mg/L concentration of WCK 771.19
Time-kill studies were performed for WCK 771 and other comparators against pneumococcal isolates, including penicillin-susceptible (N=3), penicillin-intermediate (N=3), and penicillin-resistant (N=3) ioslates. One of the penicillin-resistant isolates was also quinolone-resistant (ciprofloxacin MIC 32 mg/L). Time-kill analysis showed bactericidal activity (99.9% kill) of WCK 771 at 2× MIC after 24 h for all nine pneumococcal isolates, including the quinolone-resistant isolate. Other quinolones taken as comparators showed bactericidal activity corresponding to their respective MICs.26
All of these studies support levonadifloxacin’s potential for community use in treating respiratory infections caused by streptococcal organisms.
In Vitro Activity Against Gram-Negative Bacteria
MIC Determination Studies
In vitro activity of WCK 771 was determined against Gram-negative bacteria by Hackel et al. WCK 771 activity was compared with other quinolones against A. baumannii, E. coli, H. influenzae, K. pneumoniae, and P. aeruginosa. Against Enterobacterales (N=233), including E. coli, K. pneumoniae, Enterobacter cloacae, Proteus mirabilis, Citrobacter freundii, and Morganella morganii, WCK 771 demonstrated MIC50/90 of 0.5/>16 mg/L. In the case of P. aeruginosa (N=83) and A. baumannii (N=78), WCK 771 demonstrated MIC50 of 2 and 0.5 mg/L, respectively, whereas MIC90 was >16 mg/L for both. WCK 771 was found to be highly active (MIC50/90 of 0.03/0.06 mg/L) against H. influenzae (N=52). It should be noted that the activity of WCK 771 was comparable to levofloxacin and moxifloxacin against all the studied Gram-negative bacteria.20
Flamm et al studied the in vitro activity of WCK 771 against globally collected Gram-negative bacteria (N=4905), as part of the 2014 SENTRY antimicrobial surveillance program. WCK 771 showed good in vitro activity against Enterobacterales isolates (N=3000), with MIC50/90 of 0.5/>8 mg/L, and 73.2% of isolates inhibited at ≤4 mg/L (pharmacokinetic/pharmacodynamic [PK/PD] susceptibility breakpoint for Enterobacterales) and 70.3% of isolates inhibited at ≤2 mg/L of WCK 771. At 2 mg/L, WCK 771 inhibited 84.2% of levofloxacin-susceptible E. coli isolates. The activity spectrum of WCK 771 included the community respiratory pathogen H. influenzae, against which WCK 771 demonstrated 91.9% inhibition at ≤0.03 mg/L. WCK 771 demonstrated excellent activity against β-lactamase-positive H. influenzae (N=210) isolates (MIC50/90, 0.015/0.03 mg/L), with 99.9% of isolates inhibited at an MIC of ≤4 mg/L. Similarly, against M. catarrhalis (N=504), 97.6% inhibition was observed at ≤0.015 mg/L.19
Evaluation Against Gram-Negative Bacteria Through an In Vitro Pharmacokinetic Model (IVPM)
The pharmacodynamic activity of WCK 771 against Gram-negative isolates was studied using IVPM by Gupte et al. The clinically relevant concentrations corresponding to WCK 771 free AUC(0–24h) of 68 µg·h/mL, free Cmax of 4–5 mg/L, and t1/2 of 6.8 h were employed for Gram-negative strains (N=6) with WCK 771 MICs of 0.25–1.0 mg/L. Applying the criterion of 3 log10 kill at 48 h without resistance development as a successful outcome of drug exposure, WCK 771 was highly bactericidal for these strains, with MICs of 0.25 and 0.5 mg/L showing >3 log reduction.29
In Vitro Activity Against Anaerobes
The in vitro activity of WCK 771 was determined against 350 anaerobes, including B. fragilis, Prevotella, Porphyromona, Fusobacteria, C. perfringens, and C. difficile, by the agar dilution MIC determination method. Various anaerobic Gram-negative bacilli that were tested included B. fragilis group strains (49 of 54; 90.7%), Prevotella and Porphyromonas strains (56 of 104; 53.8%), and β-lactamase-producing Fusobacteria (2 of 47; 4.3%). MIC50 and MIC90 of WCK 771 against the isolates were 0.5 mg/L and 2 mg/L, respectively. Among the quinolones studied, WCK 771 had the lowest MICs for these anaerobic strains. A study performed by Jacobs and Appelbaum also reported good in vitro potency of WCK 771 against Propionibacterium acne, a common causative agent of acne vulgaris.30 These data suggest a potential role of WCK 771 in anaerobic and mixed bacterial infections. The MIC values of WCK 771 in comparison with metronidazole and clindamycin are shown in Table 2.23
In Vitro Activity Against Atypical Bacteria
MIC Determination Studies
In an in vitro study by Xue et al involving atypical bacteria (N=68), which included M. pneumoniae, M. genitalium, and M. hominis, WCK 771 was found to be very active, with MIC50 of 0.125–0.5 mg/L. The MIC of WCK 771 was comparable to moxifloxacin and lower than levofloxacin (eight-fold) and tetracycline (two-fold). For Ureaplasma spp. (N=20), MIC50 of WCK 771 was 0.125 mg/L, which was four- and eight-fold lower than that of moxifloxacin and levofloxacin, respectively. No difference in MICs against isolates with a high level of macrolide resistance was found. Activity against Ureaplasma isolates with tetracycline resistance (tetracycline MICs 16–32 mg/L) was also observed. Against levofloxacin-resistant Ureaplasma spp., WCK 771 was 2 to 16 times more potent than levofloxacin. For quinolone-resistant M. hominis, MICs were seven-fold higher than for susceptible isolates. Minimum bactericidal concentrations (MBCs) of WCK 771 were ≤2 dilutions higher than the MICs, suggesting its potential role in the treatment of infections caused by atypical bacteria with or without macrolide or tetracycline resistance.21 Kohlhoff et al performed WCK 771 susceptibility testing against Chlamydophila pneumoniae (N=10) using HEp-2 cells grown in 96-well microtiter plates. WCK 771 MICs against these isolates ranged from 0.5 to 4 mg/L, while MIC50/90 and MBC90 were 1/2 mg/L and 4 mg/L, respectively. WCK 771 inhibited most of the isolates (7/10) at concentrations ≤1 mg/L.22
Intracellular Activity
The intracellular activity of WCK 771 (1–2× MIC exposed for 6 days) was evaluated using the human monocyte U937 cell line infected with erythromycin-susceptible (N=1) and erythromycin-resistant (N=1) strains of Legionella pneumophila. A significant reduction (>3 log10 CFU/mL) in bacterial count was observed with WCK 771 until 7 days of exposure. Notably, in contrast to levofloxacin and moxifloxacin, where regrowth occurred after drug washout at day 3, WCK 771 showed a delayed regrowth.31
In addition to activity against streptococci and H. influenzae, activity against atypical pathogens would enable clinicians to institute empirical therapy for lower respiratory tract infections where establishing etiology at a primary care level is not possible.
Activity Against Biofilms
An in vitro study assessed the bactericidal activity of WCK 771, along with vancomycin, linezolid, and daptomycin as comparators, in planktonic and biofilm-encapsulated recent MRSA and QRSA isolates. In biofilm-embedded pathogens, WCK 771 displayed more than 90% killing. In contrast, the activities of vancomycin and linezolid were variable and daptomycin showed no killing. Consistent with the bactericidal effect, scanning electron microscope images also showed extensive disruption of the biofilm structure and a reduction in viable bacterial count by WCK 771. These data establish the activity of WCK 771 against biofilm-forming MDR S. aureus infections.32 Thus, WCK 771 shows potential for its use against biofilm-forming S. aureus strains implicated in in-dwelling medical device infection and osteomyelitis.
WCK 771 Activity Against Biodefense Bacterial Pathogens
In addition to Gram-positive and Gram-negative bacteria, the in vitro activity of WCK 771 was assessed against five tier 1 biodefense bacterial pathogens (B. anthracis, B. pseudomallei, F. tularensis, B. mallei, and Y. pestis). Antibiotic susceptibility testing was carried out by the broth microdilution method as per Clinical and Laboratory Standards Institute (CLSI) recommendations. WCK 771 demonstrated potent activity against ciprofloxacin- and ceftazidime-resistant B. pseudomallei strain, while the in vitro activity of WCK 771 was found to be two- to four-fold superior to ciprofloxacin against Y. pestis and F. tularensis. This demonstrates the potential utility of WCK 771 in the treatment of inhalational glanders and melioidosis, necessitating further evaluation in experimental and clinical studies.24 WCK 771 MIC50 and MIC90 were extremely low against B. anthracis, with eight-fold lower MICs than ciprofloxacin. WCK 771 was highly active against ciprofloxacin-resistant B. anthracis, thus paving the way for the oral treatment of anthrax through WCK 2349.
WCK 771: Determination of Quality Control (QC) Ranges
A tier 1 study was undertaken to establish the initial QC ranges for the commonly used CLSI QC strains for disk diffusion and broth microdilution MIC tests. Each QC strain was tested in multiple replicates (N=20) with a fresh inoculum preparation for each replicate.33 The QC parameters for both MIC and zone size determinations were approved and published by CLSI M100 S2634 and are summarized in Table 3.
 |
Table 1 In Vitro Activity of WCK 771 Against Staphylococcus aureus (QSSA, QRSA, VISA) Isolates |
 |
Table 2 MIC50 and MIC90 of WCK 771 in Comparison to Metronidazole and Clindamycin in Different Anaerobes |
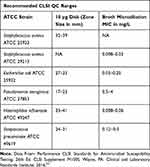 |
Table 3 WCK 771 CLSI QC Ranges for Disk Diffusion and Broth Microdilution Testing |
Hackel et al established a good correlation between agar and broth MICs for WCK 771, suggesting that regardless of the method employed, consistent WCK 771 MICs are obtained. Similarly, correlation analysis between the disk diffusion test and reference broth microdilution MIC for WCK 771 was undertaken, which established that disk diffusion is a reliable method for susceptibility testing.35
Resistance Suppression Potential of WCK 771
Several key studies have pointed towards the superior resistance suppression properties of WCK 771 in comparison to other fluoroquinolones. A large number of staphylococcal isolates express efflux-mediated fluoroquinolone resistance; however, as demonstrated by Jacobs et al, expression of the efflux pump (NorA) had no effect on the MICs of WCK 771, since it is not a substrate for the NorA efflux transporter.16 Similar findings were also reported by Gupte et al.36 High-density kill kinetic studies, a measure of the drug’s ability to tackle high inoculum cultures containing subsets of resistant clones, showed that WCK 771 elicited pronounced killing of 108 CFU/mL cultures of MRSA.37 Also, since WCK 771 shows bactericidal activity against slow-growing MRSA strains, collectively, these features may play a beneficial role in containing the development of resistance in infections involving slow-growing S. aureus.38 Furthermore, on the basis of findings reported by Bhagwat et al, the DNA gyrase preference of WCK 771 leads to several desirable resistance-suppression features, such as lower MPCs, a lower frequency of mutations, and a narrow mutation selection window. In addition to this, WCK 771 retains good potency against strains with multiple mutations in quinolone targets.14
Preclinical In vivo Studies
In Vivo Efficacy Studies Employing Mouse Infection Models
The in vivo efficacy of WCK 771 was evaluated against NorA efflux pump-expressing strains as well as MSSA (N=2) and MRSA (N=6) strains in a mouse peritonitis/systemic infection model. WCK 771 was effective by both oral and subcutaneous (SC) routes (Table 4). In MRSA infections, WCK 771 administered by the SC route exhibited superior efficacy compared to trovafloxacin and sparfloxacin.39
 |
Table 4 In Vivo Efficacy of WCK 771 in a Mouse Systemic Infection Model |
Systemic infection studies were also carried out in mice by employing S. pneumoniae strains of various serotypes, sensitivity, and resistance patterns. ED50 values of orally and subcutaneously administered WCK 771 were in the range of 18–75 mg/kg (N=5) and 3.6–50 mg/kg (N=3), respectively. In mice systemically infected with S. pneumoniae ATCC 6303, oral WCK 771 at 100 mg/kg (twice per day) for 2 days resulted in complete sterility of infected lungs, which was as good as levofloxacin.40
Patel et al demonstrated efficacy in a mouse cellulitis model employing S. aureus (MSSA and MRSA) strains. In vivo bactericidal activity of WCK 771 was observed at doses ranging from 2.5 to 50 mg/kg, which was superior to moxifloxacin and linezolid. Organ bacterial load eradication studies carried out against MRSA strain also demonstrated the bactericidal action of WCK 771 in multiple organs, including the liver, lung, spleen, and kidney. All the mouse doses of WCK 771 employed in these efficacy studies provided clinical equivalent exposures.41 Evaluation of WCK 771 in a neutropenic mouse thigh infection model demonstrated superior eradication potential against MSSA compared to vancomycin and linezolid, whereas against MRSA, the eradication efficacy was superior to vancomycin.41 In another study, with a mouse pyelonephritis model involving infection with E. coli, treatment with WCK 771 (100 mg/kg, twice a day by the oral route) resulted in complete bacterial eradication in the kidneys in 82% of mice at 48 h post-dose.12
Pharmacokinetic/Pharmacodynamic Targets of WCK 771 Against S. aureus in a Neutropenic Lung Infection Model
The relationship between free AUC/MIC, free Cmax/MIC, or percentage free time above MIC (%T>MIC) and pharmacodynamic efficacy was determined employing a 24-h dose fractionation study. Among these three pharmacodynamic indices, free AUC/MIC exhibited the best correlation with efficacy. To determine the PK/PD targets (magnitude of free AUC/MIC), nine S. aureus (MSSA N=3; MRSA/QRSA N=6) isolates were employed in a neutropenic mouse lung infection study. Neutropenic animals were infected through the intranasal route with bacterial inoculum of 5×107 CFU/mL. Subcutaneous treatment with WCK 771 was initiated at 2 h post-infection as a q24h or q12h regimen. Pharmacodynamic efficacy was assessed as the change in lung bacterial burden at 24 h compared to baseline burden at 2 h. By employing exposure response analyses, the free AUC/MIC requirement of WCK 771 for bacteriostatic effect and 1 log10 kill was 8.1±6.0 and 25.8±12.3, respectively.42
Based on the in vitro and in vivo antibacterial profile described above, upon successful development, WCK 771 and its oral prodrug WCK 2349 have the potential to offer a valuable therapeutic option for the treatment of nosocomial and community infections caused by MDR Gram-positive and Gram-negative pathogens.
WCK 771: Safety and Tolerability Studies
Patel et al undertook extensive non-clinical studies to assess the safety and tolerability of intravenous WCK 771. In acute toxicity studies on rodents, it was found that the maximum tolerated doses in Swiss mice and Wistar rats were 425 and 400 mg/kg, respectively. Similarly, in lethality studies, beagle dogs tolerated a single bolus intravenous dose of 125 mg/kg. In phototoxicity studies in mice, WCK 771 was found to be comparable to levofloxacin by exhibiting a no observed effect level (NOEL) of 200 mg/kg. In accordance with its structure, which is devoid of a halogen substituent in the fluoroquinolone core, the phototoxicity of WCK 771 was much lower than halogen substituent-bearing clinafloxacin and sparfloxacin. WCK 771 demonstrated non-mutagenic potential in mouse micronucleus and chromosomal aberration tests on repeat-dose (400 mg/kg) administration for 7 days. A 28-day subacute toxicity study in rats at doses of 175, 245, 350, and 400 mg/kg/day showed no overt toxicity or morbid changes in organs, except for mild phlebitis noticed at 350 and 400 mg/kg. Similarly, a 28-day repeat-dose toxicity study in beagle dogs at intravenous doses of 30, 50, and 80 mg/kg/day did not show overt toxicity or morbid changes in organs. Moreover, phlebitis was not noticed in beagle dogs. The phlebitis potential of WCK 771 (arginine salt) in rats was significantly lower in comparison to its sodium salt. The LD50 for WCK 771 and the sodium salt were 535 and 410 mg/kg in mice and 475 and 375 mg/kg in rats, respectively. The chondrotoxicity potential of WCK 771 was determined in beagle dog pups at doses of 15 and 30 mg/kg administered for 10 days. The NOEL for this study in pups was concluded to be 30 mg/kg.43
Clinical Evidence
Phase I Studies
Multiple phase I studies have been performed with WCK 771 and WCK 2349 in India and the USA. A brief account of phase I studies is discussed in the following sections.
Phase I Single and Multiple Ascending Dose Study (Indian Trial)
A single ascending dose study was performed in healthy Indian subjects with WCK 771 at doses ranging from 50 to 1200 mg (N=6) and oral WCK 2349 at doses ranging from 200 to 1500 mg (N=10). The mean plasma Cmax, AUC(0–infinity), and elimination half-life (t1/2) of WCK 771 after intravenous administration ranged from 1.8 to 32.3 µg/mL, 10.1 to 277.7 µg·h/mL, and 3.6 to 7.2 h, respectively, while following WCK 2349 oral administration, these parameters ranged from 5.1 to 25.6 µg/mL, 31.6 to 226.1 µg·h/mL, and 6.1 to 8.4 h, respectively. The increase in Cmax and AUC was linear across doses.44 A multiple ascending dose study was also conducted in India, with intravenous WCK 771 and oral WCK 2349 at doses of 600, 800, 1000, and 1200 mg administered every 12 h for a duration of 5 days. Both WCK 771 and WCK 2349 showed dose-proportionate increase in levonadifloxacin mean Cmax on day 5, which ranged from 26.3 to 41.26 µg/mL and 14.81 to 33.78 µg/mL respectively, while the mean AUC(0–12) ranged from 142.97 to 263.15 µg·h/mL and 91.11 to 225.21 µg·h/mL, respectively (Table 5). Taking into account both studies, the half-life of WCK 771 ranged between 6.5 and 8.7 h. Both WCK 771 and WCK 2349 were well tolerated at all doses.44 Upon absorption, WCK 2349, being an ester prodrug, is rapidly and completely cleaved to release the active parent drug (levonadifloxacin) upon exposure to esterase enzymes, which are abundantly present in the gut, blood, and liver.
 |
Table 5 Mean Levonadifloxacin Cmax and AUC on Day 5 Following Intravenous WCK 771 and Oral WCK 2349 Administered Twice Daily to Healthy Indian Subjects |
Phase I Single and Multiple Ascending Dose Study (US Trial)
The safety and pharmacokinetics (PK) of levonadifloxacin were assessed in healthy US subjects through multiple twice-daily dosing of either intravenous WCK 771 (600, 800, and 1000 mg) or oral WCK 2349 (800,1000, and 1200 mg) administered for 5 days. The PK parameters observed on day 5 of administration of WCK 771 and its prodrug are presented in Table 6. WCK 771 and WCK 2349 administered in multiple escalating doses were well tolerated. No serious adverse events or death were reported. Adverse events were generally mild to moderate in severity. No clinically significant abnormalities were observed in vital parameters, electrocardiographic (ECG), or phototoxicity assessments.45 Following intravenous WCK 771 administration, the steady-state volume of distribution (Vss) ranged from 145.34 to 172.0 L, clearance (CLss) ranged from 6.7 to 8.2 L/h, and the terminal t1/2 was 8.5–12 h. Over 5 days, the accumulation factor ranged from 0.99 to 1.1, suggesting no or minimal accumulation.46
 |
Table 6 Mean Levonadifloxacin Cmax and AUC on Day 5 Following Intravenous WCK 771 and Oral WCK 2349 Administered Twice Daily to Healthy US Subjects |
A US study by Chugh et al described the effect of food on the oral absorption of WCK 2349 and determined the absolute bioavailability of WCK 2349 at 1000 mg with respect to 800 mg of intravenous WCK 771. Compared to the fasted state, WCK 2349 administered in the fed state reduced the Cmax by 27% and delayed the time to achieve Cmax (Tmax) by 2 h. Thus, the PK profile of WCK 2349 obtained at a dose of 1000 mg was superimposable on that of WCK 771, 800 mg administered as an intravenous infusion. However, there was no change in the AUC values. Hence, the study showed that WCK 2349 could be administered irrespective of fed or fasted state. In relation to WCK 771, the absolute bioavailability of WCK 2349 was 89.35%. The concentration–time profile of levonadifloxacin generated by WCK 771 (800 mg) intravenous infusion coincided well with that of oral WCK 2349 (1000 mg).47 These results indicate that switchover therapy from an intravenous to oral formulation of levonadifloxacin is possible in hospitalized patients.
Phase I Study: PK in Hepatic Impairment
The primary pathway of metabolism of levonadifloxacin is hepatic and, hence, it is essential to understand the PK in patients with hepatic impairment. In a phase I study with a single 1000 mg oral dose of WCK 2349, a total of 24 subjects with hepatic impairment (N=8 each in Child–Pugh classes A, B, and C) and an equal number of matched controls were included. There were no significant differences (p>0.05) in the PK parameters of levonadifloxacin or its sulfate metabolite in mild or moderate hepatic impaired groups compared to normal matched control groups. In the severe hepatic impairment (Child–Pugh class C) group, AUC0–t and AUC0–∞ of levonadifloxacin were significantly higher than in the normal matched group owing to a reduction in the apparent volume of distribution. For the sulfate metabolite, only Tmax was prolonged by 1.3 h compared to controls (p<0.05). However, these differences are not considered to be clinically significant.48 This suggests that even in patients with hepatic impairment, WCK 771 and WCK 2349 can be administered safely to obtain a therapeutically relevant PK profile.
Phase I Study: Intrapulmonary PK
It is essential for a drug to achieve therapeutic concentration in lung tissue to be effective in the treatment of respiratory infections such as hospital- or community-acquired pneumonia. A study was performed in 30 healthy volunteers to determine the intrapulmonary PK of WCK 2349 (1000 mg twice daily for 5 days). The AUC(0–12) values based on mean epithelial lung fluid (ELF) and alveolar macrophage (AM) concentration were 172.6 and 35.3 µg·h/mL, respectively. The calculated AUC ratios of levonadifloxacin in ELF and AMs to the unbound fraction in plasma were 7.66 and 1.58, respectively. This suggests that the lung penetration of levonadifloxacin was excellent.49
These clinical lung penetration data establish the PK/PD basis for the therapeutic potential of levonadifloxacin for respiratory infections, which requires further evaluation in prospective clinical studies.
Phase I Study: Effect on QT Interval
The effect on ECG parameters has been one of the concerns with quinolones. Among the available quinolones, moxifloxacin is said to have the highest risk of QT prolongation and the risk is lower with gemifloxacin, levofloxacin, and ofloxacin.50 Therefore, these effects need to be evaluated in newer generation quinolones. In a thorough QT study, 48 healthy subjects were randomized to placebo, oral WCK 2349 at a supratherapeutic dose of 2600 mg, or oral moxifloxacin (400 mg) groups in a crossover fashion. Compared to the Cmax level of 17.8 µg/mL achieved with a therapeutic dose of 1000 mg twice daily, the supratherapeutic dose of WCK 2349 (2600 mg) resulted in a Cmax of 43.3 µg/mL. This supratherapeutic dose was not associated with significant changes in baseline- and placebo-corrected QTcF (QT interval corrected for heart rate by the Fridericia formula), QRS, or PR interval in healthy volunteers. A transient increment in heart rate observed in the study was clinically insignificant. Considering the safety aspects of the drug, WCK 2349 (2600 mg) was well tolerated. Twenty-six patients reported at least one treatment-emergent adverse effect (TEAE) with WCK 2349, although these were mild in severity. Only one case of moderate-severity TEAE of prolonged QT interval was possibly related to WCK 2349 at a dose of 2600 mg, resulting in subject discontinuation from the study. No deaths or serious adverse events were reported. No significant abnormalities in clinical laboratory parameters, vital signs, 12-lead ECG changes, or findings on physical examination were reported.51,52
Recent Studies
Recently, a phase III study has been completed in India, comparing levonadifloxacin (intravenous and oral) to the gold-standard anti-MRSA drug linezolid (intravenous and oral) in ABSSSI patients (ClinicalTrials.gov identifier: NCT03405064). This study aims to establish the non-inferiority of oral and intravenous levonadifloxacin to oral and intravenous linezolid in ABSSSI, including diabetic foot infection.
Conclusion
Levonadifloxacin and its ester oral prodrug, alalevonadifloxacin, are the broad-spectrum benzoquinolizine subclass of quinolones having activity against multi-drug-resistant Gram-positive pathogens including MRSA, hVISA, and VRSA, as well as quinolone-resistant strains. Activity against Gram-negative, atypical bacteria and anaerobes offers a simplified monotherapeutic advantage for the treatment of polymicrobial infections. Multiple attributes, such as not being a substrate for the NorA efflux pump, preferential DNA gyrase activity, and cidality against high-inoculum cultures and slow-growing staphylococci, contribute to resistance prevention. High systemic exposure along with higher attainment of intrapulmonary levels, as observed in ELF and AM, point towards the clinical utility of WCK 771 and WCK 2349 for the treatment of respiratory infections caused by extracellular and intracellular pathogens. In addition, improved activity in acidic pH, activity against atypical bacteria, and potent activity against anaerobic isolates excluding B. fragilis, would be of benefit in treating ABSSSI, diabetic foot, and bone and joint infection. Being devoid of potential adverse effects, such as phototoxicity, prolongation of QT interval, hepatotoxicity, and nephrotoxicity, WCK 771 and WCK 2349 are poised to offer a valuable therapeutic option for the management of complex and serious bacterial infections. The ecellent bioavailability of oral formulations in both the fasted and fed states is helpful in the smooth switch from parenteral to oral therapy. The well-established pharmacokinetics and safety of both formulations of the drug show their potential for clinical use. No significant serious or untoward clinical or laboratory side effects were seen in any phase I study, indicating that both formulations are well tolerated. The results of phase II and phase III studies should shed more light on clinical aspects of these novel antibacterials.
Acknowledgment
We thank Dr Vijay Katekhaye for his assistance in drafting, reviewing and editing the manuscript.
Disclosure
All the authors are employees of Wockhardt Ltd, Mumbai, India. Dr Sachin S. Bhagwat reports holding Wockhardt shares. The authors report no other conflicts of interest in this work.
References
1. Jasovsky D, Littmann J, Zorzet A, Cars O. Antimicrobial resistance-a threat to the world’s sustainable development. Ups J Med Sci. 2016;121(3):159–164. doi:10.1080/03009734.2016.1195900
2. Centers for Disease Control and Prevention, National Center for Emerging and Zoonotic Infectious Diseases (NCEZID), Division of Healthcare Quality Promotion (DHQP), Antibiotic/Antimicrobial Resistance (AR/AMR). Biggest threats and data. Available from: https://www.cdc.gov/drugresistance/biggest_threats.html.
3. Gajdács M. The continuing threat of methicillin-resistant staphylococcus aureus. Antibiotics. 2019;8(2):52. doi:10.3390/antibiotics8020052
4. World Health Organization. Antimicrobial resistance key facts. Available from: https://www.who.int/news-room/fact-sheets/detail/antimicrobial-resistance.
5. Gajdács M, Albericio F. Antibiotic resistance: from the bench to patients. Antibiotics. 2019;8(3):129. doi:10.3390/antibiotics8030129
6. Yu Y, Fei A. Atypical pathogen infection in community-acquired pneumonia. Biosci Trends. 2016;10(1):7–13. doi:10.5582/bst.2016.01021
7. Huong Ple T, Hien PT, Lan NT, Binh TQ, Tuan DM, Anh DD. First report on prevalence and risk factors of severe atypical pneumonia in vietnamese children aged 1–15 years. BMC Public Health. 2014;14:1304. doi:10.1186/1471-2458-14-1304
8. Gajdács M. The concept of an ideal antibiotic: implications for drug design. Molecules. 2019;24(5):892. doi:10.3390/molecules24050892
9. De Souza NJ, Gupte SV, Deshpande PK, et al. A chiral benzoquinolizine-2-carboxylic acid arginine salt active against vancomycin-resistant staphylococcus aureus. J Med Chem. 2005;48(16):5232–5242. doi:10.1021/jm050035f
10. Bhagwat SS, McGhee P, Kosowska-Shick K, Patel MV, Appelbaum PC. In vitro activity of the quinolone WCK 771 against recent U.S. hospital and community-acquired Staphylococcus aureus pathogens with various resistance types. Antimicrob Agents Chemother. 2009;53(2):811–813. doi:10.1128/AAC.01150-08
11. Dubois J, Dubois M. In vitro extracellular and intracellular activity of Levonadifloxacin (WCK 771) against S. aureus. Abstract Friday-AAR-785.
12. Upadhyay DJ, Patel MV, Gupte SV, et al. WCK 771A-an investigational fluoroquinolone (FQ) with unusual property of retaining potency in acidic medium, human urine and efficacy in mouse pyelonephritis model, abstract F-538.
13. Aldred KJ, Kerns RJ, Osheroff N. Mechanism of quinolone action and resistance. Biochemistry. 2014;53(10):1565–1574. doi:10.1021/bi5000564
14. Bhagwat SS, Mundkur LA, Gupte SV, Patel MV, Khorakiwala HF. The anti-methicillin-resistant Staphylococcus aureus quinolone WCK 771 has potent activity against sequentially selected mutants, has a narrow mutant selection window against quinolone-resistant Staphylococcus aureus, and preferentially targets DNA gyrase. Antimicrob Agents Chemother. 2006;50(11):3568–3579. doi:10.1128/AAC.00641-06
15. Bhagwat SS, Mundkur L, Gupte SV, Khorakiwala HF, Patel MV. WCK 771 selects first step mutants of s. aureus in dna gyrase. Abstract C1-1409.
16. Jacobs MR, Bajaksouzian S, Windau A, et al. In vitro activity of the new quinolone WCK 771 against staphylococci. Antimicrob Agents Chemother. 2004;48(9):3338–3342. doi:10.1128/AAC.48.9.3338-3342.2004
17. Bozdogan B, Esel D, Whitener C, Browne FA, Appelbaum PC. Antibacterial susceptibility of a vancomycin-resistant Staphylococcus aureus strain isolated at the hershey medical center. J Antimicrob Chemother. 2003;52(5):864–868. doi:10.1093/jac/dkg457
18. McHgee P, Appelbaum PC Comparative activity of WCK 771 against S. aureus with raised vancomycin and daptomycin mics and other resistotypes. Abstract F1-2129.
19. Flamm RK, Farrell DJ, Sader HS, Rhomberg PR, Jones RN. In vitro activity of WCK 771, a benzoquinolizine fluoroquinolone (levonadifloxacin) when tested against contemporary gram-positive and -negative bacteria from a global surveillance program. Sunday-456.
20. Hackel M, Bhagwat S, Kanade H, Joshi P, Patel M, Sahm D. In vitro activity of WCk771, a new benzoquinolizine quinolone in development, against key bacterial groups from the USA and Europe. F-1196. Available from: http://wockhardtdiscovery.com/wp-content/plugins/wp-pdf-stamper/stamped-files/F-1196_5702576cc94a1_e.pdf.
21. Xue G, Crabb DM, Xiao L, Liu Y, Waites KB. In vitro activities of the benzoquinolizine fluoroquinolone levonadifloxacin (WCK 771) and other antimicrobial agents against mycoplasmas and ureaplasmas in humans, including isolates with defined resistance mechanisms. Antimicrob Agents Chemother. 2018;62(11):e01348–18. doi:10.1128/AAC.01348-18
22. Kohlhoff S, Huerta N, Hammerschlag M. In vitro activity of levonadifloxacin (WCK 771) against chlamydia pneumoniae. Antimicrob Agents Chemother. 2019;63(8):e01048–19. doi:10.1128/AAC.01048-19
23. Peric M, Jacobs MR, Appelbaum PC. Anti-anaerobic activity of a novel fluoroquinolone, WCK 771, compared to those of nine other agents. Antimicrob Agents Chemother. 2004;48(8):3188–3192. doi:10.1128/AAC.48.8.3188-3192.2004
24. Heine HS, Demons ST, Miller LL, et al. In vitro activity of WCK771, a broad-spectrum anti-MRSA benzoquinolizine subclass of quinolone against five biodefense bacterial pathogens. Abstract F-1188. Available from: http://wockhardtdiscovery.com/wp-content/plugins/wp-pdf-stamper/stamped-files/F-1188_5702595e2d2aa_e.pdf.
25. Patel M, Gupte S, Upadhyay D, et al. WCK 771-predictive MRSA eradication through in vitro pharmacokinetic model (IVPM) based on human PK. Abstract A 1165.
26. Appelbaum PC, Pankuch GA, Bozdogan B, et al. Activity of the new quinolone WCK 771 against pneumococci. Clin Microbiol Infect. 2005;11(1):9–14. doi:10.1111/j.1469-0691.2004.01017.x
27. Al-Lahham A, De Souza NJ, Patel M, Rene Reinert R. Activity of the new quinolones WCK 771, WCK 1152 and WCK 1153 against clinical isolates of Streptococcus pneumoniae and Streptococcus pyogenes. J Antimicrob Chemother. 2005;56(6):1130–1133. doi:10.1093/jac/dki361
28. Pankuch GA, Jacobs MR, Khorakiwala H, De Souza N, Patel M, Appelbaum PC. Anti-pneumococcal activities of WCK 771 and WCK 919 (two new quinolones) compared to 12 other agents against 177 quinolone susceptible pneumococci, abstract F-1194.
29. Gupte S, Patel M, Jafri M, Narayanan P, Khorakiwala A. WCK 771- gram negative breakpoint determination through IVPM (in vitro pharmacokinetic model) study based on human pharmacokinetic parameters. Abstract A-440.
30. Jacobs MR, Appelbaum PC. Nadifloxacin: a quinolone for topical treatment of skin infections and potential for systemic use of its active isomer, WCK 771. Expert Opin Pharmacother. 2006;7:1957–1966. doi:10.1517/14656566.7.14.1957
31. Dubois J, Dubois M. In vitro activity of levonadifloxacin against legionella pneumophilia, abstract P0620.
32. Tellis M, Joseph J, Khande H, Bhagwat S, Patel M. In vitro bactericidal activity of Levonadifloxacin (WCK 771) against methicillin- and quinolone-resistant Staphylococcus aureus biofilms. J Med Microbial. 2019;68(8):1129–1136. doi:10.1099/jmm.0.000999
33. Hackel M, Bhagwat S, Satav J, et al. Determination of tier 1 quality control ranges for WCK 771.
34. Performance CLSI. Standards for Antimicrobial Susceptibility Testing, 26th Ed. CLSI Supplement M100S. Wayne, PA: Clinical and Laboratory Standards Institute; 2016.
35. Hackel M, Bhagwat S, Palwe S, Patel M, Sahm D. Determination of disk diffusion zone and broth dilution MIC for WCK 771.
36. Gupte SV, Patel MV, Agarwal SK, et al. The impact of NorA mediated efflux on in vitro and in vivo anti-Staphylococcal efficacy of an investigational fluoroquinolone (FQ) WCK 771 A and other FQs. Abstract F-537.
37. Patel MV, Appelbaum PC, Jacobs MR, et al. WCK 771 A - an investigational anti-staphylococcal fluoroquinolone (FQ) with strong bactericidal activity against low- and high-density cultures.
38. Upadhyay DJ, Patel MV, Gupte SK, et al. WCK 771 A-an investigational anti-MRSA fluoroquinoone (FQ) with potent concentration independent cidal action and an unusual ability to kill slow growing Staphylococci. Abstract F – 534.
39. Patel MV, De Souza NJ, Gupte SV, et al. Antistaphylococcal activity of WCK 771, a tricyclic fluoroquinolone, in animal infection models. Antimicrob Agents Chemother. 2004;48(12):4754–4761. doi:10.1128/AAC.48.12.4754-4761.2004
40. Gupte S, Patel M, Agarwal S, et al. In vitro and in vivo efficacy of an investigational fluoroquinolone (FQ) WCK 771 A against pneumococci.
41. Patel M, Gupte S, Agarwal S, et al. In vivo efficacy of fluoroquinolone (FQ) WCK 771 A in thigh infections caused by MSSA and MRSA., Abstract F-540.
42. Bhagwat S, Periasamy H, Takalkar S, et al. In vivo pharmacokinetic/pharmacodynamic targets of levonadifloxacin against Staphylococcus aureus in a neutropenic murine lung infection model. Antimicrob Agents Chemother. 2019;63:. doi:10.1128/AAC.00909-19
43. Patel M, Gupte S, Shetty N, et al. WCK 771- an investigational FQ with high intravenous tolerability.
44. Niu J, Ivaturi V, Gobburu J, Chugh R, Bhatia A. Pharmacokinetics of levonadifloxacin administered as intravenous WCK 771 and oral WCK 2349 in healthy Indian male adults.
45. Chugh R, Lakdavala F, Bhatia A. Safety and pharmacokinetics of multiple ascending doses of WCK 771 and WCK 2349. Abstract P1268. 26th Eur congr clin microbial infect dis, Amsterdam, Netherlands.
46. Mehrotra S, Ivaturi V, Gobburu J, Chugh R, Bhatia A. Pharmacokinetics of intravenous WCK 771 in healthy US adults.
47. Chugh R, Lakdavala F, Bhagwat S, Patel M, Bhatia A. Food effect and absolute bioavailability study of WCK 2349 and WCK 771 in healthy adult human volunteer in US.
48. Preston RA, Chugh R, Mastim M, Shukla U, Bhatia A. Single-center evaluation of the pharmacokinetics and safety of the novel fluoroquinolone WCK 2349 in hepatic impairment.
49. Rodvold KA, Gotfried MH, Chugh R, et al. Intrapulmonary pharmacokinetics of levonadifloxacin following oral administration of alalevonadifloxacin to healthy adult subjects. Antimicrob Agents Chemother. 2018;62(3):e02297–2317. doi:10.1128/AAC.01089-18
50. Briasoulis A, Agarwal V, Pierce WJ. QT prolongation and torsade de pointes induced by fluoroquinolones: infrequent side effects from commonly used medications. Cardiology. 2011;120(2):103–110. doi:10.1159/000334441
51. Mason JW, Chugh R, Lakdavala F, Bhatia A. Electrocardiographic effects of WCK 2349.
52. Mason JW, Chugh R, Patel A, Gutte R, Bhatia A. Electrocardiographic effects of a supratherapeutic dose of WCK 2349, a benzoquinolizine fluoroquinolone. Clin Transl Sci. 2019;12(1):47–52. doi:10.1111/cts.v12.1
 © 2019 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2019 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
