Back to Journals » Drug Design, Development and Therapy » Volume 10
Isolation and evaluation of biological efficacy of quercetol in human hepatic carcinoma cells
Authors Ali H , Dixit S, Ali D , Alkahtane AA, Alarifi S , Ali BA, Alakahtani S
Received 27 August 2015
Accepted for publication 4 November 2015
Published 6 January 2016 Volume 2016:10 Pages 155—162
DOI https://doi.org/10.2147/DDDT.S95275
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 2
Editor who approved publication: Prof. Dr. Wei Duan
Huma Ali,1 Savita Dixit,1 Daoud Ali,2 Abdullah A Alkahtane,2 Saud Alarifi,2 Bahy A Ali,2,3 Saad Alkahtani2
1Department of Chemistry, Maulana Azad National Institute of Technology, Bhopal, Madhya Pradesh, India; 2Department of Zoology, College of Science, King Saud University, Riyadh, Saudi Arabia; 3Department of Nucleic Acids Research, Genetic Engineering and Biotechnology Research Institute, City Science Research and Technology Application, Alexandria, Egypt
Abstract: Quercetol is a polyphenolic molecule present in vegetables and fruits, and is beneficial to human and animal health. The current work aimed to test cytotoxic and apoptotic effects of quercetol on HepG2 cells. Quercetol was isolated from Ocimum sanctum and characterized by gas chromatography–tandom mass spectrometry (GC-MS/MS), nuclear magnetic resonance spectroscopy, and Fourier transform infrared spectroscopy. Quercetol (50–600 µg/mL) was examined for cytotoxic activity by tetrazolium salt and neutral red uptake tests and comet assay for genotoxicity, using HepG2 cells, over 24 hours. Data from 3-(4,5-dimethylthiazol-2-yl)-2, 5-diphenyltetrazolium bromide and neutral red uptake tests demonstrated quercetol-induced cytotoxicity in HepG2 cells in a concentration-dependent manner. With 4',6-diamidino-2-phenylindole staining, a significant induction of chromosomal condensation was observed at 300 µg/mL of quercetol. DNA fragmentation analysis showed that quercetol produced cell death in HepG2 cells in a concentration-dependent manner. Thus, our study suggests that an environmentally relevant concentration of quercetol, which was a chemically standardized extract from O. sanctum, induced cell death and DNA damage in HepG2 cells.
Keywords: NMR, FTIR, quercetol, HepG2 cells, MTT assay, apoptosis, comet assay
Introduction
Ocimum sanctum Linn commonly known as Tulsi belongs to Labiateae family. A variety of constituents including flavonoids, tannins, eugenol, dimethyl benzene, ethyl benzene, saponin, and phosphorous were detected in this plant species.1 O. sanctum is a plant used in the preparation of several Ayurvedic pharmacological products.2 Chanda and Nagani3 reported a wide range of beneficial effects such as anticancer, antibacterial, antimicrobial, hepato-protective, antispasmodic, anti-inflammatory, and diaphoretic actions attributed to this plant. Some investigators quantitated quercetol content in fruits (0.002–0.25 g/kg), 0.1 g/kg in vegetables, 0.004–0.016 g/L in wine (red), 0.010–0.025 g/L in tea.4–6 Sethi et al7 reported a significant decrease in sperm count, follicle-stimulating hormone, luteinizing hormone and an increase in serum testosterone levels in O. sanctum-treated rabbits. Oridonin produced DNA damage in HepG2 cells through reactive oxygen species (ROS) generation.8 Flavonoids produce a wide range of biological effects on blood vessels and heart due to antioxidant properties.9
Quercetol exerts antioxidant properties and shows an important role in inhibiting cancer.10 Some researchers reported that quercetol inhibits lipid peroxidation and acts as an inhibitor of xanthine oxidase, oxygen radicals scavengers in vitro.11–13 Skaper et al14 reported that quercetol without ascorbic acid or with ascorbic acid inhibited oxidative damage and neurons loss in skin. Scambia et al15 reported anti-proliferative effects of quercetol in cancer cells.
The current study is an attempt to understand the anti-proliferative and apoptotic potential of quercetol isolated from O. sanctum in HepG2 cell lines.
Materials and methods
Chemicals and plants
Neutral red (NR) dye and ethidium bromide were purchased from Sigma-Aldrich Co. (St Louis, MO, USA). Antibiotics, fetal bovine serum, and DMEM/F-12 media were procured from Thermo Fisher Scientific (Waltham, MA, USA). Other reagents were high quality and purchased from local markets.
Leaves of O. sanctum were accumulated from Sanjeevani; Bhopal, Madhya Pradesh, India. The current study was approved by the ethical committee of Maulana Azad National Institute of Technology.
Extraction and isolation of quercetol
Quercetol was isolated from O. sanctum using the method shown in Figure 1.
  | Figure 1 Method for isolating quercetol from O. sanctum. |
Quercetol
Isolated compound was subjected to ultra violet (UV) spectrophotometer, FTIR gas chromatography–tandom mass spectrometry (GC-MS/MS), and NMR spectroscopy. Figure 2A shows UV spectra analysis of isolated compound. Analysis of infrared (IR) spectra appeared a broad peak for O-H stretch may be hydrogen bonded from 3,412 to 2,712/cm. Stretch 1,664, 1,649, 1,560, and 1,450/cm showed C=C stretch of benzene and 1,242/cm for C-O stretch. 941, 864, 823, 794, and 702/cm showed peaks for substituted benzene (Figure 2B). 1H NMR spectra of the compound showed proton signals at δ7.23 (1H, H-6), δ7.28 (1H, H-8), δ7.02 (1H, H-2′), δ7.33 (1H, H-5′), δ7.54 (1H, H-6′); δ3.56 (1, OH-5), δ3.51 (1, OH-7), δ2.19 (1, OH-3), δ2.03 (1, OH-3′), and δ1.56 (1, OH-4′) (Figure 2C). The (-ve) electro spray ionization mass spectra of the compound exhibited ion [M-H]− at m/e 303, demonstrating a relative weight of 302 (Figure 2D). The result showed high content of the flavonoid 3,3′,4′,5,7-pentahydroxyflavone (quercetol). The compound was purified by re-crystallization with CH3OH to produce 99% pure quercetol.
HepG2 cells and treatments
Human hepatic carcinoma (HepG2) cells were procured from National Centre For Cell Science, Pune, India. HepG2 cells were cultivated in DMEM/F-12 media with 10% fetal bovine serum and penicillin-streptomycin (100 unit/mL) in a CO2 incubator (5%, 37°C). After growth, HepG2 cells were divided into other culture plates and flasks. A stock solution of quercetol (10 mg/mL) was prepared in DMSO and diluted in cell culture media to doses (50, 100, 300, and 600 μg/mL). HepG2 cells unexposed to quercetol act as a control in each assay.
Cell shape
Shape of HepG2 cells was seen after treatment of various dosages of quercetol for 48 hours by an inverted microscope (DM IL; Leica, Wetzlar, Germany).
MTT assay
MTT test was done as described by Mossman.16 HepG2 cells were treated with quercetol (0, 50, 100, 300, and 600 μg/mL) for 24 hours.
NRU test
NRU test assay was performed according to Borenfreund and Puerner method.17
MMP
Measurement of mitochondrial membrane potential (MMP) in HepG2 cell line due to quercetol (0, 50, 100, 300, and 600 μg/mL) for 24 hours was done according to JC-1 mitochondrial membrane potential kit (Item no 10009172) from Cayman Chemical (Ann Arbor, MI, USA).
Assay for condensing of chromosome
Condensed chromosome in HepG2 cells due to quercetol exposure was observed by 2-(4-amidinophenyl)-1H-indole-6-carboxamide (DAPI) staining.
Caspase-3 activity
Twenty four hours later, HepG2 cell culture with or without quercetol were cleaned thrice and reseeded in culture media. Caspase-3 activity was determined by caspase-3 (Red-DEVD-FMK) detection kits and Glomax® multi detection system. The method was used as described by the manufacturers.
DNA strand breakage
DNA strand breakage was done by Comet test method.18
Analysis of results
The result was presented as average, and statistical analysis was done by ANOVA. P<0.05 was used as significant.
Results
Alteration in HepG2 cells
Untreated HepG2 cells are represented in Figure 3A. HepG2 cells are detached from culture plate surface and changed into round shape at 300 and 600 μg/mL quercetol exposure (Figure 3B–C).
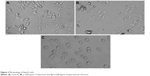  | Figure 3 Morphology of HepG2 cells. |
Viability and MMP of HepG2 cells
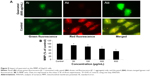
We studied the role of mitochondria (reduction of MTT) and lysosome activity (NRU) as end points of cell toxicity. MTT results confirmed a dose-related cell death after exposure to quercetol in HepG2 cells (Figure 4A). NRU test data is presented in Figure 4B. Data indicated a dose-based decayed in HepG2 cell lines treated to quercetol for 24 hours. MMP declined after 24 hours exposure of quercetol to HepG2 cells using the JC-1 fluorescent probe (Figure 5). Decline in MMP was observed as dose related and highly significant (P<0.05) at 600 μg/mL of quercetol as compared with control.
Condensing of chromosome and caspase-3 enzyme
Condensed chromosome was observed by DAPI staining. HepG2 cells treated with quercetol (50, 100, and 300 μg/mL) for 24 hours induced chromatin condensation (Figure 6). Caspase-3 was induced in cells with quercetol treatment (Figure 6C). HepG2 cells were treated with quercetol (50, 100, and 300 μg/mL) over 24 hours; the activity of caspase-3 was upgraded in a dose-based manner.
DNA fragmentation
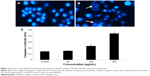
Fragmentation of DNA was quantified as percent DNA in the untreated and quercetol (50, 100, and 300 μg/mL) treated cells. The cells treated with quercetol revealed more fragmented DNA than untreated HepG2 cells. Maximum DNA fragmentation was seen at 300 μg/mL quercetol in HepG2 cells (Figure 7).
Discussion
Nutritional foods are important sources for the treatment of some types of cancers, leading to the development of potential novel agents. Several of the molecules available from foods have been shown to exert anticancer activities on cancer cells. Lee et al19 have reported these effects in animal and cells. This study exposes the quercetol effects on HepG2 cells and explores important possible mechanisms by which quercetol induces toxicity on HepG2 cell lines. This data indicates that quercetol has genotoxic and apoptotic effects on HepG2 cells. Before observing cytotoxic and genotoxic effect of quercetol, we had characterized and purified through re-crystallization with methanol to produce quercetol (99% purity).
Confliction of a few plant extracts which normally used cell toxicity assessment system has been well reported by earlier researchers. Therefore, we have used two assays, namely MTT and NRU, to find out the toxicity of quercetol in HepG2 cells because it increases validity of results. In this study, quercetol produced cell toxicity in concentration-related matter as determined by NRU and MTT tests.
During chemotherapy of several cancers, anticancerous drugs are used. Some researchers reported that ROS normally comprises H2O2, OH°, and O−2 anions, so it induces impairment in cell organelles as nucleic acid damage and finally cell death occurred.20,21 We have seen elevation of caspase-3 activity in HepG2 cells after treatment with quercetol. Quercetol may produce free radicals after their relations with cells. ROS may oxidize and reduce proteins, nucleic acids, and lipids molecule as a consequence of cell damage. In this study, quercetol significantly produced toxicity in HepG2 cells. In the present study, quercetol produced cell death by apoptosis that identified by biochemical and morphological features. Quercetol caused condensation and disintegration of chromosomes in HepG2 Cells.
Quercetol displays an incidental effect on nuclear materials due to its capability to produce ROS. Impairments of nuclear material can either produce carcinogenic cells or cell death, which disturb functions of normal cells. We found the DNA damaging effect of quercetol in HepG2 cells through single gel test that is adept at identifying double- and single-strand breaks in DNA fragmentation.22
In conclusion, these data demonstrate that quercetol has the ability to induce fragmentation of DNA in HepG2 cells. However, it is obvious that further research including cell culture and animal studies are needed to obtain more information and to make concise evaluations on the subject.
Acknowledgment
The authors would like to extend their sincere appreciation to the Deanship of Scientific Research at King Saud University for its funding of this research group NO (RG-1435-076).
Disclosure
The authors report no conflicts of interest in this work.
References
Joseph B, Nair VM. Ethano-pharmacological and phytochemical aspects of Ocimum sanctum Linn the elixir of life. BJPR. 2013;3: 273–292. | ||
Singh V, Amdekar S, Verma O. Ocimum sanctum (Tulsi): bio-pharmacological activities. Webmed Central Pharmacol. 2010;1:1–7. | ||
Chanda S, Nagani K. In vitro and In vivo methods for anticancer activity evaluation and some Indian medicinal plants possessing anticancer properties: an overview. J Pharmacog Phytochem. 2013;2: 140–152. | ||
Herrmann K. Flavonols and flavones in food plants: a review. J Food Technol. 1976;11:433–448. | ||
Hertog MGL, Hollman PCH, van der Putte B. Content of anticarcinogenic flavonoids of tea infusions, wines and fruit juices. J Agric Food Chem. 1993;41:1242–1246. | ||
Pietta PG, Mauri PL, Simonetti P, Testolin G. HPLC and MEKC determination of major flavonoids in selected food pools. Fresenius J Chem. 1995;352:788–792. | ||
Sethi J, Yadav M, Sood S, Dahiya K, Singh V. Effect of Tulsi (Ocimum sanctum Linn.) on sperm count and reproductive hormones in male albino rabbits. Int J Ayurveda Res. 2010;1:208–210. | ||
Chen G, Wang K, Yang B-Y, Tang B, Chen JX, Hua ZC. Synergistic antitumor activity of oridonin and arsenic trioxide on hepatocellular carcinoma cells. Int J Oncol. 2012;40:139–147. | ||
Middleton E Jr, Kandaswami C, Theoharides TC. The effects of plant flavonoids on mammalian cells: implications for inflammation, heart disease, and cancer. Pharmacol Rev. 2000;52:673–751. | ||
Baghel SS, Shrivastava N, Baghel RS, Agrawal P, Rajput S. A review of quercetol: antioxidant and anticancer properties. WJPPS. 2012;1:146–160. | ||
Chen YT, Zheng RL, Jia ZJ, Ju Y. Flavonoids as superoxide scavengers and antioxidants. Free Radic Biol Med. 1990;9:19–21. | ||
Chang WS, Lee YJ, Lu FJ, Chiang HC. Inhibitory effects of flavonoids on xanthine oxidase. Anticancer Res. 1993;13:2165–2170. | ||
Kerry NL, Abbey M. Red wine and fractionated phenolic compounds prepared from red wine inhibits low density lipoprotein oxidation in vitro. Atherosclerosis. 1997;135:93–102. | ||
Skaper SD, Fabris M, Ferrari V. Quercetin protects cutaneous tissue-associated cell types including sensory neurons from oxidative stress induced by glutathione depletion: cooperative effects of ascorbic acid. Free Radic Biol Med. 1997;22:669–678. | ||
Scambia G, Raneletti FO, Panici PB. Inhibitory effect of quercetin on primary ovarian and endometrial cancers and synergistic activity with cis diammine dichloro platinum (II). Gynecol Oncol. 1992;45:13–19. | ||
Mossman T. Rapid colorimetric assay for cellular growth and survival: application to proliferation and cytotoxicity assays. J Immunol Methods. 1983;65:55–63. | ||
Borenfreund E, Puerner J. A simple quantitative procedure using monolayer cultures for cytotoxicity assays (HTD/NR-90). J Tissue Culture Methods. 1984;9:7–9. | ||
Ali D, Verma A, Mujtaba F, Dwivedi A, Hans RK, Ray RS. UVB-induced apoptosis and DNA damaging potential of chrysene via reactive oxygen species in human keratinocytes. Toxicol Lett. 2011;204:199–207. | ||
Lee H Z, Hsu SL, Liu MC, Wu CH. Effects and mechanisms of aloe-emodin on cell death in human lung squamous cell carcinoma. Eur J Pharmacol. 2001;431:287–295. | ||
Ott M, Gogvadze V, Orrenius S, Zhivotovsky B. Mitochondria, oxidative stress and cell death. Apoptosis. 2007;12:913–922. | ||
Rana SV. Metals and apoptosis: recent developments. J Trace Elem Med Biol. 2008;22:262–284. | ||
Collins AR. The comet assay for DNA damage and repair: principles, applications, and limitations. Mol Biotechnol. 2004;26:249–261. |
 © 2016 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2016 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.