Back to Journals » Drug Design, Development and Therapy » Volume 13
Inhibition of Disruptor of Telomeric Silencing 1-Like Alleviated Renal Ischemia and Reperfusion Injury-Induced Fibrosis by Blocking PI3K/AKT-Mediated Oxidative Stress
Authors Yang C, Chen Z, Yu H, Liu X
Received 26 July 2019
Accepted for publication 5 December 2019
Published 27 December 2019 Volume 2019:13 Pages 4375—4387
DOI https://doi.org/10.2147/DDDT.S224909
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 2
Editor who approved publication: Prof. Dr. Yan Zhu
Chuan Yang,1,2 Zhiyuan Chen,1 Hua Yu,2 Xiuheng Liu1
1Department of Urology, Renmin Hospital of Wuhan University, Wuhan, People’s Republic of China; 2Department of Urology, The People’s Hospital of Hanchuan City, Hanchuan, People’s Republic of China
Correspondence: Xiuheng Liu
Department of Urology, Renmin Hospital of Wuhan University, No. 238 Jiefang Road, Wuhan 430060, Hubei Province, People’s Republic of China
Tel/Fax +86-027-88041911
Email [email protected]
Background: Ischemia/reperfusion (I/R) injury is a major cause of acute kidney injury, usually occurs during renal surgeries, and may eventually lead to chronic kidney diseases. However, effective therapeutic targets for renal I/R injury remain limited.
Purpose: In the present study, we investigated whether inhibition of disruptor of telomeric silencing 1-like (Dot1l) could alleviate renal I/R in vivo and in vitro, as well as the potential mechanisms involved in this process.
Methods: Sprague–Dawley rats were subjected to right renal ischemia for 45 mins and reperfusion for 0, 7, or 14 days with and without the Dot1l inhibitor EPZ004777. In addition, human renal proximal tubular epithelial cell line human kidney-2 cells were subjected to the hypoxia/reoxygenation (H/R) process (ie, 3 hrs hypoxia, 12 hrs and 24 hrs reoxygenation), with or without Dot1l inhibitor or genetic knockdown.
Results: Inhibition of Dot1l through EPZ004777 or genetic knockdown reduced the expression of alpha-smooth muscle actin, vimentin, and fibronectin in I/R- and H/R-induced injury. Moreover, H/R-induced fibrosis depended on oxidative stress in vitro. In addition, I/R- and H/R-induced generation of reactive oxygen species (ROS) was attenuated by EPZ004777 or small interfering RNA for Dot1l. Furthermore, the elevation of ROS induced by Dot1l was regulated via phosphatidylinositol 3-kinase (PI3K) and serine-threonine protein kinase (AKT) phosphorylation in vivo and in vitro.
Conclusion: Inhibition of Dot1l alleviated renal fibrosis by preventing the generation of ROS via the PI3K/AKT pathway. These results indicate that inhibitor of Dot1l could be a potential therapeutic target for renal I/R injury.
Keywords: disruptor of telomeric silencing 1-like, ischemia and reperfusion, oxidative stress, fibrosis
Introduction
Acute kidney injury (AKI) is a common clinical complication usually induced by systemic inflammation, ischemic insult, and sepsis. Renal ischemia/reperfusion (I/R) injury is one of the most common cause of AKI.1–3 Renal I/R injury is an inevitable consequence of kidney transplantation, leading to renal fibrosis and progression of graft dysfunction.4,5 Progressive tubulointerstitial fibrosis is the final way for many chronic kidney diseases (CKDs), which leads to end-stage renal failure. Currently, there are limited treatment options for I/R-induced tubulointerstitial fibrosis. Therefore, identifying the key mechanisms involved in renal I/R injury may offer protection to patients in this setting.6
Disruptor of telomeric silencing 1-like (Dot1l) is located on the nucleosome surface and the only known histone methyltransferase targeting the H3K79 position.7 Similar to its homolog gene Dot1, Dot1l catalyzes the mono-, di-, and tri-methylation of H3K79 specifically through a unique catalytic domain. It has been associated with numerous biological processes, such as cell cycle progression, DNA damage response, transcriptional regulation, somatic reprogramming, and embryonic cell development.8 In addition, other studies suggested that Dot1l plays a key role in the genesis and development of mixed lineage leukemia and tumorigenesis.9–11 As a highly conserved protein in different species, Dot1l is widely expressed in rats and humans.12,13 However, the role of Dot1l in renal I/R injury remains unclear.
I/R affects oxidative phosphorylation, resulting in an elevated production of reactive oxygen species (ROS) during the reperfusion process. The contribution of ROS in the progression of renal injury is highlighted by the capacity of several antioxidants to attenuate renal I/R.14–16 The excessive generation of ROS can induce protein, DNA, and lipid damage. In this study, we examined whether inhibition of Dot1l could modulate renal I/R-induced fibrosis in rats. We also investigated the potential mechanisms involved in the inhibition of Dot1l on the phosphatidylinositol 3-kinase/serine-threonine protein kinase (PI3K/AKT)-mediated generation of ROS.
Materials and Methods
Antibodies and Reagents
EPZ004777 (EPZ) was purchased from Selleck Chemicals (Houston, TX, USA). N-acetyl-cysteine (NAC) and polyethylene glycol–catalase were supplied by Sigma–Aldrich (St. Louis, MO, USA). Antibodies used in Western blotting (WB), namely rabbit anti-Dot1l, PI3K, AKT, phosphorylated AKT (p-AKT), and anti-β-actin were purchased from Abcam (Cambridge, UK). Antibodies against alpha-smooth muscle actin (α-SMA), vimentin, and fibronectin were obtained from Santa Cruz Biotechnology (Santa Cruz, CA, USA). Dichlorofluorescein diacetate solution was supplied by Beyotime Biotechnology (Jiangsu, China). Creatinine and urea commercial kits, the superoxide dismutase (SOD) assay kit, and the malondialdehyde (MDA) assay kit were purchased from Nanjing Jiancheng Bioengineering Institute (Nanjing, China).
Experimental Animals and the I/R Model
Adult male Sprague–Dawley rats, weighing 220–250 g, were afforded from Experimental Animal Center of the Medical College of Wuhan University (Wuhan, China), and housed under set conditions (temperature: 20–22°C; 12-h light/dark cycle). The rats had ad libitum access to water and standard chow. This study was approved by the committee of experimental animals of Wuhan University (No. W20160188), and the procedures complied with the Guidelines for the Care and Use of Laboratory Animals. EPZ was solubilized with dimethyl sulfoxide, and intraperitoneally injected once daily for 1 week in the EPZ-treated groups prior to establishing the model; different dosages were used according to the experimental design. The I/R model was performed as follows. Briefly, an intraperitoneal injection of pentobarbital (50 mg/kg) was adopted to anesthetize the animals. Subsequently, the rats were maintained at a core temperature of 37°C. During surgery, a midline incision was performed and the right kidney was excised. The left renal pedicles were clamped for 45 mins. Notably, these pedicles were not clamped in the sham group. According to the experimental design, the rats were euthanized at days 0, 7, and 14 after reperfusion.
Cell Culture
The human renal proximal tubular epithelial cell line human kidney-2 (HK-2) was supplied by the American Type Culture Collection (Manassas, VA, USA). The cell hypoxia/reoxygenation (H/R) model was established as follows. Briefly, HK-2 cells were exposed to a hypoxic condition (37°C, 1% oxygen, 94% nitrogen, and 5% carbon dioxide [CO2]) for 12 hrs in glucose- and serum-free medium to induce hypoxic injury. Subsequently, the medium was replaced, and the cells were placed under the normal condition (5% CO2) for reoxygenation (12 hrs and 24 hrs), according to the experimental design. The control group was cultured under the normal condition (5% CO2).
Real-Time Quantitative Reverse Transcription-Polymerase Chain Reaction (qRT-PCR)
RNAiso Plus (TaKaRa Biotech, Dalian, China) was used to extract total RNA from frozen kidney tissues and HK-2 cells according to the instructions provided by the manufacturer. Subsequently, the PrimeScript™ RT Reagent Kit (TaKaRa Biotech) was used for reverse transcription into cDNA. In all PCR experiments, the expression of glyceraldehyde-3-phosphate dehydrogenase was used as the internal reference. The qRT-PCR analysis was performed using the ABI ViiA7DX System (Applied Biosystems, Foster City, CA, USA). The following qRT-PCR primers for the specific target genes (listed below) were designed and synthesized by TaKaRa Biotech:
R-Dot1l: 5‘-TGACCGCACCATACTTGAAAACTA-3ʹ(F)
5‘-GGCTCTTCTTGGACTTGCGAT-3ʹ(R);
R -β-actin: 5‘-TGCTATGTTGCCCTAGACTTCG-3ʹ(F)
5‘-GTTGGCATAGAGGTCTTTACGG-3ʹ(R);
H-α-SMA: 5‘-CCTATCCCCGGGACTAAGAC-3ʹ(F), 5‘-CCATCACCCCCTGATGTCTG-3ʹ(R);
H-Vimentin: 5‘-GGAGGAGATGCTTCAGAGAGAG-3ʹ(F), 5‘-GGATTTCCTCTTCGTGGAGTTTC-3ʹ(R);
H-Fibronectin: 5‘-GCCGAATGTAGGACAAGAAGC-3ʹ(F),
5‘- AAGCACGAGTCATCCGTAGGT-3ʹ(R);
H-β-actin: 5‘-CACCCAGCACAATGAAGATCAAGAT-3ʹ(F), 5‘-CCAGTTTTTAAATCCTGAGTCAAGC-3ʹ(R);
Routine qRT-PCR was performed as follows: 94°C for 3 mins, followed by 30 cycles (25 cycles for β-actin) at 94°C for 30 s, 55°C for 30 s, and 72°C for 1 min.
WB
The total proteins were extracted from kidney tissues and HK-2 cells using a commercial kit (Beyotime, Jiangsu, China). Subsequently, a protein assay kit (Bio-Rad, Hercules, CA, USA) was used for quantification, in accordance with the protocol provided by the manufacturer. Electrophoresis on 10% sodium dodecyl sulfate-polyacrylamide gels was performed to separate equal amounts of protein (40 µg/lane), which were rapidly transferred to polyvinylidene fluoride membranes. The membranes were blocked using 5% fat-free milk for 2 hrs at room temperature. Proteins were incubated with the following primary antibodies overnight at 4ºC: anti-β-actin (1:1000), Dot1l (1:1000), α-SMA (1:1000), vimentin (1:1000), fibronectin (1:1000), PI3K (1:1000), p-AKT (1:800), and AKT (1:1000). Tris-buffered saline and Tween 20 buffer were used to remove excessive primary antibodies. Subsequently, the membranes were incubated with an appropriate secondary antibody at 37°C for 2 hrs, followed by removal of excessive secondary antibody and detection of protein bands through enhanced chemiluminescence. The levels of proteins were analyzed using the Image J Software (National Institutes of Health, Bethesda, MD, USA).
Renal Function
After reperfusion in vivo, blood samples were collected and centrifuged, and the supernatant was collected. Creatinine and urea commercial kits (Nanjing Jiancheng Bioengineering Institute, Nanjing, China) were used to evaluate the levels of blood urea nitrogen and creatinine in the serum, according to the instructions provided by the manufacturer.
Histology Staining
Hematoxylin-eosin staining was performed on sections (4-µm thick) obtained from renal tissue, which had been fixed in 4% paraformaldehyde and embedded in paraffin. Two pathologists experienced in nephropathology and blinded to the group assignments determined the morphological changes. The degree of renal tubular injury was assessed according to the pathological scores (0–5 points).17
Masson’s Trichrome Staining
After fixation in 10% phosphate-buffered formalin, the tissues were sectioned (thickness: 5 μm). Following gradual deparaffinization and hydration, the sections were stained with Masson’s trichrome stain. Subsequently, they were observed by an experienced pathologist who was blinded to the treatments.
Immunohistochemistry
A commercial chemical kit (ZSGB-BIO, Beijing, China) was used to perform immunohistochemical staining. Sections (4-µm thick) were incubated with anti-Dot1l and anti-α-SMA primary antibodies overnight at 4°C, followed by incubation with secondary antibody at 37°C for 30 mins and addition of a coloring agent. Under the microscope, five different fields were randomly selected to evaluate the intensity of staining.
Small Interfering RNA (siRNA) Transfection
SiRNAs specific to Dot1l were used to transfect HK-2 cells for 6 hrs. Meanwhile, non-targeting siRNAs (Santa Cruz Biotechnology) were employed to transfect HK-2 cells for 48 hrs as a negative control. The concentration of all transfected siRNAs was 100 µM. Transient transfections were performed with LipofectamineTM3000 (Invitrogen, Carlsbad, CA, USA). After transfection, the cells were cultured in DMEM/F12 including 0.2% fetal bovine serum for 48 hrs. Subsequently, qRT-PCR was used to ascertain the effects of siRNA transfection.
Measurement of the Level of MDA and SOD Activity
The radioimmunoprecipitation assay buffer was prepared to lyse the HK-2 cells as previously described.18 The MDA and SOD assay kits were used to determine the level of MDA and SOD activity in cell lysates according to the instructions provided by the manufacturer.
Assay for the Production of Hydrogen Peroxide (H2O2)
The Amplex Red assay was conducted to detect the production of H2O2 as previously described.19 Briefly, HK-2 cells, as well as HK-2 cells pretreated with reagents, were cultured in Dulbecco’s Modified Eagle’s Medium/F12 including 0.2% fetal bovine serum. Subsequently, the production of H2O2 in HK-2 cells was measured using the Amplex Red assay. For the detection of the level of H2O2 in kidney tissues, the kidneys were primarily perfused and homogenized as previously described.20 Amplex Red (100 μM), containing 10 U/mL horseradish peroxidase, was used to detect H2O2 in the homogenate (incubated for 1 hr at 37°C) in accordance with the recommendations of the manufacturer. Subsequently, the fluorescence readings were normalized and assessed.
Statistical Analysis
All values were normally distributed and expressed as the mean±standard error of the mean. Differences between groups were compared using one-way analysis of variance and the Student–Newman–Keuls test. P<0.05 denoted statistical significance. All statistical tests were conducted with the GraphPad Prism software version 5.0 (GraphPad Software, Inc., San Diego, CA, USA).
Results
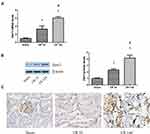
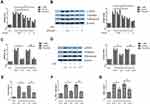
The Expression of Dot1l Was Upregulated After Renal I/R
The expression of Dot1l was initially determined at 0, 7, and 14 days after renal I/R using WB and PCR (Figure 1A and B). The expression of Dot1l was markedly increased with the extension of the reperfusion time, especially after 14 days. Meanwhile, immunohistochemical staining indicated that Dot1l was mainly located in the nucleus and its expression was elevated at 14 days of reperfusion (Figure 1C). These results suggested that Dot1l may be involved in the development of renal fibrosis after I/R, and 14-day reperfusion was used in the following experiments.
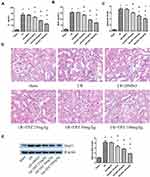
Inhibition of Dot1l Attenuated Renal Injury After I/R
EPZ was used for the inhibition of Dot1l. The results of the creatinine and blood urea nitrogen analyses showed that I/R could lead to obvious renal damage, and EPZ improved renal dysfunction, especially in the 100 mg/kg group (Figure 2A and B). The renal tissue morphology after treatment with EPZ for renal I/R is also shown in Figure 2C and D. The renal tissues were almost normal in the sham group; however, they exhibited acute damage in the proximal tubules in the I/R group, such as tubular dilatation and loss of the brush border. EPZ prevented loss of the brush border in the tubular epithelium, with the greatest protective effects observed at 100 mg/kg. WB indicated that EPZ could reduce the expression of Dot1l, with the greatest inhibitory effect noted at 100 mg/kg (Figure 2E). These results demonstrated that inhibition of Dot1l markedly attenuated renal injury after I/R.
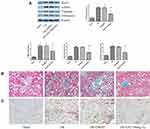
Inhibition of Dot1l Alleviated Fibrosis Induced by Renal I/R
WB indicated that EPZ could reduce the expression of α-SMA, vimentin, and fibronectin induced by I/R (Figure 3A). Masson’s trichrome staining revealed limited deposition of collagen in the sham group; however, deposition of tubulointerstitial collagen was observed in the I/R group. After treatment with EPZ, collagen deposition induced by I/R was dampened by the inhibition of Dot1l (Figure 3B). Moreover, immunohistochemical staining indicated that α-SMA was seldom found in the sham group. However, α-SMA was mainly localized in renal tubular epithelial cells in the I/R group. Moreover, the positive staining of α-SMA was reduced after treatment with EPZ (Figure 3C).
Fibrosis Induced by H/R Depended on Oxidative Stress in vitro
The results showed that H/R caused continuous accumulation of H2O2 and MDA as the reoxygenation time was extended, especially at 24 hrs. However, SOD activity was decreased with extension of the reoxygenation time (Figure 4A–C). Meanwhile, the expression of α-SMA, vimentin, and fibronectin at 24 hrs was higher than that measured at 12 hrs (Figure 4D). Therefore, 24-h reoxygenation was selected for the subsequent experiment. We investigated the relationship between fibrosis and oxidative stress using the established antioxidant NAC (1 µM) to reduce the oxidative products and stress. Following the administration of NAC, the expression of α-SMA, vimentin, and fibronectin in HK-2 cells was decreased compared with that recorded in the H/R group (Figure 4E).
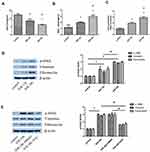
Inhibition of Dot1l Attenuated H/R-Induced Fibrosis in HK-2 Cells
Different concentrations of EPZ were used in HK-2 cells subjected to 12 hrs of hypoxia and 24 hrs of reoxygenation. The results of PCR and WB indicated that EPZ could reduce the expression of α-SMA, vimentin, and fibronectin in a dose-dependent manner (Figure 5A and B). Subsequently, siRNA against Dot1l was used to further clarify the effect of Dot1l inhibition on H/R-induced oxidative stress and fibrosis in HK-2 cells (Figure 5C and D). We observed that the expression of α-SMA, vimentin, and fibronectin was markedly decreased compared with that reported in the control group. As shown in Figure 5E–G, the levels of H2O2 production and MDA content in cells pretreated with either EPZ or siRNA against Dot1l were decreased compared with those observed in the H/R and H/R+si-NC groups. Furthermore, EPZ or siRNA against Dot1l could reverse the decreased SOD activity caused by H/R.
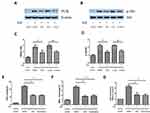
Inhibition of Dot1l Blocked Oxidative Stress via Downregulation of the PI3K/AKT Pathway in HK-2 Cells
The PI3K/AKT pathway plays a crucial role in the regulation of oxidative stress and fibrosis. The expression of PI3K and p-AKT/AKT was obviously increased in the H/R group compared with the control group. This effect could be reversed by siRNA against Dot1l or EPZ (Figure 6A–D). Wortmannin (10 µM), the inhibitor of PI3K, was administered 2 hrs prior to hypoxia to further investigate the relationship between PI3K/AKT and oxidative stress. Compared with the H/R+dimethyl sulfoxide group, the levels of H2O2 production and MDA content were notably decreased in the H/R+EPZ group or H/R+wortmannin group. In contrast, SOD activity was increased in the H/R+EPZ group or H/R+wortmannin group versus the H/R+dimethyl sulfoxide group. However, there were no obvious differences between the H/R+EPZ group and H/R+wortmannin group (Figure 6E–G). Collectively, these results revealed that inhibition of Dot1l blocked oxidative stress via inactivation of the PI3K/AKT pathway.
Inhibition of Dot1l Attenuated Oxidative Stress and Reduced PI3K/AKT Signaling in vivo
The effects of Dot1l inhibition on oxidative stress and fibrosis observed in vitro needed to be verified in vivo. As shown in Figure 7A–E, the increased expression of PI3K and p-AKT/AKT induced by I/R, as well as the production of H2O2 and MDA content, was inhibited by treatment with EPZ. Meanwhile, EPZ could reverse the decreased SOD activity induced by I/R. In summary, these results indicated that inhibition of Dot1l may prevent I/R injury by inhibiting the PI3K/AKT pathway and oxidative stress.
Discussion
In this study, we established in vivo and in vitro models of renal I/R injury. This was the first study to investigate the relationship between Dot1l and renal I/R injury. The results indicated that inhibition of Dot1l with chemical inhibitor EPZ attenuated I/R-induced damage, oxidative stress, and fibrosis in rats. In HK-2 cells, it was found that fibrosis induced by H/R depends on oxidative stress. In addition, knockdown of Dot1l or treatment with its inhibitor could reduce the H/R-induced fibrosis. Furthermore, through treatment with wortmannin (prior to H/R), we found that inhibition of Dot1l blocked oxidative stress via downregulation of the PI3K/AKT pathway in HK-2 cells. Therefore, the present study demonstrated that Dot1l may be an important therapeutic target for renal I/R injury and EPZ may be a promising agent for the treatment of AKI.
Renal I/R is an important cause of AKI which often arises after surgery, transplantation, hypovolemic conditions, and septic shock. I/R injury leads to functional and structural damage of the renal tubules by inducing tubular cell death, and these dying cells may trigger serious responses. I/R injury results in a rapid decline in kidney function and increased patient morbidity and mortality. The short- and long-term patient outcomes vary depending on the reversibility and recovery of the injury.21 In the past decades, therapeutic approaches to render organs more resistant to ischemia have been studied.22 However, effective treatment against renal I/R remains limited. In the present study, it was found that Dot1l expression was elevated with extension of reperfusion time after ischemic injury. Furthermore, the Dot1l selective inhibitor EPZ004777 could protect from renal I/R injury, alleviate tissue damage, and reverse renal dysfunction.
Renal fibrosis is a common event activated as a result of acute renal injury, subsequently leading to CKD. Renal interstitial fibrosis is typically characterized by extracellular matrix deposition and proliferation of tubulointerstitial fibroblasts in the kidney parenchyma. Hypoxia dampens tubular epithelial growth and leads to failure of remodeling by facilitating apoptosis and dedifferentiation. Profibrotic factors, including transforming growth factor-β mediating fibroblast activation and recruited leukocytes, which appear in hypoxic areas, contribute to the development of fibrosis.23 In our study, we found that I/R could lead to renal fibrosis. This finding was consistent with those reported in a previous study.24 Meanwhile, in our in vitro study, we found that the H/R process could elevate the expression of α-SMA, vimentin, and fibronectin in a time-dependent manner.
As a methyltransferase for methylation of histone H3K79, Dot1l is closely associated with tumors. A previous study reported that Dot1l played an important role in mixed lineage leukemia and also revealed several mechanisms involved in this process.25 In prostate cancer, it was found that Dot1l directly methylated the androgen receptor to regulate its activity.26 Moreover, in breast cancer cells, it was also shown that selective Dot1l inhibitors could suppress proliferation and migration.27 However, the role of Dot1l in renal ischemia remains unknown. In this study, we found that inhibition of Dot1l prevented the development of renal fibrosis induced by I/R. Furthermore, in our in vitro model, it was found that H/R increased the expression of fibrotic genes. Moreover, knockdown of Dot1l or treatment with its inhibitor could reduce the fibrosis induced by H/R.
Oxidative stress plays a crucial role in renal fibrosis. It was reported that ROS were involved in mesangial cell hypertrophy, activation of fibroblasts, and migration of tubular epithelial cells.28 In addition, ROS could increase the production of chemokines and growth factors.29 Different types of renal damage, such as AKI, result in upregulation of ROS production. The link between renal I/R and oxidative stress during fibrogenesis has been demonstrated.30 Renal I/R stimulated ROS generation; in turn, the generated ROS enhance I/R-induced damage. Herein, we replicated the observation of increased ROS generation along with the fibrotic response in HK-2 cells during H/R and in renal I/R tissues. We demonstrated that treatment with EPZ reduced the levels of I/R-induced ROS. Additionally, inhibition of Dot1l blocked oxidative stress and fibrosis via inactivation of the PI3K/AKT pathway. Furthermore, we demonstrated that inhibition of Dot1l attenuated oxidative stress and reduced PI3K/AKT signaling in vivo.
In conclusion, we identified the protective effect of Dot1l inhibition against renal I/R injury in vivo and in vitro. Furthermore, we also demonstrated that inhibition of Dot1l reduced renal fibrosis through the PI3K/AKT pathway. Overall, these results revealed that the Dot1l inhibitor can act as a promising therapeutic target for renal I/R injury.
Author Contributions
Chuan Yang performed the experiments and Zhiyuan Chen conceived and designed the experiments. All authors contributed toward data analysis, drafting and critically revising the article, agreed to be accountable for all aspects of the work, and read and approved the final version of the manuscript.
Disclosure
The authors report no conflicts of interest in this work.
References
1. Kunzendorf U, Haase M, Rolver L, Haase-Fielitz A. Novel aspects of pharmacological therapies for acute renal failure. Drugs. 2010;70(9):1099–1114. doi:10.2165/11535890-000000000-00000
2. Venkatachalam MA, Weinberg JM, Kriz W, Bidani AK. Failed tubule recovery, AKI-CKD transition, and kidney disease progression. J Am Soc Nephrol. 2015;26(8):1765–1776. doi:10.1681/ASN.2015010006
3. Xie Y, Xiao J, Fu C, Zhang Z, Ye Z, Zhang X. Ischemic preconditioning promotes autophagy and alleviates renal ischemia/reperfusion injury. Biomed Res Int. 2018;2018:8353987.
4. Zhao H, Alam A, Soo AP, George A, Ma D. Ischemia-reperfusion injury reduces long term renal graft survival: mechanism and beyond. EBioMedicine. 2018;28:31–42.
5. Javedan G, Shidfar F, Davoodi SH, et al. Conjugated linoleic acid rat pretreatment reduces renal damage in ischemia/reperfusion injury: unraveling antiapoptotic mechanisms and regulation of phosphorylated mammalian target of rapamycin. Mol Nutr Food Res. 2016;60(12):2665–2677. doi:10.1002/mnfr.201600112
6. Peng Q, Wu W, Wu KY, et al. The C5a/C5aR1 axis promotes progression of renal tubulointerstitial fibrosis in a mouse model of renal ischemia/reperfusion injury. Kidney Int. 2019;96:117–128. doi:10.1016/j.kint.2019.01.039
7. Min J, Feng Q, Li Z, Zhang Y, Xu RM. Structure of the catalytic domain of human DOT1L, a non-SET domain nucleosomal histone methyltransferase. Cell. 2003;112(5):711–723. doi:10.1016/S0092-8674(03)00114-4
8. Wong M, Tee A, Milazzo G, et al. The histone methyltransferase DOT1L promotes neuroblastoma by regulating gene transcription. Cancer Res. 2017;77(9):2522–2533. doi:10.1158/0008-5472.CAN-16-1663
9. McLean CM, Karemaker ID, van Leeuwen F. The emerging roles of DOT1L in leukemia and normal development. Leukemia. 2014;28(11):2131–2138. doi:10.1038/leu.2014.169
10. Kim W, Kim R, Park G, Park JW, Kim JE. Deficiency of H3K79 histone methyltransferase Dot1-like protein (DOT1L) inhibits cell proliferation. J Biol Chem. 2012;287(8):5588–5599. doi:10.1074/jbc.M111.328138
11. Oktyabri D, Ishimura A, Tange S, Terashima M, Suzuki T. DOT1L histone methyltransferase regulates the expression of BCAT1 and is involved in sphere formation and cell migration of breast cancer cell lines. Biochimie. 2016;123:20–31.
12. Gao Y, Ge W. The histone methyltransferase DOT1L inhibits osteoclastogenesis and protects against osteoporosis. Cell Death Dis. 2018;9(2):33. doi:10.1038/s41419-017-0040-5
13. Castano BM, Cailotto F, Kerkhof HJ, et al. Genome-wide association and functional studies identify the DOT1L gene to be involved in cartilage thickness and hip osteoarthritis. Proc Natl Acad Sci U S A. 2012;109(21):8218–8223. doi:10.1073/pnas.1119899109
14. Chatterjee PK, Cuzzocrea S, Brown PA, et al. Tempol, a membrane-permeable radical scavenger, reduces oxidant stress-mediated renal dysfunction and injury in the rat. Kidney Int. 2000;58(2):658–673. doi:10.1046/j.1523-1755.2000.00212.x
15. Noiri E, Nakao A, Uchida K, et al. Oxidative and nitrosative stress in acute renal ischemia. Am J Physiol Renal Physiol. 2001;281(5):F948–F957. doi:10.1152/ajprenal.2001.281.5.F948
16. de Araujo M, Andrade L, Coimbra TM, Rodrigues AJ, Seguro AC. Magnesium supplementation combined with N-acetylcysteine protects against postischemic acute renal failure. J Am Soc Nephrol. 2005;16(11):3339–3349. doi:10.1681/ASN.2004100832
17. Zhang YL, Zhang J, Cui LY, Yang S. Autophagy activation attenuates renal ischemia-reperfusion injury in rats. Exp Biol Med (Maywood). 2015;240(12):1590–1598. doi:10.1177/1535370215581306
18. Yuan XP, Liu LS, Chen CB, et al. MicroRNA-423-5p facilitates hypoxia/reoxygenation-induced apoptosis in renal proximal tubular epithelial cells by targeting GSTM1 via endoplasmic reticulum stress. Oncotarget. 2017;8(47):82064–82077. doi:10.18632/oncotarget.18289
19. Tong X, Khandelwal AR, Qin Z, et al. Role of smooth muscle Nox4-based NADPH oxidase in neointimal hyperplasia. J Mol Cell Cardiol. 2015;89(Pt B):185–194. doi:10.1016/j.yjmcc.2015.11.013
20. Babelova A, Avaniadi D, Jung O, et al. Role of Nox4 in murine models of kidney disease. Free Radic Biol Med. 2012;53(4):842–853. doi:10.1016/j.freeradbiomed.2012.06.027
21. Pefanis A, Ierino FL, Murphy JM, Cowan PJ. Regulated necrosis in kidney ischemia-reperfusion injury. Kidney Int. 2019;96:291–301. doi:10.1016/j.kint.2019.02.009
22. Chen X, Liu X, Wan X, Wu Y, Chen Y, Cao C. Ischemic preconditioning attenuates renal ischemia-reperfusion injury by inhibiting activation of IKKbeta and inflammatory response. Am J Nephrol. 2009;30(3):287–294. doi:10.1159/000225928
23. Tanaka T. A mechanistic link between renal ischemia and fibrosis. Med Mol Morphol. 2017;50(1):1–8. doi:10.1007/s00795-016-0146-3
24. Wang L, Chen H, Liu XH, et al. Ozone oxidative preconditioning inhibits renal fibrosis induced by ischemia and reperfusion injury in rats. Exp Ther Med. 2014;8(6):1764–1768. doi:10.3892/etm.2014.2004
25. De Vos D, Frederiks F, Terweij M, et al. Progressive methylation of ageing histones by Dot1 functions as a timer. EMBO Rep. 2011;12(9):956–962. doi:10.1038/embor.2011.131
26. Vlaming H, van Leeuwen F. The upstreams and downstreams of H3K79 methylation by DOT1L. Chromosoma. 2016;125(4):593–605. doi:10.1007/s00412-015-0570-5
27. Onder TT, Kara N, Cherry A, et al. Chromatin-modifying enzymes as modulators of reprogramming. Nature. 2012;483(7391):598–602. doi:10.1038/nature10953
28. Liu Y. Cellular and molecular mechanisms of renal fibrosis. Nat Rev Nephrol. 2011;7(12):684–696. doi:10.1038/nrneph.2011.149
29. Richter K, Konzack A, Pihlajaniemi T, Heljasvaara R, Kietzmann T. Redox-fibrosis: impact of TGFbeta1 on ROS generators, mediators and functional consequences. Redox Biol. 2015;6:344–352.
30. Qin T, Yin S, Yang J, et al. Sinomenine attenuates renal fibrosis through Nrf2-mediated inhibition of oxidative stress and TGFbeta signaling. Toxicol Appl Pharmacol. 2016;304:1–8.
 © 2019 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2019 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.